The Potential Use of Propolis as a Primary or an Adjunctive Therapy in Respiratory Tract-Related Diseases and Disorders: A Systematic Scoping Review
et al., Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2021.112595, Feb 2022
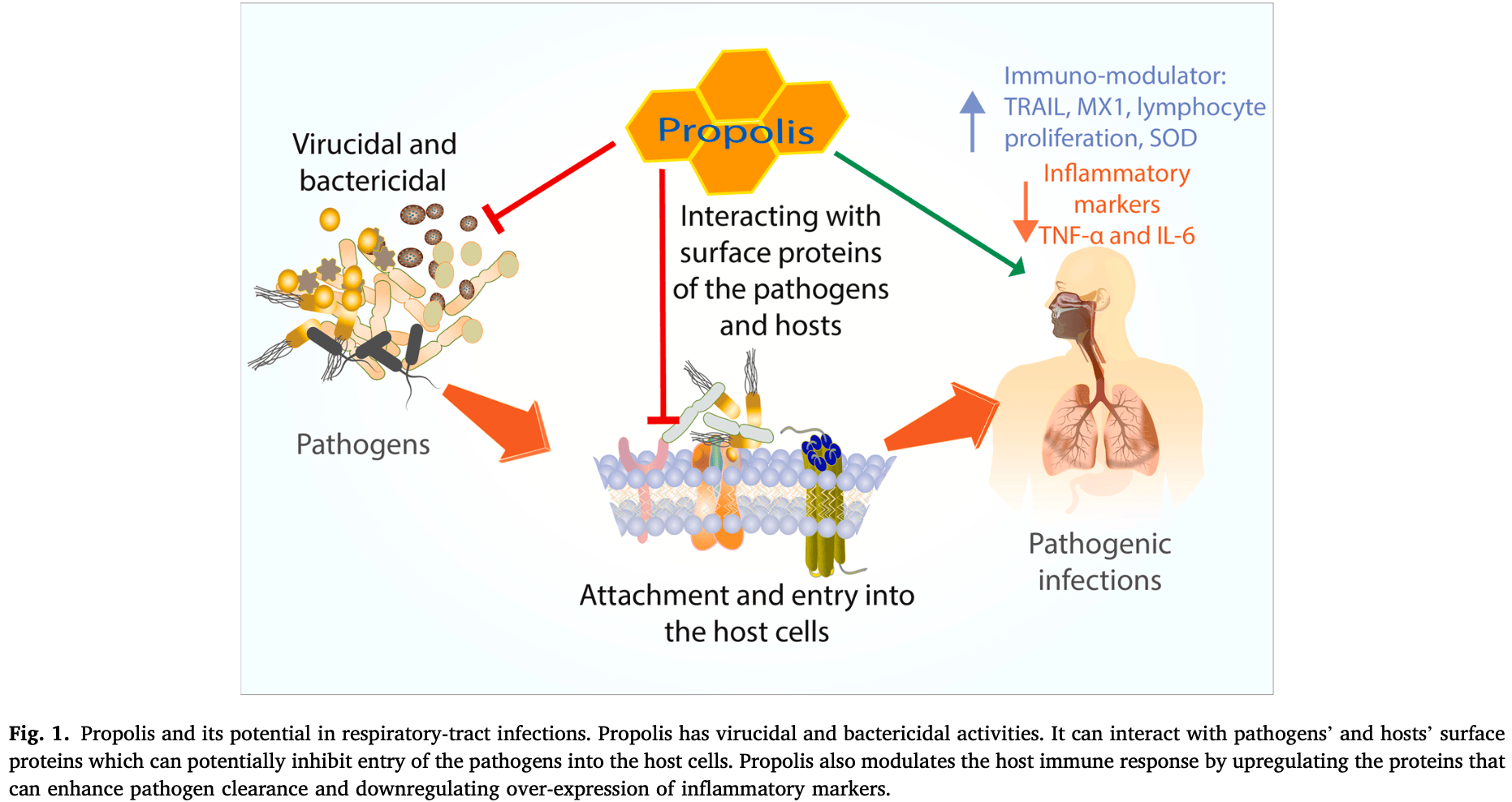
Review of the potential therapeutic benefits of propolis in treating respiratory tract-related diseases and disorders. Authors overview extensive in vitro, in silico, animal, and human clinical trial evidence demonstrating antiviral, antimicrobial, anti-inflammatory, anticancer, and immunomodulatory properties of propolis against respiratory illnesses like COVID-19, influenza, pneumonia, asthma, and more. Propolis bioactive compounds appear to inhibit pathogens' attachment and entry into host cells, enhance viral clearance, suppress inflammatory cytokines, upregulate antioxidants, and beneficially modulate immune responses. Authors conclude propolis and its compounds show promise as primary or adjunctive therapies for respiratory diseases, with mostly mild adverse effects in a handful of reports.
1.
Kustiawan et al., New insights of propolis nanoformulation and its therapeutic potential in human diseases, ADMET and DMPK, doi:10.5599/admet.2128.
2.
Ożarowski et al., The Effects of Propolis on Viral Respiratory Diseases, Molecules, doi:10.3390/molecules28010359.
3.
Zullkiflee et al., Propolis: Its Role and Efficacy in Human Health and Diseases, Molecules, doi:10.3390/molecules27186120.
Zulhendri et al., 28 Feb 2022, peer-reviewed, 9 authors.
Contact: felix.zulhendri@kebunefi.com, c.perera@auckland.ac.nz, steven.tandean@usu.ac.id, herry.herman@unpad.ac.id, andreas21008@mail.unpad.ac.id, dr.ckavita@gmail.com, arfiza_putra@yahoo.com, ronny@unpad.ac.id.
The Potential Use of Propolis as a Primary or an Adjunctive Therapy in Respiratory Tract-Related Diseases and Disorders: A Systematic Scoping Review
Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2021.112595
Propolis is a resinous beehive product that is collected by the bees from plant resin and exudates, to protect and maintain hive homeostasis. Propolis has been used by humans therapeutically to treat many ailments including respiratory tract-related diseases and disorders. The aim of the present systematic scoping review is to evaluate the experimental evidence to support the use of propolis as a primary or an adjunctive therapy in respiratory tract-related diseases and disorders. After applying the exclusion criteria, 158 research publications were retrieved and identified from Scopus, Web of Science, Pubmed, and Google Scholar. The key themes of the included studies were pathogenic infection-related diseases and disorders, inflammation-related disorders, lung cancers, and adverse effects. Furthermore, the potential molecular and biochemical mechanisms of action of propolis in alleviating respiratory tract-related diseases and disorders are discussed. In conclusion, the therapeutic benefits of propolis have been demonstrated by various in vitro studies, in silico studies, animal models, and human clinical trials. Based on the weight and robustness of the available experimental and clinical evidence, propolis is effective, either as a primary or an adjunctive therapy, in treating respiratory tract-related diseases.
Declaration of conflicting interests Kebun Efi produces the Indonesian stingless bee propolis extracts. All other authors declare no competing financial interests and no conflict of interest.
Appendix A. Supporting information Supplementary data associated with this article can be found in the online version at doi:10.1016/j.biopha.2021.112595.
References
Abu Almaaty, El-Aziz, Omar, Abdeen, Afifi et al., Antioxidant Property of the Egyptian Propolis Extract Versus Aluminum Silicate Intoxication on a Rat's Lung: Histopathological Studies, Molecules, doi:10.3390/molecules25245821
Ali, Blicharska, Shilpi, Seidel, Investigation of the anti-TB potential of selected propolis constituents using a molecular docking approach, Sci. Rep, doi:10.1038/s41598-018-30209-y
Almansour, Jarrar, Protective effect of propolis against pulmonary histological alterations induced by 10 nm naked gold nanoparticles, Chiang Mai J. Sci
Araújo, Mattar, Reis, Serra, Fialho et al., Pharmacognostic and acute toxicological evaluation of Scaptotrigona aff. postica propolis extract in preclinical assays, Nat. Prod. Res, doi:10.1080/14786419.2010.482059
Aru, Guzelmeric, Akgul, Demirel, Kirmizibekmez, Antiproliferative Activity of Chemically Characterized Propolis from Turkey and Its Mechanisms of Action, Chem. Biodivers, doi:10.1002/cbdv.201900189
Bae, Lee, -H, Kim, Chrysin suppresses mast cell-mediated allergic inflammation: Involvement of calcium, caspase-1 and nuclear factor-κB, Toxicol. Appl. Pharmacol, doi:10.1016/j.taap.2011.04.008
Banskota, Nagaoka, Sumioka, Tezuka, Awale et al., Antiproliferative activity of the Netherlands propolis and its active principles in cancer cell lines, J. Ethnopharmacol, doi:10.1016/S0378-8741(02)00022-3
Barroso, Cattani-Cavalieri, De Brito-Gitirana, Fautrel, Lagente et al., Propolis reversed cigarette smoke-induced emphysema through macrophage alternative activation independent of Nrf2, Bioorganic, Med. Chem, doi:10.1016/j.bmc.2017.08.026
Basista, Filipek, Allergy to propolis in Polish beekeepers, Post. DERMATOLOGII I Alergol, doi:10.5114/pdia.2012.32391
Bharti, Kumar, Kaur, Protective effect of bee propolis against antituberculosis drugs (Rifampicin and isoniazid)-induced hematological toxicity in sprague dawley rats, Asian, J. Pharm. Clin. Res, doi:10.22159/ajpcr.2017.v10i3.15991
Bilgin, Kismet, Kuru, Kaya, Senes et al., Ultrastructural investigation of the protective effects of propolis on bleomycin induced pulmonary fibrosis, Biotech. Histochem, doi:10.3109/10520295.2015.1123294
Bjermer, Westman, Holmström, Wickman, The complex pathophysiology of allergic rhinitis: Scientific rationale for the development of an alternative treatment option, Allergy, Asthma Clin. Immunol, doi:10.1186/s13223-018-0314-1
Brihoum, Maiza, Sahali, Boulmeltout, Barratt et al., Dual effect of Algerian propolis on lung cancer: Antitumor and chemopreventive effects involving antioxidant activity, Brazilian, J. Pharm. Sci, doi:10.1590/s2175-97902018000117396
Calhelha, Falcão, Queiroz, Vilas-Boas, Ferreira, Cytotoxicity of portuguese propolis: The proximity of the in vitro doses for tumor and normal cell lines, Biomed. Res. Int, doi:10.1155/2014/897361
Chen, Lee, Chang, Lee, Wei, Hot-pressurized fluid extraction of flavonoids and phenolic acids from Brazilian propolis and their cytotoxic assay in vitro, J. Chin. Inst. Chem. Eng, doi:10.1016/j.jcice.2007.04.004
Cohen, Varsano, Kahan, Sarrell, Uziel, Effectiveness of an Herbal Preparation Containing Echinacea, Propolis, and Vitamin C in Preventing Respiratory Tract Infections in Children: A Randomized, Double-blind, Placebo-Controlled, Multicenter Study, Arch. Pediatr. Adolesc. Med, doi:10.1001/archpedi.158.3.217
Cuzzolin, Francini-Pesenti, Verlato, Joppi, Baldelli et al., Use of herbal products among 392 Italian pregnant women: Focus on pregnancy outcome, Pharmacoepidemiol. Drug Saf, doi:10.1002/pds.2040
De Farias, Reis, Araújo, Araújo, Assunção et al., Effects of stingless bee propolis on experimental asthma, Evid. -Based Complement, Altern. Med, doi:10.1155/2014/951478
De Lima, Almeida, Alves, Rodrigues, Crotti et al., Biological properties of volatile oil from Brazilian brown propolis, Rev. Bras. Farm. J. Pharmacogn, doi:10.1016/j.bjp.2019.07.004
Debiaggi, Tateo, Pagani, Luini, Romero, Effects of propolis flavonoids on virus infectivity and replication, Microbiologica
Demir, Aliyazicioglu, Turan, Misir, Mentese et al., Antiproliferative and proapoptotic activity of Turkish propolis on human lung cancer cell line, Nutr. Cancer, doi:10.1080/01635581.2016.1115096
Dewi, Sahlan, Pratami, Agus, Salim et al., Identifying propolis compounds potential to be covid-19 therapies by targeting sars-cov-2 main protease, Int. J. Appl. Pharm, doi:10.22159/ijap.2021.v13s2.20
Drago, Mombelli, Vecchi, Fassina, Tocalli et al., In vitro antimicrobial activity of propolis dry extract, J. Chemother, doi:10.1179/joc.2000.12.5.390
Drago, Vecchi, Nicola, Gismondo, In vitro antimicrobial activity of a novel propolis formulation (Actichelated propolis), J. Appl. Microbiol, doi:10.1111/j.1365-2672.2007.03421.x
Eguchi, Matsunaga, Endo, Ichihara, Ikari, Kaempferide enhances chemosensitivity of human lung adenocarcinoma A549 cells mediated by the decrease in phosphorylation of Akt and claudin-2 expression, Nutrients, doi:10.3390/nu12041190
El-Aidy, Ebeid, Sallam, Muhammad, Abbas et al., Evaluation of propolis, honey, and royal jelly in amelioration of peripheral blood leukocytes and lung inflammation in mouse conalbumin-induced asthma model, Saudi J. Biol. Sci, doi:10.1016/j.sjbs.2014.11.005
El-Anwar, Abdelmonem, Abdelsameea, Alshawadfy, El-Kashishy, The Effect of Propolis in Healing Injured Nasal Mucosa: An Experimental Study, Int. Arch. Otorhinolaryngol, doi:10.1055/s-0036-1579664
El-Shouny, Muagam, Sadik, Hamza, Antimicrobial activity of propolis extract on URT infections in pediatric patients admitted to al-thowrah hospital, Hodeidah City, Yemen, World, J. Med. Sci, doi:10.5829/idosi.wjms.2012.7.3.6442
Elwakil, Shaaban, Bekhit, El-Naggar, Olama, Potential anti-COVID-19 activity of Egyptian propolis using computational modeling, Future Virol, doi:10.2217/fvl-2020-0329
Esposito, Garzarella, Bocchino, D'avino, Caruso et al., A standardized polyphenol mixture extracted from poplar-type propolis for remission of symptoms of uncomplicated upper respiratory tract infection (URTI): A monocentric, randomized, double-blind, placebo-controlled clinical trial, Phytomedicine, doi:10.1016/j.phymed.2020.153368
Fan, Ma, Zhang, Xu, Suolangzhaxi et al., Song, Microemulsion can improve the immune-enhancing activity of propolis flavonoid on immunosuppression and immune response, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2013.09.039
Fan, Wang, Liu, Hu, Zhao et al., Adjuvanticity of epimedium polysaccharide-propolis flavone on inactivated vaccines against AI and ND virus, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2012.08.025
Fang, Yan, Xu, He, Zhou et al., Tectochrysin ameliorates murine allergic airway inflammation by suppressing Th2 response and oxidative stress, Eur. J. Pharm, doi:10.1016/j.ejphar.2021.174100
Ferkol, Schraufnagel, The global burden of respiratory disease, Ann. Am. Thorac. Soc, doi:10.1513/AnnalsATS.201311-405PS
Fiorini, Scorza, De Almeida, Fonseca, Finsterer et al., Antiviral activity of brazilian green propolis extract against sars-cov-2 (Severe acute respiratory syndrome-coronavirus 2) infection: Case report and review, Clinics, doi:10.6061/clinics/2021/e2357
Frión-Herrera, Díaz-García, Ruiz-Fuentes, Rodríguez-Sánchez, Sforcin, Brazilian green propolis induced apoptosis in human lung cancer A549 cells through mitochondrial-mediated pathway, J. Pharm. Pharm, doi:10.1111/jphp.12449
Garegnani, Madrid, Meza, Misleading clinical evidence and systematic reviews on ivermectin for COVID-19, BMJ Evid. -Based Med, doi:10.1136/bmjebm-2021-111678
Goodwin, Jenkins, Molecular endotyping of pulmonary fibrosis, Chest, doi:10.1378/chest.15-1511
Governa, Cusi, Borgonetti, Sforcin, Terrosi et al., Beyond the biological effect of a chemically characterized poplar propolis: Antibacterial and antiviral activity and comparison with flurbiprofen in cytokines release by LPS-stimulated human mononuclear cells, Biomedicines, doi:10.3390/biomedicines7040073
Gu, Zhang, Du, Shen, Zhu, Pinocembrin attenuates allergic airway inflammation via inhibition of NF-κB pathway in mice, Int. Immunopharmacol, doi:10.1016/j.intimp.2017.10.005
Guler, Tatar, Yildiz, Belduz, Kolayli, Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study, Arch. Microbiol, doi:10.1007/s00203-021-02351-1
Guzmán-Gutiérrez, Nieto-Camacho, Castillo-Arellano, Huerta-Salazar, Hernández-Pasteur et al., Mexican propolis: A source of antioxidants and anti-inflammatory compounds, and isolation of a novel chalcone and ε-caprolactone derivative, Molecules, doi:10.3390/molecules23020334
Hayakari, Matsumiya, Xing, Tayone, Dempoya et al., Effects of Brazilian green propolis on double-stranded RNA-mediated induction of interferon-inducible gene and inhibition of recruitment of polymorphonuclear cells, J. Sci. Food Agric, doi:10.1002/jsfa.5892
Hemmati, Dianat, Jalali, Evaluation of the Effect of Caffeic Acid Phenethyl Ester (CAPE) on Pharmacological Responses of Isolated Rat Trachea in vitro, Tanaffos
Hirayama, Lee, Binns, Taniguchi, Dietary supplementation by Japanese patients with chronic obstructive pulmonary disease, Complement. Ther. Med, doi:10.1016/j.ctim.2008.02.007
Ilhan-Ayisigi, Ulucan, Saygili, Saglam-Metiner, Gulce-Iz et al., Nano-vesicular formulation of propolis and cytotoxic effects in a 3D spheroid model of lung cancer, J. Sci. Food Agric, doi:10.1002/jsfa.10400
Inamura, Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification, Front. Oncol, doi:10.3389/fonc.2017.00193
Indasari, Marhendra, Wardhana, Extract Bee Propolis (Trigona sp) for Preventive Increase Protease Activity and Defect of Trachea Histology in Rats (Rattus norvegicus) Exposed to Cigarette Smoke, Ser. Earth Environ. Sci, doi:10.1088/1755-1315/391/1/012048
Ippolito, Floreno, Rinaldi, Trodella, Meroni et al., Efficacy of a propolis-based syrup (FARINGEL) in preventing radiation-induced Esophagitis in locally advanced lung cancer, Chemotherapy, doi:10.1159/000487897
Ismail, Farag, Propolis protects against bleomycin-induced pulmonary fibrosis through mitochondrial-dependent pathway: A histological study, Egypt, J. Histol, doi:10.1097/01.EHX.0000473798.21269.f1
Jabir, Al Ali, Biochemical study and gene expression of Glutathione-Stransferase (GST) in induced asthma in rat, Orient. J. Chem, doi:10.13005/ojc/310337
Joshi, Thakur, Mayank, Poduri, Exploring insights of hydroxychloroquine, a controversial drug in Covid-19: An update, Food Chem. Toxicol, doi:10.1016/j.fct.2021.112106
Jung, Lee, Choi, Yea, Choi et al., Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced asthma, Life Sci, doi:10.1016/j.lfs.2008.01.014
Jung, Lee, Kim, Choi, Park et al., Antiinflammatory activity of caffeic acid phenethyl ester (CAPE) extracted from Rhodiola sacra against lipopolysaccharide-induced inflammatory responses in mice, Process Biochem, doi:10.1016/j.procbio.2008.03.004
Kai, Obuchi, Yoshida, Watanabe, Tsutsumi et al., In vitro and in vivo anti-influenza virus activities of flavonoids and related compounds as components of Brazilian propolis (AF-08), J. Funct. Foods, doi:10.1016/j.jff.2014.03.019
Kao, Chang-Chien, Chang, Yeh, Wang, Propolis inhibits TGF-beta 1-induced epithelial-mesenchymal transition in human alveolar epithelial cells via PPAR gamma activation, Int. Immunopharmacol, doi:10.1016/j.intimp.2012.12.018
Kavaz, Kurnaz, Guvenc, Yarim, Aksoy, Effects of Oral Propolis on Mucosal Wound Healing after Endoscopic Nasal Surgery in a Rabbit Model, TURKISH, Arch. Otorhinolaryngol, doi:10.5152/tao.2019.4164
Khacha-Ananda, Tragoolpua, Chantawannakul, Tragoolpua, Antioxidant and anti-cancer cell proliferation activity of propolis extracts from two extraction methods, Asian Pac, J. Cancer Prev, doi:10.7314/APJCP.2013.14.11.6991
Khacha-Ananda, Tragoolpua, Chantawannakul, Tragoolpua, Propolis extracts from the northern region of Thailand suppress cancer cell growth through induction of apoptosis pathways, Invest. N. Drugs, doi:10.1007/s10637-016-0392-1
Khayrani, Irdiani, Aditama, Pratami, Lischer et al., Evaluating the potency of Sulawesi propolis compounds as ACE-2 inhibitors through molecular docking for COVID-19 drug discovery preliminary study, J. King Saud. Univ. -Sci, doi:10.1016/j.jksus.2020.101297
Khayyal, El-Ghazaly, El-Khatib, Hatem, De Vries et al., A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients, Fundam. Clin. Pharmacol, doi:10.1046/j.1472-8206.2003.00117.x
Khosravi, Alheidary, Nikaein, Asghari, Aspergillus fumigatus conidia stimulate lung epithelial cells (TC-1 JHU-1) to produce IL-12, IFN-γ, IL-13 and IL-17 cytokines: Modulatory effect of propolis extract | Effet de la propolis sur les cytokines induites par Aspergillus fumigatus, J. Mycol. Med, doi:10.1016/j.mycmed.2018.09.006
Kimoto, Koya-Miyata, Hino, Micallef, Hanaya et al., Pulmonary carcinogenesis induced by ferric nitrilotriacetate in mice and protection from it by Brazilian propolis and artepillin C, Virchows Arch, doi:10.1007/s004280000350
Koc, Tekeli, Kanbur, Karayigit, Liman, The effects of chrysin on lipopolysaccharide-induced sepsis in rats, J. Food Biochem, doi:10.1111/jfbc.13359
Koksel, Kaplan, Ozdulger, Tamer, Degirmenci et al., Oleic acid-induced lung injury in rats and effects of caffeic acid phenethyl ester, Exp. Lung Res, doi:10.1080/01902140590918876
Koksel, Ozdulger, Tamer, Cinel, Ercil et al., Effects of caffeic acid phenethyl ester on lipopolysaccharide-induced lung injury in rats, Pulm. Pharmacol. Ther, doi:10.1016/j.pupt.2005.03.006
Koo, Lee, Kim, Lee, Detoxification effects of aloe polysaccharide and propolis on the urinary excretion of metabolites in smokers, FOOD Chem. Toxicol, doi:10.1016/j.fct.2019.05.029
Kosari, Noureddini, Khamechi, Najafi, Ghaderi et al., The effect of propolis plus Hyoscyamus niger L. methanolic extract on clinical symptoms in patients with acute respiratory syndrome suspected to COVID-19: A clinical trial, Phyther. Res, doi:10.1002/ptr.7116
Kowacz, Pollack, Cells in new light: Ion concentration, voltage, and pressure gradients across a hydrogel membrane, ACS Omega, doi:10.1021/acsomega.0c02595
Kucukgul, Erdogan, Inhibition of cigarette smoke induced-inflammation and oxidative damage by caffeic acid phenethyl ester in A549 Cells, Asian J. Pharm
Kudo, Ishigatsubo, Aoki, Pathology of asthma, Front. Microbiol, doi:10.3389/fmicb.2013.00263
Kujumgiev, Tsvetkova, Serkedjieva, Bankova, Christov et al., Antibacterial, antifungal and antiviral activity of propolis of different geographic origin, J. Ethnopharmacol, doi:10.1016/S0378-8741(98)00131-7
Kumar, Dhanjal, Bhargava, Kaul, Wang et al., Withanone and Withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells, J. Biomol. Struct. Dyn. Jun, doi:10.1080/07391102.2020.1775704
Kumar, Dhanjal, Kaul, Wadhwa, Sundar, Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1772108
Kuropatnicki, Szliszka, Krol, Historical aspects of propolis research in modern times, Evid. -Based Complement, Altern. Med
Kustiawan, Phuwapraisirisan, Puthong, Palaga, Arung et al., Propolis from the stingless bee Trigona incisa from East Kalimantan, Indonesia, induces in vitro cytotoxicity and apoptosis in cancer cell lines, Asian Pac, J. Cancer Prev, doi:10.7314/APJCP.2015.16.15.6581
Kuwata, Takemura, Urushisaki, Fukuoka, Hosokawa-Muto et al., 3,4-dicaffeoylquinic acid, a major constituent of Brazilian propolis, increases TRAIL expression and extends the lifetimes of mice infected with the influenza a virus, Evid. -Based Complement, Altern. Med, doi:10.1155/2012/946867
Kwon, Shin, Perumalsamy, Wang, Ahn, Antiviral effects and possible mechanisms of action of constituents from Brazilian propolis and related compounds, J. Apic. Res, doi:10.1080/00218839.2019.1695715
La, Vázquez, Ruiz, Ortiz, Sierra, Stingless Bee's honey from Yucatán: Culture, traditional uses and nutraceutical potential
Larki, Hemmati, Arzi, Ghafurian, Borujerdnia et al., Regulatory effect of caffeic acid phenethyl ester on type I collagen and interferon-gamma in bleomycin-induced pulmonary fibrosis in rat, Res. Pharm. Sci
Larki-Harchegani, Hemmati, Arzi, Ghafurian-Boroojerdnia, Shabib et al., Evaluation of the Effects of Caffeic Acid Phenethyl Ester on Prostaglandin E-2 and Two Key Cytokines Involved in Bleomycin-induced Pulmonary Fibrosis, Iran, J. Basic Med. Sci
Lee, Jeong, Kim, Lee, Choi et al., Preparation of Caffeic Acid Phenethyl Ester-Incorporated Nanoparticles and Their Biological Activity, J. Pharm. Sci, doi:10.1002/jps.24278
Li, Awale, Tezuka, Kadota, Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship, Bioorganic, Med. Chem, doi:10.1016/j.bmc.2008.04.016
Li, Awale, Tezuka, Kadota, Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship, Bioorganic, Med. Chem, doi:10.1016/j.bmc.2008.04.016
Li, Awale, Tezuka, Kadota, Cytotoxic constituents of propolis from Myanmar and their structure-activity relationship, Biol. Pharm. Bull, doi:10.1248/bpb.32.2075
Li, Awale, Tezuka, Kadota, Cytotoxicity of constituents from Mexican propolis against a panel of six different cancer cell lines, Nat. Prod. Commun, doi:10.1177/1934578x1000501018
Liang, Feng, Wu, Zhong, Gao et al., Caffeic acid phenethyl ester suppressed growth and metastasis of nasopharyngeal carcinoma cells by inactivating the NF-kappa B pathway, DRUG Des. Dev. Ther, doi:10.2147/DDDT.S199182
Liao, Hsu, Chu, Fu, Caffeic acid phenethyl ester suppresses the induction of eotaxin in human lung fibroblast cells, J. Asthma, doi:10.3109/02770900903556405
Lim, Kim, Jeong, Choi, Jung, Chrysin increases the therapeutic efficacy of Docetaxel and mitigates Docetaxel-Induced edema, Integr. Cancer Ther, doi:10.1177/1534735416645184
Lin, Hsu, Chen, Chu, Fu, Caffeic acid phenethyl ester suppresses eotaxin secretion and nuclear p-STAT6 in human lung fibroblast cells, J. Microbiol. Immunol. Infect, doi:10.1016/j.jmii.2011.04.008
Lin, Liang, Lee, Chuang, Tseng, Antitumor progression potential of caffeic acid phenethyl ester involving p75 NTR in C6 glioma cells, Chem. Biol. Interact, doi:10.1016/j.cbi.2010.09.002
Lin, Tseng, Chang, Lee, Pulmonary tumour with high carcinoembryonic antigen titre caused by chronic propolis aspiration, Eur. Respir. J, doi:10.1183/09031936.00141706
Linawati, Wande, Dwija, Wiryawan, Sugiritama et al., Immunomodulator potency of Euphorbia milii and propolis combination tea (Emp) through the secretion of granzyme b that is connected with lung damage and liver toxicity in mycobacterium tuberculosis infected mice, Pharmakeftiki
Lirdprapamongkol, Sakurai, Abdelhamed, Yokoyama, Athikomkulchai et al., Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation, Int. J. Oncol, doi:10.3892/ijo.2013.1926
Lirdprapamongkol, Sakurai, Abdelhamed, Yokoyama, Maruyama et al., A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells, Oncol. Rep, doi:10.3892/or.2013.2667
Lobsiger, Borer-Reinhold, Weiss, Muller, Helbling, Allergy to propolis: Behind the symptoms of an immediate-type reaction a T-cell sensitization may be hiding, Allergologie, doi:10.5414/ALX01451
Lopes, Ferreira, Nesi, Lanzetti, Pires et al., Antioxidant action of propolis on mouse lungs exposed to short-term cigarette smoke, Bioorganic, Med. Chem, doi:10.1016/j.bmc.2013.10.044
Luo, Jiang, Wang, Fitzgerald, Hu et al., Analysis on herbal medicines utilized for treatment of COVID-19, Acta Pharm. Sin. B, doi:10.1016/j.apsb.2020.05.007
Ma, Zhang, Liu, Ge, Gu et al., Caffeic acid phenethyl ester alleviates asthma by regulating the airway microenvironment via the ROS-responsive MAPK/Akt pathway, Free Radic, Biol. Med, doi:10.1016/j.freeradbiomed.2016.09.012
Machado, Assunção, Da Silva, Reis, Costa et al., Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity, Evid. -Based Complement, Altern. Med, doi:10.1155/2012/157652
Mahani, Sulaeman, Anwar, Damanik, Ploeger, Efficacy of Propolis Supplementation to Accelerate Healing Process and Body Weight Recovery of Pulmonary Tuberculosis Patients, J. GIZI DAN Pangan, doi:10.25182/jgp.2018.13.1.1-10
Malik, Kumar, Kaul, Wadhwa, Sundar, Computational insights into the potential of withaferin-a, withanone and caffeic acid phenethyl ester for treatment of aberrant-EGFR driven lung cancers, Biomolecules, doi:10.3390/biom11020160
Marchisio, Esposito, Bianchini, Desantis, Galeone et al., Effectiveness of a propolis and zinc solution in preventing acute otitis media in children with a history of recurrent acute otitis media, Int. J. Immunopathol. Pharmacol, doi:10.1177/039463201002300219
Marco, Piccioni, Pagiotti, Pietrella, Antibiofilm and Antioxidant Activity of Propolis and Bud Poplar Resins versus Pseudomonas aeruginosa, Evid. -Based Complement. Altern. Med, doi:10.1155/2017/5163575
Marti, López, Gascón, Julve, Propolis nasal spray effectively improves recovery from infectious acute rhinitis and common cold symptoms in children: a pilot study, J. Biol. Regul. Homeost. Agents
Maruhashi, Eguchi, Akizuki, Hamada, Furuta et al., Chrysin enhances anticancer drug-induced toxicity mediated by the reduction of claudin-1 and 11 expression in a spheroid culture model of lung squamous cell carcinoma cells, Sci. Rep, doi:10.1038/s41598-019-50276-z
Menniti-Ippolito, Mazzanti, Santuccio, Moro, Calapai et al., Surveillance of suspected adverse reactions to natural health products in Italy, Pharmacoepidemiol. Drug Saf
Munn, Peters, Stern, Tufanaru, Mcarthur et al., Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach, BMC Med. Res. Methodol, doi:10.1186/s12874-018-0611-x
Nagaoka, Banskota, Tezuka, Harimaya, Koizumi et al., Inhibitory effects of caffeic acid phenethyl ester analogues on experimental lung metastasis of murine colon 26-L5 carcinoma cells, Biol. Pharm. Bull
Nurmagambetov, Kuwahara, Garbe, The economic burden of asthma in the United States, 2008-2013, Ann. Am. Thorac. Soc, doi:10.1513/AnnalsATS.201703-259OC
Oguzkaya-Artan, Koc, Silici, Ozturk, Activity of propolis in an experimental model of Pneumocystosis, Saudi Med. J
Ohkuma, Kanno, Asama, Doi-Takaki, Kawaguchi et al., Effect of dietary supplement containing brazilian propolis on the common cold, Pharmacometrics
Oliveira, Lima, Neto Mde, Bastos, Da Silva Filho et al., Evaluation of genotoxicity and antigenotoxicity of Artepillin C in V79 cells by the comet and micronucleus assays, Nutr. Cancer, doi:10.1080/01635581.2013.815233
Onlen, Duran, Atik, Savas, Altug et al., Antibacterial activity of propolis against MRSA and synergism with topical mupirocin, J. Altern. Complement. Med, doi:10.1089/acm.2007.7021
Ophori, Wemabu, Antimicrobial activity of propolis extract on bacteria isolated from nasopharynx of patients with upper respiratory tract infection admitted to Central Hospital, Benin City, Nigeria, AFRICAN, J. Microbiol. Res
Orodan, Vodnar, Toiu, Pop, Vlase et al., Phytochemical analysis, antimicrobial and antioxidant effect of some gemmotherapic remedies used in respiratory diseases, Farmacia
Orsolic, Sver, Terzic, Tadic, Basic, Inhibitory Effect of Water-Soluble Derivative of Propolis and Its Polyphenolic Compounds on Tumor Growth and Metastasizing Ability: A Possible Mode of Antitumor Action, Nutr. Cancer, doi:10.1207/s15327914nc4702_8
Oršolic, Šver, Terzić, Bašić, Peroral application of water-soluble derivative of propolis (WSDP) and its related polyphenolic compounds and their influence on immunological and antitumour activity, Vet. Res. Commun, doi:10.1007/s11259-005-3303-z
Oršolić, Bašić, Antitumor, hematostimulative and radioprotective action of water-soluble derivative of propolis (WSDP), Biomed. Pharmacother, doi:10.1016/j.biopha.2005.03.013
Oršolić, Bašić, Immunomodulation by water-soluble derivative of propolis: A factor of antitumor reactivity, J. Ethnopharmacol, doi:10.1016/S0378-8741(02)00329-X
Oršolić, Terzić, Šver, Bašić, Honey-bee products in prevention and/or therapy of murine transplantable tumours, J. Sci. Food Agric, doi:10.1002/jsfa.2041
Ozyurt, Sogut, Yildirim, Kart, Iraz et al., Inhibitory effect of caffeic acid phenethyl ester on bleomycineinduced lung fibrosis in rats, Clin. Chim. ACTA, doi:10.1016/j.cccn.2003.09.015
Pai, Lee, Chen, Leu, Weng, Propolin C Inhibited Migration and Invasion via Suppression of EGFR-Mediated Epithelial-to-Mesenchymal Transition in Human Lung Cancer Cells, Evid. -Based Complement. Altern. Med, doi:10.1155/2018/7202548
Paris, Peraza Lope, Masson, Delgado Kú, Ojeda, The organization of stingless beekeeping (Meliponiculture) at Mayapán, Yucatan, Mexico, J. Anthropol. Archaeol, doi:10.1016/j.jaa.2018.07.004
Patterson, Mcintyre, Clough, Rushton, Societal Impacts of Pandemics: Comparing COVID-19 With History to Focus Our Response, Front. Public Heal, doi:10.3389/fpubh.2021.630449
Peng, Yuan, Shen, Niu, Du et al., Immunopotentiation of four natural adjuvants co-administered with a highly pathogenic porcine reproductive and respiratory syndrome virus glycoprotein 5 subunit, Virus Genes, doi:10.1007/s11262-016-1299-9
Permatasari, Hasan, The effect of ethanol extract propolis (EEP) on the level of IFN-F and superoxide dismutase (SOD) activities in patients with MDR tuberculosis, Respirology. Conference
Peters, Godfrey, Khalil, Mcinerney, Parker et al., Guidance for conducting systematic scoping reviews, Int. J. Evid. Based Health, doi:10.1097/XEB.0000000000000050
Piñeros, De Lima, Rodrigues, Gembre, Bertolini et al., Green propolis increases myeloid suppressor cells and CD4+Foxp3+ cells and reduces Th2 inflammation in the lungs after allergen exposure, J. Ethnopharmacol, doi:10.1016/j.jep.2019.112496
Ponte, Silva, Maia, Bee-honey, propolis and Eucalyptus globulus extract: Pre-clinical toxicity study in Rodents, Pharmacogn. Mag
Popova, Dimitrova, Al-Lawati, Tsvetkova, Najdenski et al., Omani propolis: Chemical profiling, antibacterial activity and new propolis plant sources, Chem. Cent. J, doi:10.1186/1752-153X-7-158
Rasmussen, Cameron, Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal, Biol, J. Linn. Soc, doi:10.1111/j.1095-8312.2009.01341.x
Raso, Wistuba, Molecular pathogenesis of early-stage non-small cell lung cancer and a proposal for tissue banking to facilitate identification of new biomarkers, J. Thorac. Oncol, doi:10.1097/JTO.0b013e318074fe42
Refaat, Mady, Sarhan, Rateb, Alaaeldin, Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19, Int. J. Pharm, doi:10.1016/j.ijpharm.2020.120028
Rossi, Longo, Russo, Borrelli, Sautebin, The role of the phenethyl ester of caffeic acid (CAPE) in the inhibition of rat lung cyclooxygenase activity by propolis, Fitoterapia, doi:10.1016/S0367-326X(02)00188-0
Sahlan, Irdiani, Flamandita, Aditama, Alfarraj et al., Molecular interaction analysis of Sulawesi propolis compounds with SARS-CoV-2 main protease as preliminary study for COVID-19 drug discovery, J. King Saud. Univ. -Sci, doi:10.1016/j.jksus.2020.101234
Scheller, Dworniczak, Waldemar-Klimmek, Rajca, Tomczyk et al., Synergism between ethanolic extract of propolis (EEP) and antituberculosis drugs on growth of mycobacteria, Z. Fur Naturforsch. -Sect. C. J. Biosci, doi:10.1515/znc-1999-7-814
Scheller, Kawalski, Oklek, Dworniczak, Matsuno et al., Correlation between virulence of various strains of mycobacteria and their susceptibility to ethanolic extract of propolis (EEP), ZEITSCHRIFT FUR Naturforsch. C-A, J. Biosci
Serkedjieva, Manolova, Bankova, Anti-influenza virus effect of some propolis constituents and their analogues (esters of substituted cinnamic acids), J. Nat. Prod, doi:10.1021/np50081a003
Sezer, Sahin, Solak, Fidan, Kara et al., Effects of caffeic acid phenethyl ester on the histopathological changes in the lungs of cigarette smokeexposed rabbits, BASIC Clin. Pharmacol. Toxicol, doi:10.1111/j.1742-7843.2007.00111.x
Seçilmis, Silici, Bee product efficacy in children with upper respiratory tract infections, Turk. J. Pediatr, doi:10.24953/turkjped.2020.04.013
Shaha, Mizuguchi, Kitamura, Fujino, Yabumoto et al., Effect of royal jelly and brazilian green propolis on the signaling for histamine H1 receptor and interleukin-9 gene expressions responsible for the pathogenesis of the allergic rhinitis, Biol. Pharm. Bull, doi:10.1248/bpb.b18-00325
Shaldam, Yahya, Mohamed, Abdel-Daim, Naggar, In silico screening of potent bioactive compounds from honeybee products against COVID-19 target enzymes, Environ. Sci. Pollut. Res, doi:10.1007/s11356-021-14195-9
Shimizu, Hino, Tsutsumi, Yong, Watanabe et al., Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice, Antivir. Chem. Chemother, doi:10.1177/095632020801900102
Shinmei, Yano, Kagawa, Izawa, Akagi et al., Effect of Brazilian propolis on sneezing and nasal rubbing in experimental allergic rhinitis of mice, Immunopharmacol. Immunotoxicol, doi:10.3109/08923970903078443
Silveira, Capcha, Sanches, De Sousa Moreira, Garnica et al., Green propolis extract attenuates acute kidney injury and lung injury in a rat model of sepsis, Sci. Rep, doi:10.1038/s41598-021-85124-6
Silveira, Jong, Berretta, Galvão, Ribeiro et al., Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.111526
Sobočanec, Šverko, Balog, Šarić, Rusak et al., Oxidant/antioxidant properties of croatian native propolis, J. Agric. Food Chem, doi:10.1021/jf0612023
Sonoki, Tanimae, Furuta, Endo, Matsunaga et al., Caffeic acid phenethyl ester down-regulates claudin-2 expression at the transcriptional and post-translational levels and enhances chemosensitivity to doxorubicin in lung adenocarcinoma A549 cells, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2018.02.016
Speciale, Costanzo, Puglisi, Musumeci, Catania et al., Antibacterial activity of Propolis and its active principles alone and in combination with macrolides, beta-lactams and fluoroquinolones against microorganisms responsible for respiratory infections, J. Chemother, doi:10.1179/joc.2006.18.2.164
Sy, Wu, Chiang, Wang, Wu, Propolis extracts exhibit an immunoregulatory activity in an OVA-sensitized airway inflammatory animal model, Int. Immunopharmacol, doi:10.1016/j.intimp.2006.01.015
Sy, Yang, Chiu, Wu, The immunoregulatory effects of caffeic acid phenethyl ester on the cytokine secretion of peripheral blood mononuclear cells from asthmatic children, Pediatr. Neonatol, doi:10.1016/j.pedneo.2011.08.005
Takeshita, Watanabe, Toyama, Hayashi, Honda et al., Effect of Brazilian propolis on exacerbation of respiratory syncytial virus infection in mice exposed to tetrabromobisphenol A, a brominated flame retardant, Evid. -Based Complement, Altern. Med, doi:10.1155/2013/698206
Tani, Hasumi, Tatefuji, Hashimoto, Koshino et al., Inhibitory activity of Brazilian green propolis components and their derivatives on the release of cys-leukotrienes, Bioorg. Med. Chem, doi:10.1016/j.bmc.2009.11.007
Teerasripreecha, Phuwapraisirisan, Puthong, Kimura, Okuyama et al., In vitro antiproliferative/cytotoxic activity on cancer cell lines of a cardanol and a cardol enriched from Thai Apis mellifera propolis, BMC Complement. Altern. Med, doi:10.1186/1472-6882-12-27
Thirugnanasampandan, Raveendran, Jayakumar, Analysis of chemical composition and bioactive property evaluation of Indian propolis, Asian Pac, J. Trop. Biomed, doi:10.1016/S2221-1691(12)60114-2
Trabace, Tucci, Ciuffreda, Matteo, Fortunato et al., Natural" relief of pregnancy-related symptoms and neonatal outcomes: Above all do no harm, J. Ethnopharmacol, doi:10.1016/j.jep.2015.08.046
Trucchi, Paganino, Orsi, Amicizia, Tisa et al., Hospital and economic burden of influenza-like illness and lower respiratory tract infection in adults ≥50 yearsold, BMC Health Serv. Res, doi:10.1186/s12913-019-4412-7
Turkyilmaz, Alhan, Ercin, Vanizor, Kaklikkaya et al., Effects of Caffeic Acid Phenethyl Ester on Pancreatitis in Rats, J. Surg. Res, doi:10.1016/j.jss.2007.04.019
Umthong, Phuwapraisirisan, Puthong, Chanchao, In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines, BMC Complement. Altern. Med, doi:10.1186/1472-6882-11-37
Urushisaki, Takemura, Tazawa, Fukuoka, Hosokawa-Muto et al., Caffeoylquinic acids are major constituents with potent anti-influenza effects in brazilian green propolis water extract, Evid. -Based Complement. Altern. Med, doi:10.1155/2011/254914
Valcic, Montenegro, Mujica, Avila, Franzblau et al., Phytochemical, morphological, and biological investigations of propolis from Central Chile, Z. Fur Naturforsch. -Sect. C. J. Biosci, doi:10.1515/znc-1999-5-617
Vale, De, Rodrigues, Carvalho, Moura et al., Evidences of High Genetic Differentiation among Populations of the Stingless Bee Scaptotrigona depilis (Moure, 1942) in Piauí, Brazil, Bee World, doi:10.1080/0005772x.2020.1854998
Vekic, Ivanisevic, Zeljkovic, Spasojevic-Kalimanovska, Bogavac-Stanojevic et al., Effect of propolis and N-acetylcysteine supplementation on lipoprotein subclasses distribution and paraoxonase 1 activity in subjects with acute respiratory infection, J. Med. Biochem, doi:10.5937/jomb0-24695
Wang, Chu, Liang, Lin, Chiang, Caffeic acid phenethyl ester inhibits nuclear factor-kappaB and protein kinase B signalling pathways and induces caspase-3 expression in primary human CD4+ T cells, Clin. Exp. Immunol, doi:10.1111/j.1365-2249.2009.04067.x
Wang, Lin, Liang, Yang, Lee et al., The effect of caffeic acid phenethyl ester on the functions of human monocyte-derived dendritic cells, BMC Immunol, doi:10.1186/1471-2172-10-39
Wang, Sankarapandian, Cheng, Woo, Kwon et al., Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea, BMC Complement. Altern. Med, doi:10.1186/s12906-016-1043-y
Weng, Liao, Chen, Wu, Lin, Propolin H from Taiwanese propolis induces G1 arrest in human lung carcinoma cells, J. Agric. Food Chem, doi:10.1021/jf070201n
Wu, Hsu, Propolis-induced descending necrotizing mediastinitis and aspiration pneumonia, Ann. Thorac. Surg, doi:10.1016/j.athoracsur.2012.09.086
Yang, Guan, Li, Li, Li, Chrysin attenuates carrageenan-induced pleurisy and lung injury via activation of SIRT1/NRF2 pathway in rats, Eur. J. Pharm, doi:10.1016/j.ejphar.2018.08.015
Yang, Jit, Leung, Zheng, Feng et al., The economic burden of influenza-associated outpatient visits and hospitalizations in China: A retrospective survey, Infect. Dis. Poverty, doi:10.1186/s40249-015-0077-6
Yangi, Cengiz, Ustuner, Dincer, Ozbayer et al., Propolis protects endotoxin induced acute lung and liver inflammation through attenuating inflammatory responses and oxidative stress, J. Med. Food, doi:10.1089/jmf.2017.0151
Yildirim, Hacievliyagil, Kutlu, Aydin, Kurkcuoglu et al., Effect of water extract of Turkish propolis on tuberculosis infection in guinea-pigs, Pharmacol. Res, doi:10.1016/j.phrs.2003.10.007
Zaeemzadeh, Hemmati, Arzi, Jalali, Rashidi, Protective effect of caffeic acid phenethyl ester (CAPE) on amiodarone-induced pulmonary fibrosis in rat, Iran, J. Pharm. Res
Zhang, Liu, Liu, Du, Fu et al., Economic burden for lung cancer survivors in urban China, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph14030308
Zhang, Yan, Allergic contact stomatitis caused by propolis throat candies, Contact Dermat, doi:10.1111/cod.13525
Zhu, Wang, Ming, Chen, Zhang, Disease burden of COPD in china: A systematic review, Int. J. COPD, doi:10.2147/COPD.S161555
Zorlu, COVID-19 and Anatolian propolis: A case report, Acta Med. Mediterr, doi:10.19193/0393-6384_2021_2_188
Zujovic, The Randomized, Double-Blind, Placebo-Controlled Study Of Efficacy And Safety Of Propolis And N-Acetylcysteine Compared To Placebo In Adults In Acute Condition With Sputum Production, Am. J. Respir. Crit. Care Med
Zujovic, Zugic, The randomized, double-blind, placebo-controlled study of efficacy and safety of propolis and n-acetylcysteine compared to placebo in adults in acute condition with sputum production, Am. J. Respir. Crit. Care Med
Zujovic, Zuza, Djordjevic, Assessment of the Quality of Life of Patients with Acute Bronchitis on the Propolis with N-Acetylcisteine Versus N-Acetylcisteine, A7773-A7773, Am. J. Respir. Crit. Care Med, doi:10.1164/ajrccm-conference.2020.201.1_meetingabstracts.a7773
Zulhendri, Perera, Tandean, Can propolis be a useful adjuvant in brain and neurological disorders and injuries? A systematic scoping review of the latest experimental evidence, Biomedicines, doi:10.3390/biomedicines9091227
Živanović, Pavlović, Stojanović, Veljković, Attitudes to and prevalence of bee product usage in pediatric pulmonology patients, Eur, J. Integr. Med, doi:10.1016/j.eujim.2019.02.001
DOI record:
{
"DOI": "10.1016/j.biopha.2021.112595",
"ISSN": [
"0753-3322"
],
"URL": "http://dx.doi.org/10.1016/j.biopha.2021.112595",
"alternative-id": [
"S0753332221013822"
],
"article-number": "112595",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "The Potential Use of Propolis as a Primary or an Adjunctive Therapy in Respiratory Tract-Related Diseases and Disorders: A Systematic Scoping Review"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Biomedicine & Pharmacotherapy"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.biopha.2021.112595"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Author(s). Published by Elsevier Masson SAS."
}
],
"author": [
{
"affiliation": [],
"family": "Zulhendri",
"given": "Felix",
"sequence": "first"
},
{
"affiliation": [],
"family": "Perera",
"given": "Conrad O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tandean",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdulah",
"given": "Rizky",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herman",
"given": "Herry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Christoper",
"given": "Andreas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chandrasekaran",
"given": "Kavita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Putra",
"given": "Arfiza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lesmana",
"given": "Ronny",
"sequence": "additional"
}
],
"container-title": "Biomedicine & Pharmacotherapy",
"container-title-short": "Biomedicine & Pharmacotherapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"em-consulte.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
30
]
],
"date-time": "2021-12-30T11:46:48Z",
"timestamp": 1640864808000
},
"deposited": {
"date-parts": [
[
2023,
4,
17
]
],
"date-time": "2023-04-17T22:33:19Z",
"timestamp": 1681770799000
},
"funder": [
{
"DOI": "10.13039/501100015690",
"doi-asserted-by": "publisher",
"name": "Universitas Padjadjaran"
}
],
"indexed": {
"date-parts": [
[
2023,
11,
14
]
],
"date-time": "2023-11-14T08:13:26Z",
"timestamp": 1699949606214
},
"is-referenced-by-count": 9,
"issued": {
"date-parts": [
[
2022,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T00:00:00Z",
"timestamp": 1643673600000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
23
]
],
"date-time": "2021-12-23T00:00:00Z",
"timestamp": 1640217600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0753332221013822?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0753332221013822?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "112595",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
2
]
]
},
"published-print": {
"date-parts": [
[
2022,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Societal Impacts of Pandemics: Comparing COVID-19 With History to Focus Our Response",
"author": "Patterson",
"journal-title": "Front. Public Heal",
"key": "10.1016/j.biopha.2021.112595_bib1",
"volume": "9",
"year": "2021"
},
{
"article-title": "Misleading clinical evidence and systematic reviews on ivermectin for COVID-19",
"author": "Garegnani",
"journal-title": "BMJ Evid. -Based Med",
"key": "10.1016/j.biopha.2021.112595_bib2",
"year": "2021"
},
{
"DOI": "10.1016/j.fct.2021.112106",
"article-title": "Exploring insights of hydroxychloroquine, a controversial drug in Covid-19: An update",
"author": "Joshi",
"doi-asserted-by": "crossref",
"journal-title": "Food Chem. Toxicol.",
"key": "10.1016/j.biopha.2021.112595_bib3",
"volume": "151",
"year": "2021"
},
{
"DOI": "10.1513/AnnalsATS.201311-405PS",
"article-title": "The global burden of respiratory disease",
"author": "Ferkol",
"doi-asserted-by": "crossref",
"first-page": "404",
"journal-title": "Ann. Am. Thorac. Soc.",
"key": "10.1016/j.biopha.2021.112595_bib4",
"volume": "11",
"year": "2014"
},
{
"DOI": "10.1186/s40249-015-0077-6",
"article-title": "The economic burden of influenza-associated outpatient visits and hospitalizations in China: A retrospective survey",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "44",
"journal-title": "Infect. Dis. Poverty",
"key": "10.1016/j.biopha.2021.112595_bib5",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.3390/ijerph14030308",
"article-title": "Economic burden for lung cancer survivors in urban China",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "308",
"journal-title": "Int. J. Environ. Res. Public Health",
"key": "10.1016/j.biopha.2021.112595_bib6",
"volume": "14",
"year": "2017"
},
{
"DOI": "10.2147/COPD.S161555",
"article-title": "Disease burden of COPD in china: A systematic review",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "1353",
"journal-title": "Int. J. COPD",
"key": "10.1016/j.biopha.2021.112595_bib7",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1186/s12913-019-4412-7",
"article-title": "Hospital and economic burden of influenza-like illness and lower respiratory tract infection in adults ≥50 years-old",
"author": "Trucchi",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "BMC Health Serv. Res",
"key": "10.1016/j.biopha.2021.112595_bib8",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1513/AnnalsATS.201703-259OC",
"article-title": "The economic burden of asthma in the United States, 2008-2013",
"author": "Nurmagambetov",
"doi-asserted-by": "crossref",
"first-page": "348",
"journal-title": "Ann. Am. Thorac. Soc.",
"key": "10.1016/j.biopha.2021.112595_bib9",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1016/j.apsb.2020.05.007",
"article-title": "Analysis on herbal medicines utilized for treatment of COVID-19",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "1192",
"journal-title": "Acta Pharm. Sin. B.",
"key": "10.1016/j.biopha.2021.112595_bib10",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1155/2013/964149",
"article-title": "Historical aspects of propolis research in modern times",
"author": "Kuropatnicki",
"doi-asserted-by": "crossref",
"journal-title": "Evid. -Based Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.112595_bib11",
"volume": "2013",
"year": "2013"
},
{
"author": "De La Luz Ortiz Vázquez",
"key": "10.1016/j.biopha.2021.112595_bib12",
"series-title": "Stingless Bee’s honey from Yucatán: Culture, traditional uses and nutraceutical potential",
"year": "2016"
},
{
"DOI": "10.1016/j.jaa.2018.07.004",
"article-title": "The organization of stingless beekeeping (Meliponiculture) at Mayapán, Yucatan, Mexico",
"author": "Paris",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J. Anthropol. Archaeol.",
"key": "10.1016/j.biopha.2021.112595_bib13",
"volume": "52",
"year": "2018"
},
{
"DOI": "10.1097/XEB.0000000000000050",
"article-title": "Guidance for conducting systematic scoping reviews",
"author": "Peters",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Int. J. Evid. Based Health",
"key": "10.1016/j.biopha.2021.112595_bib14",
"volume": "13",
"year": "2015"
},
{
"DOI": "10.1186/s12874-018-0611-x",
"article-title": "Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach",
"author": "Munn",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "BMC Med. Res. Methodol.",
"key": "10.1016/j.biopha.2021.112595_bib15",
"volume": "18",
"year": "2018"
},
{
"article-title": "Effects of propolis flavonoids on virus infectivity and replication",
"author": "Debiaggi",
"first-page": "207",
"journal-title": "Microbiologica",
"key": "10.1016/j.biopha.2021.112595_bib16",
"volume": "13",
"year": "1990"
},
{
"DOI": "10.1016/j.ijpharm.2020.120028",
"article-title": "Optimization and evaluation of propolis liposomes as a promising therapeutic approach for COVID-19",
"author": "Refaat",
"doi-asserted-by": "crossref",
"journal-title": "Int. J. Pharm.",
"key": "10.1016/j.biopha.2021.112595_bib17",
"volume": "592",
"year": "2021"
},
{
"article-title": "Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity",
"author": "Kumar",
"first-page": "1",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "10.1016/j.biopha.2021.112595_bib18",
"year": "2020"
},
{
"article-title": "Withanone and Withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells",
"author": "Kumar",
"first-page": "1",
"journal-title": "J. Biomol. Struct. Dyn. Jun.",
"key": "10.1016/j.biopha.2021.112595_bib19",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1007/s00203-021-02351-1",
"article-title": "Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study",
"author": "Guler",
"doi-asserted-by": "crossref",
"first-page": "3557",
"journal-title": "Arch. Microbiol.",
"key": "10.1016/j.biopha.2021.112595_bib20",
"volume": "203",
"year": "2021"
},
{
"DOI": "10.1016/j.jksus.2020.101297",
"article-title": "Evaluating the potency of Sulawesi propolis compounds as ACE-2 inhibitors through molecular docking for COVID-19 drug discovery preliminary study",
"author": "Khayrani",
"doi-asserted-by": "crossref",
"journal-title": "J. King Saud. Univ. - Sci.",
"key": "10.1016/j.biopha.2021.112595_bib21",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.2217/fvl-2020-0329",
"article-title": "Potential anti-COVID-19 activity of Egyptian propolis using computational modeling",
"author": "Elwakil",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Future Virol.",
"key": "10.1016/j.biopha.2021.112595_bib22",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1007/s11356-021-14195-9",
"article-title": "In silico screening of potent bioactive compounds from honeybee products against COVID-19 target enzymes",
"author": "Shaldam",
"doi-asserted-by": "crossref",
"first-page": "40507",
"journal-title": "Environ. Sci. Pollut. Res.",
"key": "10.1016/j.biopha.2021.112595_bib23",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.22159/ijap.2021.v13s2.20",
"article-title": "Identifying propolis compounds potential to be covid-19 therapies by targeting sars-cov-2 main protease",
"author": "Dewi",
"doi-asserted-by": "crossref",
"first-page": "103",
"journal-title": "Int. J. Appl. Pharm.",
"key": "10.1016/j.biopha.2021.112595_bib24",
"volume": "13",
"year": "2021"
},
{
"article-title": "COVID-19 and Anatolian propolis: A case report",
"author": "Zorlu",
"first-page": "1229",
"journal-title": "Acta Med. Mediterr.",
"key": "10.1016/j.biopha.2021.112595_bib25",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.6061/clinics/2021/e2357",
"article-title": "Antiviral activity of brazilian green propolis extract against sars-cov-2 (Severe acute respiratory syndrome-coronavirus 2) infection: Case report and review",
"author": "Fiorini",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Clinics",
"key": "10.1016/j.biopha.2021.112595_bib26",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2021.111526",
"article-title": "BeeCovid Team., Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial",
"author": "Silveira",
"doi-asserted-by": "crossref",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.biopha.2021.112595_bib27",
"volume": "138",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7116",
"article-title": "The effect of propolis plus Hyoscyamus niger L. methanolic extract on clinical symptoms in patients with acute respiratory syndrome suspected to COVID‐19: A clinical trial",
"author": "Kosari",
"doi-asserted-by": "crossref",
"first-page": "4000",
"journal-title": "Phyther. Res.",
"key": "10.1016/j.biopha.2021.112595_bib28",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1021/np50081a003",
"article-title": "Anti-influenza virus effect of some propolis constituents and their analogues (esters of substituted cinnamic acids)",
"author": "Serkedjieva",
"doi-asserted-by": "crossref",
"first-page": "294",
"journal-title": "J. Nat. Prod.",
"key": "10.1016/j.biopha.2021.112595_bib29",
"volume": "55",
"year": "1992"
},
{
"DOI": "10.1155/2011/254914",
"article-title": "Caffeoylquinic acids are major constituents with potent anti-influenza effects in brazilian green propolis water extract",
"author": "Urushisaki",
"doi-asserted-by": "crossref",
"journal-title": "Evid. -Based Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.112595_bib30",
"volume": "2011",
"year": "2011"
},
{
"DOI": "10.1016/j.jff.2014.03.019",
"article-title": "In vitro and in vivo anti-influenza virus activities of flavonoids and related compounds as components of Brazilian propolis (AF-08)",
"author": "Kai",
"doi-asserted-by": "crossref",
"first-page": "214",
"journal-title": "J. Funct. Foods",
"key": "10.1016/j.biopha.2021.112595_bib31",
"volume": "8",
"year": "2014"
},
{
"DOI": "10.1016/S0378-8741(98)00131-7",
"article-title": "Antibacterial, antifungal and antiviral activity of propolis of different geographic origin",
"author": "Kujumgiev",
"doi-asserted-by": "crossref",
"first-page": "235",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib32",
"volume": "64",
"year": "1999"
},
{
"DOI": "10.3390/biomedicines7040073",
"article-title": "Beyond the biological effect of a chemically characterized poplar propolis: Antibacterial and antiviral activity and comparison with flurbiprofen in cytokines release by LPS-stimulated human mononuclear cells",
"author": "Governa",
"doi-asserted-by": "crossref",
"first-page": "73",
"journal-title": "Biomedicines",
"key": "10.1016/j.biopha.2021.112595_bib33",
"volume": "7",
"year": "2019"
},
{
"DOI": "10.1177/095632020801900102",
"article-title": "Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice",
"author": "Shimizu",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Antivir. Chem. Chemother.",
"key": "10.1016/j.biopha.2021.112595_bib34",
"volume": "19",
"year": "2008"
},
{
"DOI": "10.1515/znc-1999-5-617",
"article-title": "Phytochemical, morphological, and biological investigations of propolis from Central Chile",
"author": "Valcic",
"doi-asserted-by": "crossref",
"first-page": "406",
"journal-title": "Z. Fur Naturforsch. - Sect. C. J. Biosci.",
"key": "10.1016/j.biopha.2021.112595_bib35",
"volume": "54",
"year": "1999"
},
{
"article-title": "In vitro antimicrobial activity of propolis dry extract",
"author": "Drago",
"first-page": "102",
"journal-title": "J. Chemother.",
"key": "10.1016/j.biopha.2021.112595_bib36",
"volume": "13",
"year": "2001"
},
{
"DOI": "10.1179/joc.2006.18.2.164",
"article-title": "Antibacterial activity of Propolis and its active principles alone and in combination with macrolides, beta-lactams and fluoroquinolones against microorganisms responsible for respiratory infections",
"author": "Speciale",
"doi-asserted-by": "crossref",
"first-page": "164",
"journal-title": "J. Chemother.",
"key": "10.1016/j.biopha.2021.112595_bib37",
"volume": "18",
"year": "2006"
},
{
"DOI": "10.1089/acm.2007.7021",
"article-title": "Antibacterial activity of propolis against MRSA and synergism with topical mupirocin",
"author": "Onlen",
"doi-asserted-by": "crossref",
"first-page": "713",
"journal-title": "J. Altern. Complement. Med",
"key": "10.1016/j.biopha.2021.112595_bib38",
"volume": "13",
"year": "2007"
},
{
"article-title": "Antimicrobial activity of propolis extract on bacteria isolated from nasopharynx of patients with upper respiratory tract infection admitted to Central Hospital, Benin City, Nigeria, AFRICAN",
"author": "Ophori",
"first-page": "1719",
"journal-title": "J. Microbiol. Res.",
"key": "10.1016/j.biopha.2021.112595_bib39",
"volume": "4",
"year": "2010"
},
{
"DOI": "10.1186/1752-153X-7-158",
"article-title": "Omani propolis: Chemical profiling, antibacterial activity and new propolis plant sources",
"author": "Popova",
"doi-asserted-by": "crossref",
"first-page": "158",
"journal-title": "Chem. Cent. J.",
"key": "10.1016/j.biopha.2021.112595_bib40",
"volume": "7",
"year": "2013"
},
{
"article-title": "Phytochemical analysis, antimicrobial and antioxidant effect of some gemmotherapic remedies used in respiratory diseases",
"author": "Orodan",
"first-page": "224",
"journal-title": "Farmacia",
"key": "10.1016/j.biopha.2021.112595_bib41",
"volume": "64",
"year": "2016"
},
{
"DOI": "10.1155/2017/5163575",
"article-title": "Antibiofilm and Antioxidant Activity of Propolis and Bud Poplar Resins versus Pseudomonas aeruginosa",
"author": "De Marco",
"doi-asserted-by": "crossref",
"journal-title": "Evid. -Based Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.112595_bib42",
"volume": "2017",
"year": "2017"
},
{
"article-title": "Correlation between virulence of various strains of mycobacteria and their susceptibility to ethanolic extract of propolis (EEP), ZEITSCHRIFT FUR Naturforsch. C-A",
"author": "Scheller",
"first-page": "1040",
"journal-title": "J. Biosci.",
"key": "10.1016/j.biopha.2021.112595_bib43",
"volume": "53",
"year": "1998"
},
{
"DOI": "10.1515/znc-1999-7-814",
"article-title": "Synergism between ethanolic extract of propolis (EEP) and anti-tuberculosis drugs on growth of mycobacteria",
"author": "Scheller",
"doi-asserted-by": "crossref",
"first-page": "549",
"journal-title": "Z. Fur Naturforsch. - Sect. C. J. Biosci.",
"key": "10.1016/j.biopha.2021.112595_bib44",
"volume": "54",
"year": "1999"
},
{
"DOI": "10.3390/molecules23020334",
"article-title": "Mexican propolis: A source of antioxidants and anti-inflammatory compounds, and isolation of a novel chalcone and ε-caprolactone derivative",
"author": "Guzmán-Gutiérrez",
"doi-asserted-by": "crossref",
"first-page": "334",
"journal-title": "Molecules",
"key": "10.1016/j.biopha.2021.112595_bib45",
"volume": "23",
"year": "2018"
},
{
"DOI": "10.1016/j.bjp.2019.07.004",
"article-title": "Biological properties of volatile oil from Brazilian brown propolis",
"author": "de Lima",
"doi-asserted-by": "crossref",
"first-page": "807",
"journal-title": "Rev. Bras. Farm. J. Pharmacogn.",
"key": "10.1016/j.biopha.2021.112595_bib46",
"volume": "29",
"year": "2019"
},
{
"DOI": "10.1001/archpedi.158.3.217",
"article-title": "Effectiveness of an Herbal Preparation Containing Echinacea, Propolis, and Vitamin C in Preventing Respiratory Tract Infections in Children: A Randomized, Double-blind, Placebo-Controlled, Multicenter Study",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "217",
"journal-title": "Arch. Pediatr. Adolesc. Med.",
"key": "10.1016/j.biopha.2021.112595_bib47",
"volume": "158",
"year": "2004"
},
{
"DOI": "10.1177/039463201002300219",
"article-title": "Effectiveness of a propolis and zinc solution in preventing acute otitis media in children with a history of recurrent acute otitis media",
"author": "Marchisio",
"doi-asserted-by": "crossref",
"first-page": "567",
"journal-title": "Int. J. Immunopathol. Pharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib48",
"volume": "23",
"year": "2010"
},
{
"article-title": "Propolis nasal spray effectively improves recovery from infectious acute rhinitis and common cold symptoms in children: a pilot study",
"author": "Marti",
"first-page": "943",
"journal-title": "J. Biol. Regul. Homeost. Agents",
"key": "10.1016/j.biopha.2021.112595_bib49",
"volume": "31",
"year": "2017"
},
{
"DOI": "10.1016/j.phymed.2020.153368",
"article-title": "A standardized polyphenol mixture extracted from poplar-type propolis for remission of symptoms of uncomplicated upper respiratory tract infection (URTI): A monocentric, randomized, double-blind, placebo-controlled clinical trial",
"author": "Esposito",
"doi-asserted-by": "crossref",
"journal-title": "Phytomedicine",
"key": "10.1016/j.biopha.2021.112595_bib50",
"volume": "80",
"year": "2021"
},
{
"DOI": "10.24953/turkjped.2020.04.013",
"article-title": "Bee product efficacy in children with upper respiratory tract infections",
"author": "Seçilmiş",
"doi-asserted-by": "crossref",
"first-page": "634",
"journal-title": "Turk. J. Pediatr.",
"key": "10.1016/j.biopha.2021.112595_bib51",
"volume": "62",
"year": "2020"
},
{
"DOI": "10.5937/jomb0-24695",
"article-title": "Effect of propolis and N-acetylcysteine supplementation on lipoprotein subclasses distribution and paraoxonase 1 activity in subjects with acute respiratory infection",
"author": "Vekic",
"doi-asserted-by": "crossref",
"first-page": "467",
"journal-title": "J. Med. Biochem",
"key": "10.1016/j.biopha.2021.112595_bib52",
"volume": "39",
"year": "2020"
},
{
"article-title": "3,4-dicaffeoylquinic acid, a major constituent of Brazilian propolis, increases TRAIL expression and extends the lifetimes of mice infected with the influenza a virus",
"author": "Kuwata",
"journal-title": "Evid. -Based Complement. Altern. Med",
"key": "10.1016/j.biopha.2021.112595_bib53",
"volume": "2012",
"year": "2012"
},
{
"DOI": "10.1155/2013/698206",
"article-title": "Effect of Brazilian propolis on exacerbation of respiratory syncytial virus infection in mice exposed to tetrabromobisphenol A, a brominated flame retardant",
"author": "Takeshita",
"doi-asserted-by": "crossref",
"journal-title": "Evid. -Based Complement. Altern. Med",
"key": "10.1016/j.biopha.2021.112595_bib54",
"volume": "2013",
"year": "2013"
},
{
"DOI": "10.1002/jsfa.5892",
"article-title": "Effects of Brazilian green propolis on double-stranded RNA-mediated induction of interferon-inducible gene and inhibition of recruitment of polymorphonuclear cells",
"author": "Hayakari",
"doi-asserted-by": "crossref",
"first-page": "646",
"journal-title": "J. Sci. Food Agric.",
"key": "10.1016/j.biopha.2021.112595_bib55",
"volume": "93",
"year": "2013"
},
{
"DOI": "10.1016/j.ijbiomac.2012.08.025",
"article-title": "Adjuvanticity of epimedium polysaccharide-propolis flavone on inactivated vaccines against AI and ND virus",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "1028",
"journal-title": "Int. J. Biol. Macromol.",
"key": "10.1016/j.biopha.2021.112595_bib56",
"volume": "51",
"year": "2012"
},
{
"DOI": "10.1016/j.ijbiomac.2013.09.039",
"article-title": "Microemulsion can improve the immune-enhancing activity of propolis flavonoid on immunosuppression and immune response",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "126",
"journal-title": "Int. J. Biol. Macromol.",
"key": "10.1016/j.biopha.2021.112595_bib57",
"volume": "63",
"year": "2014"
},
{
"key": "10.1016/j.biopha.2021.112595_bib58",
"unstructured": "A. Permatasari, H. Hasan, The effect of ethanol extract propolis (EEP) on the level of IFN-F and superoxide dismutase (SOD) activities in patients with MDR tuberculosis, Respirology. Conference (2014) 58."
},
{
"DOI": "10.25182/jgp.2018.13.1.1-10",
"article-title": "Efficacy of Propolis Supplementation to Accelerate Healing Process and Body Weight Recovery of Pulmonary Tuberculosis Patients",
"author": "Mahani",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J. GIZI DAN Pangan.",
"key": "10.1016/j.biopha.2021.112595_bib59",
"volume": "13",
"year": "2018"
},
{
"article-title": "Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship, Bioorganic",
"author": "Li",
"first-page": "5434",
"journal-title": "Med. Chem.",
"key": "10.1016/j.biopha.2021.112595_bib60",
"volume": "16",
"year": "2008"
},
{
"DOI": "10.1248/bpb.32.2075",
"article-title": "Cytotoxic constituents of propolis from Myanmar and their structure-activity relationship",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "2075",
"journal-title": "Biol. Pharm. Bull.",
"key": "10.1016/j.biopha.2021.112595_bib61",
"volume": "32",
"year": "2009"
},
{
"article-title": "Cytotoxicity of constituents from Mexican propolis against a panel of six different cancer cell lines",
"author": "Li",
"first-page": "1601",
"journal-title": "Nat. Prod. Commun.",
"key": "10.1016/j.biopha.2021.112595_bib62",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1186/1472-6882-12-27",
"article-title": "In vitro antiproliferative/cytotoxic activity on cancer cell lines of a cardanol and a cardol enriched from Thai Apis mellifera propolis",
"author": "Teerasripreecha",
"doi-asserted-by": "crossref",
"first-page": "27",
"journal-title": "BMC Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.112595_bib63",
"volume": "12",
"year": "2012"
},
{
"DOI": "10.1186/1472-6882-11-37",
"article-title": "In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines",
"author": "Umthong",
"doi-asserted-by": "crossref",
"first-page": "37",
"journal-title": "BMC Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.112595_bib64",
"volume": "11",
"year": "2011"
},
{
"DOI": "10.1007/s10637-016-0392-1",
"article-title": "Propolis extracts from the northern region of Thailand suppress cancer cell growth through induction of apoptosis pathways",
"author": "Khacha-ananda",
"doi-asserted-by": "crossref",
"first-page": "707",
"journal-title": "Invest. N. Drugs",
"key": "10.1016/j.biopha.2021.112595_bib65",
"volume": "34",
"year": "2016"
},
{
"DOI": "10.1016/S2221-1691(12)60114-2",
"article-title": "Analysis of chemical composition and bioactive property evaluation of Indian propolis, Asian Pac",
"author": "Thirugnanasampandan",
"doi-asserted-by": "crossref",
"first-page": "651",
"journal-title": "J. Trop. Biomed.",
"key": "10.1016/j.biopha.2021.112595_bib66",
"volume": "2",
"year": "2012"
},
{
"DOI": "10.7314/APJCP.2015.16.15.6581",
"article-title": "Propolis from the stingless bee Trigona incisa from East Kalimantan, Indonesia, induces in vitro cytotoxicity and apoptosis in cancer cell lines",
"author": "Kustiawan",
"doi-asserted-by": "crossref",
"first-page": "6581",
"journal-title": "Asian Pac. J. Cancer Prev.",
"key": "10.1016/j.biopha.2021.112595_bib67",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1080/01635581.2016.1115096",
"article-title": "Antiproliferative and proapoptotic activity of Turkish propolis on human lung cancer cell line",
"author": "Demir",
"doi-asserted-by": "crossref",
"first-page": "165",
"journal-title": "Nutr. Cancer",
"key": "10.1016/j.biopha.2021.112595_bib68",
"volume": "68",
"year": "2016"
},
{
"DOI": "10.1016/S0378-8741(02)00022-3",
"article-title": "Antiproliferative activity of the Netherlands propolis and its active principles in cancer cell lines",
"author": "Banskota",
"doi-asserted-by": "crossref",
"first-page": "67",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib69",
"volume": "80",
"year": "2002"
},
{
"DOI": "10.1007/s004280000350",
"article-title": "Pulmonary carcinogenesis induced by ferric nitrilotriacetate in mice and protection from it by Brazilian propolis and artepillin C",
"author": "Kimoto",
"doi-asserted-by": "crossref",
"first-page": "259",
"journal-title": "Virchows Arch.",
"key": "10.1016/j.biopha.2021.112595_bib70",
"volume": "438",
"year": "2001"
},
{
"DOI": "10.1080/01635581.2013.815233",
"article-title": "Evaluation of genotoxicity and antigenotoxicity of Artepillin C in V79 cells by the comet and micronucleus assays",
"author": "de Oliveira",
"doi-asserted-by": "crossref",
"first-page": "1098",
"journal-title": "Nutr. Cancer",
"key": "10.1016/j.biopha.2021.112595_bib71",
"volume": "65",
"year": "2013"
},
{
"DOI": "10.1021/jf070201n",
"article-title": "Propolin H from Taiwanese propolis induces G1 arrest in human lung carcinoma cells",
"author": "Weng",
"doi-asserted-by": "crossref",
"first-page": "5289",
"journal-title": "J. Agric. Food Chem.",
"key": "10.1016/j.biopha.2021.112595_bib72",
"volume": "55",
"year": "2007"
},
{
"DOI": "10.1155/2018/7202548",
"article-title": "Propolin C Inhibited Migration and Invasion via Suppression of EGFR-Mediated Epithelial-to-Mesenchymal Transition in Human Lung Cancer Cells",
"author": "Pai",
"doi-asserted-by": "crossref",
"journal-title": "Evid. -Based Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.112595_bib73",
"volume": "2018",
"year": "2018"
},
{
"DOI": "10.1207/s15327914nc4702_8",
"article-title": "Inhibitory Effect of Water-Soluble Derivative of Propolis and Its Polyphenolic Compounds on Tumor Growth and Metastasizing Ability: A Possible Mode of Antitumor Action",
"author": "Orsolic",
"doi-asserted-by": "crossref",
"first-page": "156",
"journal-title": "Nutr. Cancer",
"key": "10.1016/j.biopha.2021.112595_bib74",
"volume": "47",
"year": "2003"
},
{
"DOI": "10.1016/S0378-8741(02)00329-X",
"article-title": "Immunomodulation by water-soluble derivative of propolis: A factor of antitumor reactivity",
"author": "Oršolić",
"doi-asserted-by": "crossref",
"first-page": "265",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib75",
"volume": "84",
"year": "2003"
},
{
"DOI": "10.1016/j.biopha.2005.03.013",
"article-title": "Antitumor, hematostimulative and radioprotective action of water-soluble derivative of propolis (WSDP)",
"author": "Oršolić",
"doi-asserted-by": "crossref",
"first-page": "561",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.biopha.2021.112595_bib76",
"volume": "59",
"year": "2005"
},
{
"DOI": "10.3892/or.2013.2667",
"article-title": "A flavonoid chrysin suppresses hypoxic survival and metastatic growth of mouse breast cancer cells",
"author": "Lirdprapamongkol",
"doi-asserted-by": "crossref",
"first-page": "2357",
"journal-title": "Oncol. Rep.",
"key": "10.1016/j.biopha.2021.112595_bib77",
"volume": "30",
"year": "2013"
},
{
"DOI": "10.3892/ijo.2013.1926",
"article-title": "Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation",
"author": "Lirdprapamongkol",
"doi-asserted-by": "crossref",
"first-page": "329",
"journal-title": "Int. J. Oncol.",
"key": "10.1016/j.biopha.2021.112595_bib78",
"volume": "43",
"year": "2013"
},
{
"DOI": "10.1177/1534735416645184",
"article-title": "Chrysin increases the therapeutic efficacy of Docetaxel and mitigates Docetaxel-Induced edema",
"author": "Lim",
"doi-asserted-by": "crossref",
"first-page": "496",
"journal-title": "Integr. Cancer Ther.",
"key": "10.1016/j.biopha.2021.112595_bib79",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1038/s41598-019-50276-z",
"article-title": "Chrysin enhances anticancer drug-induced toxicity mediated by the reduction of claudin-1 and 11 expression in a spheroid culture model of lung squamous cell carcinoma cells",
"author": "Maruhashi",
"doi-asserted-by": "crossref",
"first-page": "13753",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.biopha.2021.112595_bib80",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.3390/nu12041190",
"article-title": "Kaempferide enhances chemosensitivity of human lung adenocarcinoma A549 cells mediated by the decrease in phosphorylation of Akt and claudin-2 expression",
"author": "Eguchi",
"doi-asserted-by": "crossref",
"first-page": "1190",
"journal-title": "Nutrients",
"key": "10.1016/j.biopha.2021.112595_bib81",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1111/jphp.12449",
"article-title": "Brazilian green propolis induced apoptosis in human lung cancer A549 cells through mitochondrial-mediated pathway",
"author": "Frión-Herrera",
"doi-asserted-by": "crossref",
"first-page": "1448",
"journal-title": "J. Pharm. Pharm.",
"key": "10.1016/j.biopha.2021.112595_bib82",
"volume": "67",
"year": "2015"
},
{
"DOI": "10.2147/DDDT.S199182",
"article-title": "Caffeic acid phenethyl ester suppressed growth and metastasis of nasopharyngeal carcinoma cells by inactivating the NF-kappa B pathway",
"author": "Liang",
"doi-asserted-by": "crossref",
"first-page": "1335",
"journal-title": "DRUG Des. Dev. Ther.",
"key": "10.1016/j.biopha.2021.112595_bib83",
"volume": "13",
"year": "2019"
},
{
"DOI": "10.1016/j.jnutbio.2018.02.016",
"article-title": "Caffeic acid phenethyl ester down-regulates claudin-2 expression at the transcriptional and post-translational levels and enhances chemosensitivity to doxorubicin in lung adenocarcinoma A549 cells",
"author": "Sonoki",
"doi-asserted-by": "crossref",
"first-page": "205",
"journal-title": "J. Nutr. Biochem.",
"key": "10.1016/j.biopha.2021.112595_bib84",
"volume": "56",
"year": "2018"
},
{
"DOI": "10.1097/JTO.0b013e318074fe42",
"article-title": "Molecular pathogenesis of early-stage non-small cell lung cancer and a proposal for tissue banking to facilitate identification of new biomarkers",
"author": "Raso",
"doi-asserted-by": "crossref",
"journal-title": "J. Thorac. Oncol.",
"key": "10.1016/j.biopha.2021.112595_bib85",
"volume": "2",
"year": "2007"
},
{
"DOI": "10.3389/fonc.2017.00193",
"article-title": "Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification",
"author": "Inamura",
"doi-asserted-by": "crossref",
"first-page": "193",
"journal-title": "Front. Oncol.",
"key": "10.1016/j.biopha.2021.112595_bib86",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1016/S0367-326X(02)00188-0",
"article-title": "The role of the phenethyl ester of caffeic acid (CAPE) in the inhibition of rat lung cyclooxygenase activity by propolis",
"author": "Rossi",
"doi-asserted-by": "crossref",
"journal-title": "Fitoterapia",
"key": "10.1016/j.biopha.2021.112595_bib87",
"volume": "73",
"year": "2002"
},
{
"DOI": "10.1089/jmf.2017.0151",
"article-title": "Propolis protects endotoxin induced acute lung and liver inflammation through attenuating inflammatory responses and oxidative stress",
"author": "Yangi",
"doi-asserted-by": "crossref",
"first-page": "1096",
"journal-title": "J. Med. Food",
"key": "10.1016/j.biopha.2021.112595_bib88",
"volume": "21",
"year": "2018"
},
{
"DOI": "10.1155/2012/157652",
"article-title": "Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity",
"author": "Machado",
"doi-asserted-by": "crossref",
"journal-title": "Evid. -Based Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.112595_bib89",
"volume": "2012",
"year": "2012"
},
{
"DOI": "10.1016/j.pupt.2005.03.006",
"article-title": "Effects of caffeic acid phenethyl ester on lipopolysaccharide-induced lung injury in rats",
"author": "Koksel",
"doi-asserted-by": "crossref",
"first-page": "90",
"journal-title": "Pulm. Pharmacol. Ther.",
"key": "10.1016/j.biopha.2021.112595_bib90",
"volume": "19",
"year": "2006"
},
{
"DOI": "10.1111/jfbc.13359",
"article-title": "The effects of chrysin on lipopolysaccharide-induced sepsis in rats",
"author": "Koc",
"doi-asserted-by": "crossref",
"journal-title": "J. Food Biochem.",
"key": "10.1016/j.biopha.2021.112595_bib91",
"volume": "44",
"year": "2020"
},
{
"DOI": "10.1038/s41598-021-85124-6",
"article-title": "Green propolis extract attenuates acute kidney injury and lung injury in a rat model of sepsis",
"author": "Silveira",
"doi-asserted-by": "crossref",
"first-page": "5925",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.biopha.2021.112595_bib92",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.cccn.2003.09.015",
"article-title": "Inhibitory effect of caffeic acid phenethyl ester on bleomycine-induced lung fibrosis in rats",
"author": "Ozyurt",
"doi-asserted-by": "crossref",
"first-page": "65",
"journal-title": "Clin. Chim. ACTA",
"key": "10.1016/j.biopha.2021.112595_bib93",
"volume": "339",
"year": "2004"
},
{
"article-title": "Regulatory effect of caffeic acid phenethyl ester on type I collagen and interferon-gamma in bleomycin-induced pulmonary fibrosis in rat",
"author": "Larki",
"first-page": "243",
"journal-title": "Res. Pharm. Sci.",
"key": "10.1016/j.biopha.2021.112595_bib94",
"volume": "8",
"year": "2013"
},
{
"article-title": "Evaluation of the Effects of Caffeic Acid Phenethyl Ester on Prostaglandin E-2 and Two Key Cytokines Involved in Bleomycin-induced Pulmonary Fibrosis, Iran",
"author": "Larki-Harchegani",
"first-page": "850",
"journal-title": "J. Basic Med. Sci.",
"key": "10.1016/j.biopha.2021.112595_bib95",
"volume": "16",
"year": "2013"
},
{
"DOI": "10.1097/01.EHX.0000473798.21269.f1",
"article-title": "Propolis protects against bleomycin-induced pulmonary fibrosis through mitochondrial-dependent pathway: A histological study",
"author": "Ismail",
"doi-asserted-by": "crossref",
"first-page": "732",
"journal-title": "Egypt. J. Histol.",
"key": "10.1016/j.biopha.2021.112595_bib96",
"volume": "38",
"year": "2015"
},
{
"DOI": "10.3109/10520295.2015.1123294",
"article-title": "Ultrastructural investigation of the protective effects of propolis on bleomycin induced pulmonary fibrosis",
"author": "Bilgin",
"doi-asserted-by": "crossref",
"first-page": "195",
"journal-title": "Biotech. Histochem.",
"key": "10.1016/j.biopha.2021.112595_bib97",
"volume": "91",
"year": "2016"
},
{
"DOI": "10.1016/j.jss.2007.04.019",
"article-title": "Effects of Caffeic Acid Phenethyl Ester on Pancreatitis in Rats",
"author": "Turkyilmaz",
"doi-asserted-by": "crossref",
"first-page": "19",
"journal-title": "J. Surg. Res.",
"key": "10.1016/j.biopha.2021.112595_bib98",
"volume": "145",
"year": "2008"
},
{
"article-title": "Protective effect of caffeic acid phenethyl ester (CAPE) on amiodarone-induced pulmonary fibrosis in rat, Iran",
"author": "Zaeemzadeh",
"first-page": "321",
"journal-title": "J. Pharm. Res.",
"key": "10.1016/j.biopha.2021.112595_bib99",
"volume": "10",
"year": "2011"
},
{
"DOI": "10.1016/j.sjbs.2014.11.005",
"article-title": "Evaluation of propolis, honey, and royal jelly in amelioration of peripheral blood leukocytes and lung inflammation in mouse conalbumin-induced asthma model",
"author": "El-Aidy",
"doi-asserted-by": "crossref",
"first-page": "780",
"journal-title": "Saudi J. Biol. Sci.",
"key": "10.1016/j.biopha.2021.112595_bib100",
"volume": "22",
"year": "2015"
},
{
"DOI": "10.1016/j.ejphar.2018.08.015",
"article-title": "Chrysin attenuates carrageenan-induced pleurisy and lung injury via activation of SIRT1/NRF2 pathway in rats",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Eur. J. Pharm.",
"key": "10.1016/j.biopha.2021.112595_bib101",
"volume": "836",
"year": "2018"
},
{
"DOI": "10.3390/molecules25245821",
"article-title": "Antioxidant Property of the Egyptian Propolis Extract Versus Aluminum Silicate Intoxication on a Rat’s Lung: Histopathological Studies",
"author": "Abu Almaaty",
"doi-asserted-by": "crossref",
"first-page": "5821",
"journal-title": "Molecules",
"key": "10.1016/j.biopha.2021.112595_bib102",
"volume": "25",
"year": "2020"
},
{
"article-title": "Protective effect of propolis against pulmonary histological alterations induced by 10 nm naked gold nanoparticles",
"author": "Almansour",
"first-page": "449",
"journal-title": "Chiang Mai J. Sci.",
"key": "10.1016/j.biopha.2021.112595_bib103",
"volume": "44",
"year": "2017"
},
{
"article-title": "Propolis reversed cigarette smoke-induced emphysema through macrophage alternative activation independent of Nrf2, Bioorganic",
"author": "Barroso",
"first-page": "5557",
"journal-title": "Med. Chem.",
"key": "10.1016/j.biopha.2021.112595_bib104",
"volume": "25",
"year": "2017"
},
{
"article-title": "Extract Bee Propolis (Trigona sp) for Preventive Increase Protease Activity and Defect of Trachea Histology in Rats (Rattus norvegicus) Exposed to Cigarette Smoke",
"author": "Indasari",
"key": "10.1016/j.biopha.2021.112595_bib105",
"series-title": "Ser. Earth Environ. Sci.",
"year": "2019"
},
{
"article-title": "Antioxidant action of propolis on mouse lungs exposed to short-term cigarette smoke, Bioorganic",
"author": "Lopes",
"first-page": "7570",
"journal-title": "Med. Chem.",
"key": "10.1016/j.biopha.2021.112595_bib106",
"volume": "21",
"year": "2013"
},
{
"DOI": "10.1111/j.1742-7843.2007.00111.x",
"article-title": "Effects of caffeic acid phenethyl ester on the histopathological changes in the lungs of cigarette smoke-exposed rabbits",
"author": "Sezer",
"doi-asserted-by": "crossref",
"first-page": "187",
"journal-title": "BASIC Clin. Pharmacol. Toxicol.",
"key": "10.1016/j.biopha.2021.112595_bib107",
"volume": "101",
"year": "2007"
},
{
"article-title": "Inhibition of cigarette smoke induced-inflammation and oxidative damage by caffeic acid phenethyl ester in A549 Cells",
"author": "Kucukgul",
"first-page": "S711",
"journal-title": "Asian J. Pharm.",
"key": "10.1016/j.biopha.2021.112595_bib108",
"volume": "10",
"year": "2016"
},
{
"DOI": "10.1016/j.fct.2019.05.029",
"article-title": "Detoxification effects of aloe polysaccharide and propolis on the urinary excretion of metabolites in smokers",
"author": "Koo",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "FOOD Chem. Toxicol.",
"key": "10.1016/j.biopha.2021.112595_bib109",
"volume": "130",
"year": "2019"
},
{
"DOI": "10.1378/chest.15-1511",
"article-title": "Molecular endotyping of pulmonary fibrosis",
"author": "Goodwin",
"doi-asserted-by": "crossref",
"first-page": "228",
"journal-title": "Chest",
"key": "10.1016/j.biopha.2021.112595_bib110",
"volume": "149",
"year": "2016"
},
{
"DOI": "10.1016/j.procbio.2008.03.004",
"article-title": "Anti-inflammatory activity of caffeic acid phenethyl ester (CAPE) extracted from Rhodiola sacra against lipopolysaccharide-induced inflammatory responses in mice",
"author": "Jung",
"doi-asserted-by": "crossref",
"first-page": "783",
"journal-title": "Process Biochem",
"key": "10.1016/j.biopha.2021.112595_bib111",
"volume": "43",
"year": "2008"
},
{
"DOI": "10.3109/08923970903078443",
"article-title": "Effect of Brazilian propolis on sneezing and nasal rubbing in experimental allergic rhinitis of mice",
"author": "Shinmei",
"doi-asserted-by": "crossref",
"first-page": "688",
"journal-title": "Immunopharmacol. Immunotoxicol.",
"key": "10.1016/j.biopha.2021.112595_bib112",
"volume": "31",
"year": "2009"
},
{
"DOI": "10.1016/j.bmc.2009.11.007",
"article-title": "Inhibitory activity of Brazilian green propolis components and their derivatives on the release of cys-leukotrienes",
"author": "Tani",
"doi-asserted-by": "crossref",
"first-page": "151",
"journal-title": "Bioorg. Med. Chem.",
"key": "10.1016/j.biopha.2021.112595_bib113",
"volume": "18",
"year": "2010"
},
{
"DOI": "10.1016/j.taap.2011.04.008",
"article-title": "Chrysin suppresses mast cell-mediated allergic inflammation: Involvement of calcium, caspase-1 and nuclear factor-κB",
"author": "Bae",
"doi-asserted-by": "crossref",
"first-page": "56",
"journal-title": "Toxicol. Appl. Pharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib114",
"volume": "254",
"year": "2011"
},
{
"DOI": "10.1016/j.intimp.2017.10.005",
"article-title": "Pinocembrin attenuates allergic airway inflammation via inhibition of NF-κB pathway in mice",
"author": "Gu",
"doi-asserted-by": "crossref",
"first-page": "90",
"journal-title": "Int. Immunopharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib115",
"volume": "53",
"year": "2017"
},
{
"DOI": "10.1248/bpb.b18-00325",
"article-title": "Effect of royal jelly and brazilian green propolis on the signaling for histamine H1 receptor and interleukin-9 gene expressions responsible for the pathogenesis of the allergic rhinitis",
"author": "Shaha",
"doi-asserted-by": "crossref",
"first-page": "1440",
"journal-title": "Biol. Pharm. Bull.",
"key": "10.1016/j.biopha.2021.112595_bib116",
"volume": "41",
"year": "2018"
},
{
"DOI": "10.1186/1471-2172-10-39",
"article-title": "The effect of caffeic acid phenethyl ester on the functions of human monocyte-derived dendritic cells",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "BMC Immunol.",
"key": "10.1016/j.biopha.2021.112595_bib117",
"volume": "10",
"year": "2009"
},
{
"DOI": "10.1016/j.pedneo.2011.08.005",
"article-title": "The immunoregulatory effects of caffeic acid phenethyl ester on the cytokine secretion of peripheral blood mononuclear cells from asthmatic children",
"author": "Sy",
"doi-asserted-by": "crossref",
"first-page": "327",
"journal-title": "Pediatr. Neonatol.",
"key": "10.1016/j.biopha.2021.112595_bib118",
"volume": "52",
"year": "2011"
},
{
"DOI": "10.1016/j.intimp.2006.01.015",
"article-title": "Propolis extracts exhibit an immunoregulatory activity in an OVA-sensitized airway inflammatory animal model",
"author": "Sy",
"doi-asserted-by": "crossref",
"first-page": "1053",
"journal-title": "Int. Immunopharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib119",
"volume": "6",
"year": "2006"
},
{
"DOI": "10.1016/j.lfs.2008.01.014",
"article-title": "Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced asthma",
"author": "Jung",
"doi-asserted-by": "crossref",
"first-page": "797",
"journal-title": "Life Sci.",
"key": "10.1016/j.biopha.2021.112595_bib120",
"volume": "82",
"year": "2008"
},
{
"DOI": "10.1155/2014/951478",
"article-title": "Effects of stingless bee propolis on experimental asthma",
"author": "De Farias",
"doi-asserted-by": "crossref",
"journal-title": "Evid. -Based Complement. Altern. Med",
"key": "10.1016/j.biopha.2021.112595_bib121",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.1016/j.ejphar.2021.174100",
"article-title": "Tectochrysin ameliorates murine allergic airway inflammation by suppressing Th2 response and oxidative stress",
"author": "Fang",
"doi-asserted-by": "crossref",
"journal-title": "Eur. J. Pharm.",
"key": "10.1016/j.biopha.2021.112595_bib122",
"volume": "902",
"year": "2021"
},
{
"DOI": "10.13005/ojc/310337",
"article-title": "Biochemical study and gene expression of Glutathione-S-transferase (GST) in induced asthma in rat",
"author": "Jabir",
"doi-asserted-by": "crossref",
"first-page": "1587",
"journal-title": "Orient. J. Chem.",
"key": "10.1016/j.biopha.2021.112595_bib123",
"volume": "31",
"year": "2015"
},
{
"DOI": "10.1016/j.jep.2019.112496",
"article-title": "Green propolis increases myeloid suppressor cells and CD4+Foxp3+ cells and reduces Th2 inflammation in the lungs after allergen exposure",
"author": "Piñeros",
"doi-asserted-by": "crossref",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib124",
"volume": "252",
"year": "2020"
},
{
"DOI": "10.1016/j.freeradbiomed.2016.09.012",
"article-title": "Caffeic acid phenethyl ester alleviates asthma by regulating the airway microenvironment via the ROS-responsive MAPK/Akt pathway",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "163",
"journal-title": "Free Radic. Biol. Med.",
"key": "10.1016/j.biopha.2021.112595_bib125",
"volume": "101",
"year": "2016"
},
{
"DOI": "10.1046/j.1472-8206.2003.00117.x",
"article-title": "A clinical pharmacological study of the potential beneficial effects of a propolis food product as an adjuvant in asthmatic patients",
"author": "Khayyal",
"doi-asserted-by": "crossref",
"first-page": "93",
"journal-title": "Fundam. Clin. Pharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib126",
"volume": "17",
"year": "2003"
},
{
"article-title": "The Randomized, Double-Blind, Placebo-Controlled Study Of Efficacy And Safety Of Propolis And N-Acetylcysteine Compared To Placebo In Adults In Acute Condition With Sputum Production",
"author": "Zujovic",
"first-page": "A2675",
"journal-title": "Am. J. Respir. Crit. Care Med. 195 MA-",
"key": "10.1016/j.biopha.2021.112595_bib127",
"year": "2017"
},
{
"article-title": "The randomized, double-blind, placebo-controlled study of efficacy and safety of propolis and n-acetylcysteine compared to placebo in adults in acute condition with sputum production",
"author": "Zujovic",
"first-page": "A3196",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "10.1016/j.biopha.2021.112595_bib128",
"volume": "197",
"year": "2018"
},
{
"article-title": "Assessment of the Quality of Life of Patients with Acute Bronchitis on the Propolis with N-Acetylcisteine Versus N-Acetylcisteine",
"author": "Zujovic",
"journal-title": "Am. J. Respir. Crit. Care Med. 201 MA-",
"key": "10.1016/j.biopha.2021.112595_bib129",
"year": "2020"
},
{
"DOI": "10.1016/j.ctim.2008.02.007",
"article-title": "Dietary supplementation by Japanese patients with chronic obstructive pulmonary disease",
"author": "Hirayama",
"doi-asserted-by": "crossref",
"first-page": "37",
"journal-title": "Complement. Ther. Med.",
"key": "10.1016/j.biopha.2021.112595_bib130",
"volume": "17",
"year": "2009"
},
{
"article-title": "Attitudes to and prevalence of bee product usage in pediatric pulmonology patients, Eur",
"author": "Živanović",
"first-page": "1",
"journal-title": "J. Integr. Med",
"key": "10.1016/j.biopha.2021.112595_bib131",
"volume": "27",
"year": "2019"
},
{
"DOI": "10.1186/s13223-018-0314-1",
"article-title": "The complex pathophysiology of allergic rhinitis: Scientific rationale for the development of an alternative treatment option",
"author": "Bjermer",
"doi-asserted-by": "crossref",
"first-page": "24",
"journal-title": "Allergy, Asthma Clin. Immunol.",
"key": "10.1016/j.biopha.2021.112595_bib132",
"volume": "15",
"year": "2019"
},
{
"DOI": "10.3389/fmicb.2013.00263",
"article-title": "Pathology of asthma",
"author": "Kudo",
"doi-asserted-by": "crossref",
"first-page": "263",
"journal-title": "Front. Microbiol.",
"key": "10.1016/j.biopha.2021.112595_bib133",
"volume": "4",
"year": "2013"
},
{
"DOI": "10.1183/09031936.00141706",
"article-title": "Pulmonary tumour with high carcinoembryonic antigen titre caused by chronic propolis aspiration",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "1227",
"journal-title": "Eur. Respir. J.",
"key": "10.1016/j.biopha.2021.112595_bib134",
"volume": "30",
"year": "2007"
},
{
"DOI": "10.1016/j.athoracsur.2012.09.086",
"article-title": "Propolis-induced descending necrotizing mediastinitis and aspiration pneumonia",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "e87",
"journal-title": "Ann. Thorac. Surg.",
"key": "10.1016/j.biopha.2021.112595_bib135",
"volume": "95",
"year": "2013"
},
{
"DOI": "10.1002/pds.1566",
"article-title": "Surveillance of suspected adverse reactions to natural health products in Italy",
"author": "Menniti-Ippolito",
"doi-asserted-by": "crossref",
"first-page": "626",
"journal-title": "Pharmacoepidemiol. Drug Saf.",
"key": "10.1016/j.biopha.2021.112595_bib136",
"volume": "17",
"year": "2008"
},
{
"DOI": "10.5414/ALX01451",
"article-title": "Allergy to propolis: Behind the symptoms of an immediate-type reaction a T-cell sensitization may be hiding",
"author": "Lobsiger",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Allergologie",
"key": "10.1016/j.biopha.2021.112595_bib137",
"volume": "35",
"year": "2012"
},
{
"DOI": "10.5114/pdia.2012.32391",
"article-title": "Allergy to propolis in Polish beekeepers",
"author": "Basista",
"doi-asserted-by": "crossref",
"first-page": "440",
"journal-title": "Post. DERMATOLOGII I Alergol.",
"key": "10.1016/j.biopha.2021.112595_bib138",
"volume": "29",
"year": "2012"
},
{
"DOI": "10.1111/cod.13525",
"article-title": "Allergic contact stomatitis caused by propolis throat candies",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "58",
"journal-title": "Contact Dermat.",
"key": "10.1016/j.biopha.2021.112595_bib139",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1002/pds.2040",
"article-title": "Use of herbal products among 392 Italian pregnant women: Focus on pregnancy outcome",
"author": "Cuzzolin",
"doi-asserted-by": "crossref",
"first-page": "1151",
"journal-title": "Pharmacoepidemiol. Drug Saf.",
"key": "10.1016/j.biopha.2021.112595_bib140",
"volume": "19",
"year": "2010"
},
{
"DOI": "10.1016/j.jep.2015.08.046",
"article-title": "“Natural” relief of pregnancy-related symptoms and neonatal outcomes: Above all do no harm",
"author": "Trabace",
"doi-asserted-by": "crossref",
"first-page": "396",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib141",
"volume": "174",
"year": "2015"
},
{
"article-title": "Bee-honey, propolis and Eucalyptus globulus extract: Pre-clinical toxicity study in Rodents",
"author": "Ponte",
"first-page": "278",
"journal-title": "Pharmacogn. Mag.",
"key": "10.1016/j.biopha.2021.112595_bib142",
"volume": "4",
"year": "2008"
},
{
"DOI": "10.1080/14786419.2010.482059",
"article-title": "Pharmacognostic and acute toxicological evaluation of Scaptotrigona aff. postica propolis extract in pre-clinical assays",
"author": "Araújo",
"doi-asserted-by": "crossref",
"first-page": "1037",
"journal-title": "Nat. Prod. Res.",
"key": "10.1016/j.biopha.2021.112595_bib143",
"volume": "25",
"year": "2011"
},
{
"DOI": "10.3390/biomedicines9091227",
"article-title": "Can propolis be a useful adjuvant in brain and neurological disorders and injuries? A systematic scoping review of the latest experimental evidence",
"author": "Zulhendri",
"doi-asserted-by": "crossref",
"first-page": "1227",
"journal-title": "Biomedicines",
"key": "10.1016/j.biopha.2021.112595_bib144",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1111/j.1095-8312.2009.01341.x",
"article-title": "Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal, Biol",
"author": "Rasmussen",
"doi-asserted-by": "crossref",
"first-page": "206",
"journal-title": "J. Linn. Soc.",
"key": "10.1016/j.biopha.2021.112595_bib145",
"volume": "99",
"year": "2010"
},
{
"DOI": "10.1080/0005772X.2020.1854998",
"article-title": "Evidences of High Genetic Differentiation among Populations of the Stingless Bee Scaptotrigona depilis (Moure, 1942) in Piauí, Brazil",
"author": "Vale",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Bee World",
"key": "10.1016/j.biopha.2021.112595_bib146",
"volume": "98",
"year": "2021"
},
{
"article-title": "Antimicrobial activity of propolis extract on URT infections in pediatric patients admitted to al-thowrah hospital, Hodeidah City, Yemen, World",
"author": "El-Shouny",
"first-page": "172",
"journal-title": "J. Med. Sci.",
"key": "10.1016/j.biopha.2021.112595_bib147",
"volume": "7",
"year": "2012"
},
{
"DOI": "10.1038/s41598-018-30209-y",
"article-title": "Investigation of the anti-TB potential of selected propolis constituents using a molecular docking approach",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "12238",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.biopha.2021.112595_bib148",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.1016/j.phrs.2003.10.007",
"article-title": "Effect of water extract of Turkish propolis on tuberculosis infection in guinea-pigs",
"author": "Yildirim",
"doi-asserted-by": "crossref",
"first-page": "287",
"journal-title": "Pharmacol. Res.",
"key": "10.1016/j.biopha.2021.112595_bib149",
"volume": "49",
"year": "2004"
},
{
"article-title": "Activity of propolis in an experimental model of Pneumocystosis",
"author": "Oguzkaya-Artan",
"first-page": "1115",
"journal-title": "Saudi Med. J.",
"key": "10.1016/j.biopha.2021.112595_bib150",
"volume": "29",
"year": "2008"
},
{
"article-title": "Protective effect of bee propolis against anti-tuberculosis drugs (Rifampicin and isoniazid)-induced hematological toxicity in sprague dawley rats, Asian",
"author": "Bharti",
"first-page": "188",
"journal-title": "J. Pharm. Clin. Res",
"key": "10.1016/j.biopha.2021.112595_bib151",
"volume": "10",
"year": "2017"
},
{
"article-title": "Immunomodulator potency of Euphorbia milii and propolis combination tea (Emp) through the secretion of granzyme b that is connected with lung damage and liver toxicity in mycobacterium tuberculosis infected mice",
"author": "Linawati",
"first-page": "50",
"journal-title": "Pharmakeftiki",
"key": "10.1016/j.biopha.2021.112595_bib152",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.1186/s12906-016-1043-y",
"article-title": "Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "65",
"journal-title": "BMC Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.112595_bib153",
"volume": "16",
"year": "2016"
},
{
"DOI": "10.1080/00218839.2019.1695715",
"article-title": "Antiviral effects and possible mechanisms of action of constituents from Brazilian propolis and related compounds",
"author": "Kwon",
"doi-asserted-by": "crossref",
"first-page": "413",
"journal-title": "J. Apic. Res.",
"key": "10.1016/j.biopha.2021.112595_bib154",
"volume": "59",
"year": "2020"
},
{
"DOI": "10.1016/j.jksus.2020.101234",
"article-title": "Molecular interaction analysis of Sulawesi propolis compounds with SARS-CoV-2 main protease as preliminary study for COVID-19 drug discovery",
"author": "Sahlan",
"doi-asserted-by": "crossref",
"journal-title": "J. King Saud. Univ. - Sci.",
"key": "10.1016/j.biopha.2021.112595_bib155",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1007/s11262-016-1299-9",
"article-title": "Immunopotentiation of four natural adjuvants co-administered with a highly pathogenic porcine reproductive and respiratory syndrome virus glycoprotein 5 subunit",
"author": "Peng",
"doi-asserted-by": "crossref",
"first-page": "261",
"journal-title": "Virus Genes",
"key": "10.1016/j.biopha.2021.112595_bib156",
"volume": "52",
"year": "2016"
},
{
"DOI": "10.1111/j.1365-2672.2007.03421.x",
"article-title": "In vitro antimicrobial activity of a novel propolis formulation (Actichelated propolis)",
"author": "Drago",
"doi-asserted-by": "crossref",
"first-page": "1914",
"journal-title": "J. Appl. Microbiol",
"key": "10.1016/j.biopha.2021.112595_bib157",
"volume": "103",
"year": "2007"
},
{
"DOI": "10.1021/acsomega.0c02595",
"article-title": "Cells in new light: Ion concentration, voltage, and pressure gradients across a hydrogel membrane",
"author": "Kowacz",
"doi-asserted-by": "crossref",
"first-page": "21024",
"journal-title": "ACS Omega",
"key": "10.1016/j.biopha.2021.112595_bib158",
"volume": "5",
"year": "2020"
},
{
"article-title": "Effect of dietary supplement containing brazilian propolis on the common cold",
"author": "Ohkuma",
"first-page": "43",
"journal-title": "Pharmacometrics",
"key": "10.1016/j.biopha.2021.112595_bib159",
"volume": "79",
"year": "2010"
},
{
"DOI": "10.1016/j.jcice.2007.04.004",
"article-title": "Hot-pressurized fluid extraction of flavonoids and phenolic acids from Brazilian propolis and their cytotoxic assay in vitro",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "191",
"journal-title": "J. Chin. Inst. Chem. Eng.",
"key": "10.1016/j.biopha.2021.112595_bib160",
"volume": "38",
"year": "2007"
},
{
"article-title": "Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship, Bioorganic",
"author": "Li",
"first-page": "5434",
"journal-title": "Med. Chem.",
"key": "10.1016/j.biopha.2021.112595_bib161",
"volume": "16",
"year": "2008"
},
{
"DOI": "10.1016/j.cbi.2010.09.002",
"article-title": "Antitumor progression potential of caffeic acid phenethyl ester involving p75NTR in C6 glioma cells",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "607",
"journal-title": "Chem. Biol. Interact.",
"key": "10.1016/j.biopha.2021.112595_bib162",
"volume": "188",
"year": "2010"
},
{
"DOI": "10.7314/APJCP.2013.14.11.6991",
"article-title": "Antioxidant and anti-cancer cell proliferation activity of propolis extracts from two extraction methods",
"author": "Khacha-ananda",
"doi-asserted-by": "crossref",
"first-page": "6991",
"journal-title": "Asian Pac. J. Cancer Prev.",
"key": "10.1016/j.biopha.2021.112595_bib163",
"volume": "14",
"year": "2013"
},
{
"DOI": "10.1155/2014/897361",
"article-title": "Cytotoxicity of portuguese propolis: The proximity of the in vitro doses for tumor and normal cell lines",
"author": "Calhelha",
"doi-asserted-by": "crossref",
"journal-title": "Biomed. Res. Int.",
"key": "10.1016/j.biopha.2021.112595_bib164",
"volume": "2014",
"year": "2014"
},
{
"article-title": "Dual effect of Algerian propolis on lung cancer: Antitumor and chemopreventive effects involving antioxidant activity, Brazilian",
"author": "Brihoum",
"journal-title": "J. Pharm. Sci.",
"key": "10.1016/j.biopha.2021.112595_bib165",
"volume": "54",
"year": "2018"
},
{
"DOI": "10.1002/cbdv.201900189",
"article-title": "Antiproliferative Activity of Chemically Characterized Propolis from Turkey and Its Mechanisms of Action",
"author": "Aru",
"doi-asserted-by": "crossref",
"journal-title": "Chem. Biodivers.",
"key": "10.1016/j.biopha.2021.112595_bib166",
"volume": "16",
"year": "2019"
},
{
"DOI": "10.1002/jsfa.10400",
"article-title": "Nano-vesicular formulation of propolis and cytotoxic effects in a 3D spheroid model of lung cancer",
"author": "Ilhan-Ayisigi",
"doi-asserted-by": "crossref",
"first-page": "3525",
"journal-title": "J. Sci. Food Agric.",
"key": "10.1016/j.biopha.2021.112595_bib167",
"volume": "100",
"year": "2020"
},
{
"DOI": "10.3390/biom11020160",
"article-title": "Computational insights into the potential of withaferin-a, withanone and caffeic acid phenethyl ester for treatment of aberrant-EGFR driven lung cancers",
"author": "Malik",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Biomolecules",
"key": "10.1016/j.biopha.2021.112595_bib168",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1007/s11259-005-3303-z",
"article-title": "Peroral application of water-soluble derivative of propolis (WSDP) and its related polyphenolic compounds and their influence on immunological and antitumour activity",
"author": "Oršolic",
"doi-asserted-by": "crossref",
"first-page": "575",
"journal-title": "Vet. Res. Commun.",
"key": "10.1016/j.biopha.2021.112595_bib169",
"volume": "29",
"year": "2005"
},
{
"DOI": "10.1002/jsfa.2041",
"article-title": "Honey-bee products in prevention and/or therapy of murine transplantable tumours",
"author": "Oršolić",
"doi-asserted-by": "crossref",
"first-page": "363",
"journal-title": "J. Sci. Food Agric.",
"key": "10.1016/j.biopha.2021.112595_bib170",
"volume": "85",
"year": "2005"
},
{
"DOI": "10.1248/bpb.26.638",
"article-title": "Inhibitory effects of caffeic acid phenethyl ester analogues on experimental lung metastasis of murine colon 26-L5 carcinoma cells",
"author": "Nagaoka",
"doi-asserted-by": "crossref",
"first-page": "638",
"journal-title": "Biol. Pharm. Bull.",
"key": "10.1016/j.biopha.2021.112595_bib171",
"volume": "26",
"year": "2003"
},
{
"DOI": "10.1002/jps.24278",
"article-title": "Preparation of Caffeic Acid Phenethyl Ester-Incorporated Nanoparticles and Their Biological Activity",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "144",
"journal-title": "J. Pharm. Sci.",
"key": "10.1016/j.biopha.2021.112595_bib172",
"volume": "104",
"year": "2015"
},
{
"DOI": "10.1159/000487897",
"article-title": "Efficacy of a propolis-based syrup (FARINGEL) in preventing radiation-induced Esophagitis in locally advanced lung cancer",
"author": "Ippolito",
"doi-asserted-by": "crossref",
"first-page": "76",
"journal-title": "Chemotherapy",
"key": "10.1016/j.biopha.2021.112595_bib173",
"volume": "63",
"year": "2018"
},
{
"DOI": "10.3109/02770900903556405",
"article-title": "Caffeic acid phenethyl ester suppresses the induction of eotaxin in human lung fibroblast cells",
"author": "Liao",
"doi-asserted-by": "crossref",
"first-page": "233",
"journal-title": "J. Asthma",
"key": "10.1016/j.biopha.2021.112595_bib174",
"volume": "47",
"year": "2010"
},
{
"DOI": "10.1016/j.intimp.2012.12.018",
"article-title": "Propolis inhibits TGF-beta 1-induced epithelial-mesenchymal transition in human alveolar epithelial cells via PPAR gamma activation",
"author": "Kao",
"doi-asserted-by": "crossref",
"first-page": "565",
"journal-title": "Int. Immunopharmacol.",
"key": "10.1016/j.biopha.2021.112595_bib175",
"volume": "15",
"year": "2013"
},
{
"DOI": "10.1016/j.mycmed.2018.09.006",
"article-title": "Aspergillus fumigatus conidia stimulate lung epithelial cells (TC-1 JHU-1) to produce IL-12, IFN-γ, IL-13 and IL-17 cytokines: Modulatory effect of propolis extract | Effet de la propolis sur les cytokines induites par Aspergillus fumigatus",
"author": "Khosravi",
"doi-asserted-by": "crossref",
"first-page": "594",
"journal-title": "J. Mycol. Med.",
"key": "10.1016/j.biopha.2021.112595_bib176",
"volume": "28",
"year": "2018"
},
{
"DOI": "10.1080/01902140590918876",
"article-title": "Oleic acid-induced lung injury in rats and effects of caffeic acid phenethyl ester",
"author": "Koksel",
"doi-asserted-by": "crossref",
"first-page": "483",
"journal-title": "Exp. Lung Res.",
"key": "10.1016/j.biopha.2021.112595_bib177",
"volume": "31",
"year": "2005"
},
{
"DOI": "10.1021/jf0612023",
"article-title": "Oxidant/antioxidant properties of croatian native propolis",
"author": "Sobočanec",
"doi-asserted-by": "crossref",
"first-page": "8018",
"journal-title": "J. Agric. Food Chem.",
"key": "10.1016/j.biopha.2021.112595_bib178",
"volume": "54",
"year": "2006"
},
{
"DOI": "10.1055/s-0036-1579664",
"article-title": "The Effect of Propolis in Healing Injured Nasal Mucosa: An Experimental Study",
"author": "El-Anwar",
"doi-asserted-by": "crossref",
"first-page": "222",
"journal-title": "Int. Arch. Otorhinolaryngol.",
"key": "10.1016/j.biopha.2021.112595_bib179",
"volume": "20",
"year": "2016"
},
{
"DOI": "10.1016/j.jmii.2011.04.008",
"article-title": "Caffeic acid phenethyl ester suppresses eotaxin secretion and nuclear p-STAT6 in human lung fibroblast cells",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "435",
"journal-title": "J. Microbiol. Immunol. Infect.",
"key": "10.1016/j.biopha.2021.112595_bib180",
"volume": "44",
"year": "2011"
},
{
"DOI": "10.1111/j.1365-2249.2009.04067.x",
"article-title": "Caffeic acid phenethyl ester inhibits nuclear factor-kappaB and protein kinase B signalling pathways and induces caspase-3 expression in primary human CD4+ T cells",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "223",
"journal-title": "Clin. Exp. Immunol.",
"key": "10.1016/j.biopha.2021.112595_bib181",
"volume": "160",
"year": "2010"
},
{
"article-title": "Evaluation of the Effect of Caffeic Acid Phenethyl Ester (CAPE) on Pharmacological Responses of Isolated Rat Trachea in vitro",
"author": "Hemmati",
"first-page": "256",
"journal-title": "Tanaffos",
"key": "10.1016/j.biopha.2021.112595_bib182",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.5152/tao.2019.4164",
"article-title": "Effects of Oral Propolis on Mucosal Wound Healing after Endoscopic Nasal Surgery in a Rabbit Model, TURKISH",
"author": "Kavaz",
"doi-asserted-by": "crossref",
"first-page": "68",
"journal-title": "Arch. Otorhinolaryngol.",
"key": "10.1016/j.biopha.2021.112595_bib183",
"volume": "57",
"year": "2019"
}
],
"reference-count": 183,
"references-count": 183,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0753332221013822"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology",
"General Medicine"
],
"subtitle": [],
"title": "The Potential Use of Propolis as a Primary or an Adjunctive Therapy in Respiratory Tract-Related Diseases and Disorders: A Systematic Scoping Review",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "146"
}