Two-way pharmacodynamic modeling of drug combinations and its application to pairs of repurposed Ebola and SARS-CoV-2 agents
et al., Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01015-23, Apr 2024
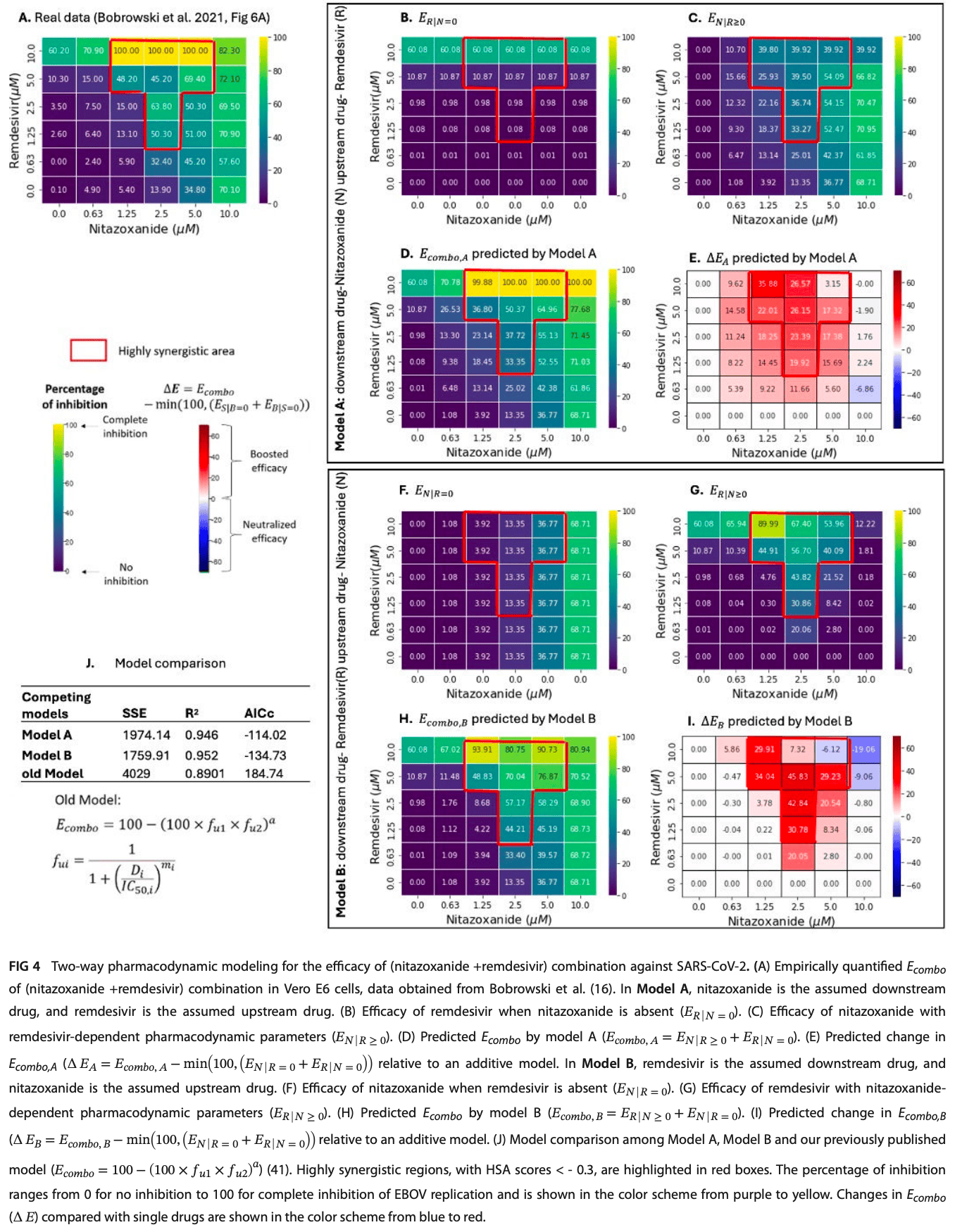
In silico study supporting the synergistic combination of nitazoxanide and remdesivir for SARS-CoV-2. Authors developed a two-way pharmacodynamic modeling approach to capture the concentration-dependent drug-drug interactions and combined efficacy of nitazoxanide and remdesivir using previously published in vitro dose-response data.
6 preclinical studies support the efficacy of nitazoxanide for COVID-19:
Study covers nitazoxanide and remdesivir.
1.
Xu et al., Two-way pharmacodynamic modeling of drug combinations and its application to pairs of repurposed Ebola and SARS-CoV-2 agents, Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01015-23.
2.
Rajoli et al., Dose prediction for repurposing nitazoxanide in SARS‐CoV‐2 treatment or chemoprophylaxis, British Journal of Clinical Pharmacology, doi:10.1111/bcp.14619.
Xu et al., 3 Apr 2024, USA, peer-reviewed, 7 authors.
Contact: jschiffe@fredhutch.org.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
Two-way pharmacodynamic modeling of drug combinations and its application to pairs of repurposed Ebola and SARS-CoV-2 agents
Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01015-23
Existing pharmacodynamic (PD) mathematical models for drug combina tions discriminate antagonistic, additive, multiplicative, and synergistic effects, but fail to consider how concentration-dependent drug interaction effects may vary across an entire dose-response matrix. We developed a two-way pharmacodynamic (TWPD) model to capture the PD of two-drug combinations. TWPD captures interactions between upstream and downstream drugs that act on different stages of viral replication, by quantifying upstream drug efficacy and concentration-dependent effects on down stream drug pharmacodynamic parameters. We applied TWPD to previously published in vitro drug matrixes for repurposed potential anti-Ebola and anti-SARS-CoV-2 drug pairs. Depending on the drug pairing, the model recapitulated combined efficacies as or more accurately than existing models and can be used to infer efficacy at untested drug concentrations. TWPD fits the data slightly better in one direction for all drug pairs, meaning that we can tentatively infer the upstream drug. Based on its high accuracy, TWPD could be used in concert with PK models to estimate the therapeutic effects of drug pairs in vivo.
AUTHOR CONTRIBUTIONS Shuang
ADDITIONAL FILES The following material is available online.
Supplemental Material Supplemental material (AAC01015-23-s0001.docx). Figures S1 to S7; Tables S1 to S10 .
References
Andersen, Ianevski, Lysvand, Vitkauskiene, Oksenych et al., Discovery and development of safe-in-man broad-spectrum antiviral agents, Int J Infect Dis, doi:10.1093/infdis/jiy304
Berenbaum, What is synergy?, Pharmacol Rev
Bliss, The toxicity of poisons applied jointly 1, Ann Appl Biol, doi:10.1111/j.1744-7348.1939.tb06990.x
Bobrowski, Chen, Eastman, Itkin, Shinn et al., Synergistic and antagonistic drug combinations against SARS-CoV-2, Mol Ther, doi:10.1016/j.csbj.2015.09.001
Box, Draper, Empirical model-building and response surfaces, doi:10.1016/j.csbj.2015.09.001
Brun, Dennis, Greco, Bernacki, Pera et al., Modeling the combination of amphotericin B, micafungin, and nikkomycin Z against Aspergillus fumigatus in vitro using a novel response surface paradigm, Antimicrob Agents Chemother, doi:10.1128/AAC.01007-06
Bulitta, Landersdorfer, Forrest, Brown, Neely et al., Relevance of pharmacokinetic and pharmacodynamic modeling to clinical care of critically ill patients, Curr Pharm Biotechnol, doi:10.1080/19466315.2018.1437071
Bösl, Ianevski, Than, Andersen, Kuivanen et al., Common nodes of virus-host interaction revealed through an integrated network analysis, Front Immunol, doi:10.1016/j.csbj.2015.09.001
Chaudhuri, Symons, Deval, Innovation and trends in the development and approval of antiviral medicines: 1987-2017 and beyond, Antiviral Res, doi:10.1093/infdis/jiy304
Cheng, Williamson, Zheng, Improving therapy of severe infections through drug repurposing of synergistic combinations, Curr Opin Pharmacol, doi:10.1016/j.csbj.2015.09.001
De Mello, Tao, Kim, Bulitta, Rodriquez et al., Zika virus replication is substantially inhibited by novel favipiravir and interferon alpha combination regimens, Antimicrob Agents Chemother, doi:10.1016/j.csbj.2015.09.001
Degregorio, Wurz, Taras, Erkkola, Halonen et al., Pharmacokinetics of (deaminohydroxy) toremifene in humans: a new, selective estrogen-receptor modulator, Eur J Clin Pharmacol, doi:10.1007/s002280000176
Derendorf, Meibohm, Modeling of pharmacokinetic/ pharmacodynamic (PK/PD) relationships: concepts and perspectives, Pharm Res, doi:10.1016/j.drudis.2008.01.003
Drusano, Argenio, Preston, Barone, Symonds et al., Use of drug effect interaction modeling with Monte Carlo simulation to examine the impact of dosing interval on the projected antiviral activity of the combination of abacavir and amprenavir, Antimicrob Agents Chemother, doi:10.1128/AAC.44.6.1655-1659.2000
Drusano, Neely, Van Guilder, Schumitzky, Brown et al., Analysis of combination drug therapy to develop regimens with shortened duration of treatment for tuberculo sis, PLoS One, doi:10.1371/journal.pone.0101311
Dyall, Nelson, Dewald, Guha, Hart et al., Identification of combinations of approved drugs with synergistic activity against Ebola virus in cell cultures, J Infect Dis, doi:10.1093/infdis/jiy304
Esmaeili, Owens, Wagoner, Polyak, Schiffer, A unifying model to explain nirmatrelvir / ritonavir's high efficacy during early treatment and low efficacy as post-exposure prophylaxis, and to predict viral rebound, medRxiv, doi:10.1101/2023.08.23.23294505
Finch, Dyall, Xu, Nelson, Postnikova et al., Formulation, stability, pharmacokinetic, and modeling studies for tests of synergistic combinations of orally available approved drugs against Ebola virus in vivo, Microorganisms, doi:10.3390/microorganisms9030566
Finch, Dyall, Xu, Nelson, Postnikova et al., Formulation, stability, pharmacokinetic, and modeling studies for tests of synergistic combinations of orally available approved drugs against Ebola virus in vivo, Microorganisms, doi:10.3390/microorganisms9030566
Foucquier, Guedj, Analysis of drug combinations: current methodological landscape, Pharmacol Res Perspect, doi:10.1002/prp2.149
Franco, Tao, Hanrahan, Zhou, Bulitta et al., Combination regimens of favipiravir plus interferon alpha inhibit chikungunya virus replication in clinically relevant human cell lines, Microorganisms, doi:10.3390/microorganisms9020307
Greco, Bravo, Parsons, The search for synergy: a critical review from a response surface perspective, Pharmacol Rev, doi:10.1016/j.csbj.2015.09.001
Haffizulla, Hartman, Hoppers, Resnick, Samudrala et al., Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial, Lancet Infect Dis, doi:10.1016/S1473-3099(14)70717-0
Hakamaki, Apoil, Arstila, Timmer, Lehtonen, Bepridil in the elderly. A pharmacokinetic and clinical monitoring study, Curr Ther Res
Herring, Oda, Wagoner, Kirchmeier, Connor et al., Inhibition of arenaviruses by combinations of orally available approved drugs, Antimicrob Agents Chemother, doi:10.1016/j.csbj.2015.09.001
Hulseberg, Fénéant, Wijs, Kessler, Nelson et al., Arbidol and other low-molecular-weight drugs that inhibit Lassa and Ebola viruses, J Virol, doi:10.1128/JVI.02185-18
Humeniuk, Mathias, Cao, Osinusi, Shen et al., Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects, Clin Transl Sci, doi:10.1111/cts.12840
Ianevski, Andersen, Merits, Bjørås, Kainov, Expanding the activity spectrum of antiviral agents, Drug Discov Today, doi:10.1016/j.drudis.2019.04.006
Ianevski, Giri, Aittokallio, SynergyFinder 2.0: visual analytics of multi-drug combination synergies, Nucleic Acids Res, doi:10.1016/j.csbj.2015.09.001
Ianevski, Giri, Aittokallio, SynergyFinder 3.0: an interactive analysis and consensus interpretation of multi-drug synergies across multiple samples, Nucleic Acids Res, doi:10.1016/j.csbj.2015.09.001
Ianevski, He, Aittokallio, Tang, SynergyFinder: a web application for analyzing drug combination dose-response matrix data, Bioinformatics, doi:10.1016/j.csbj.2015.09.001
Ianevski, Yao, Biza, Zusinaite, Mannik et al., Identification and tracking of antiviral drug combinations, Viruses, doi:10.1016/j.csbj.2015.09.001
Jilek, Zarr, Sampah, Rabi, Bullen et al., A quantitative basis for antiretroviral therapy for HIV-1 infection, Nat Med, doi:10.1038/nm.2649
Johansen, Brannan, Delos, Shoemaker, Stossel et al., FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection, Sci Transl Med, doi:10.1126/scitranslmed.3005471
Johansen, Dewald, Shoemaker, Hoffstrom, Lear-Rooney et al., A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity, Sci Transl Med, doi:10.1126/scitranslmed.aaa5597
Joshua, Swan, Corey, Ossig, Ruebsamen-Schaeff et al., Mathematical modeling of herpes simplex virus-2 suppression with pritelivir predicts trial outcomes, Sci Transl Med, doi:10.1126/scitranslmed.aad6654
Landersdorfer, Ly, Xu, Tsuji, Bulitta, Quantifying subpopulation synergy for antibiotic combinations via mechanismbased modeling and a sequential dosing design, Antimicrob Agents Chemother, doi:10.1080/19466315.2018.1437071
Liu, Yin, Languino, Altieri, Evaluation of drug combina tion effect using a bliss independence dose-response surface model, Stat Biopharm Res
Lo, Jordan, Arvey, Sudhamsu, Shrivastava-Ranjan et al., GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses, Sci Rep, doi:10.1038/srep43395
Loewe, The problem of synergism and antagonism of combined drugs, Arzneim-Forsch
Maharao, Antontsev, Wright, Varshney, Entering the era of computationally driven drug development, Drug Metab Rev, doi:10.1080/03602532.2020.1726944
Park, A review of modeling approaches to predict drug response in clinical oncology, Yonsei Med J, doi:10.3349/ymj.2017.58.1.1
Pizzorno, Padey, Dubois, Julien, Traversier et al., In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2, Antiviral Res, doi:10.1016/j.csbj.2015.09.001
Pizzorno, Padey, Terrier, Rosa-Calatrava, Drug repurposing approaches for the treatment of influenza viral infection: reviving old drugs to fight against a long-lived enemy, Front Immunol, doi:10.3389/fimmu.2019.00531
Pomeroy, Drusano, Rodriquez, Brown, Searching for synergy: identifying optimal antiviral combination therapy using Hepatitis C virus (HCV) agents in a replicon system, Antiviral Res, doi:10.1016/j.antiviral.2017.09.001
Prichard, Aseltine, Shipman, MacSynergyTM II
Prichard, Shipman, A three-dimensional model to analyze drug-drug interactions, Antiviral Res, doi:10.1016/j.csbj.2015.09.001
Pécheur, Borisevich, Halfmann, Morrey, Smee et al., The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses, J Virol, doi:10.1128/JVI.02077-15
Rajman, PK/PD modelling and simulations: utility in drug development, Drug Discov Today, doi:10.1016/j.drudis.2008.01.003
Reeves, Mayer, Decamp, Huang, Zhang et al., High monoclonal neutralization titers reduced breakthrough HIV-1 viral loads in the antibody mediated prevention trials, Nat Commun, doi:10.1038/s41467-023-43384-y
Ronfeld, Tremaine, Wilner, Pharmacokinetics of sertraline and its N-demethyl metabolite in elderly and young male and female volunteers, Clin Pharmacokinet, doi:10.2165/00003088-199700321-00004
Rossignol, Nitazoxanide: a first-in-class broad-spectrum antiviral agent, Antiviral Research, doi:10.1016/j.antiviral.2014.07.014
Santos Nascimento, De Aquino, Da, Ef, Drug repurposing: a strategy for discovering inhibitors against emerging viral infections, Curr Med Chem, doi:10.1016/j.csbj.2015.09.001
Schiffer, Swan, Corey, Rapid viral expansion and short drug half-life explain the incomplete effectiveness of current herpes simplex virus 2-directed antiviral agents, Antimicrob Agents Chemother, doi:10.1128/AAC.01114-13
Sheahan, Sims, Graham, Menachery, Gralinski et al., Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses, Sci Transl Med, doi:10.1126/scitranslmed.aal3653
Snyder, Argenio, Weislow, Bilello, Drusano, The triple combination indinavir-zidovudine-lamivudine is highly synergistic, Antimicrob Agents Chemother, doi:10.1128/AAC.44.4.1051-1058.2000
Snyder, Goebel, Koide, Ptak, Kalkeri, Synergistic antiviral activity of Sofosbuvir and type-I interferons (α and β) against Zika virus, J Med Virol, doi:10.1016/j.csbj.2015.09.001
Sun, He, Martínez-Romero, Kouznetsova, Tawa et al., Synergistic drug combination effectively blocks Ebola virus infection, Antiviral Res, doi:10.1016/j.csbj.2015.09.001
Sun, He, Qiu, Zhu, Zhao et al., Pharmacokinetics of single and multiple oral doses of arbidol in healthy Chinese volunteers, Int J Clin Pharmacol Ther, doi:10.5414/CP201843
Sun, Sanderson, Zheng, Drug combination therapy increases successful drug repositioning, Drug Discov Today, doi:10.1016/j.drudis.2016.05.015
Wagoner, Herring, Hsiang, Ianevski, Biering et al., Combinations of host-and virus-targeting antiviral drugs confer synergistic suppression of SARS-CoV-2, Microbiol Spectr, doi:10.1016/j.csbj.2015.09.001
Warren, Jordan, Lo, Ray, Mackman et al., Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys, Nature, doi:10.1038/nature17180
Wicha, Chen, Clewe, Simonsson, A general pharmaco dynamic interaction model identifies perpetrators and victims in drug interactions, Nat Commun, doi:10.1038/s41467-017-01929-y
Wicha, Kees, Kuss, Kloft, Pharmacodynamic and response surface analysis of linezolid or vancomycin combined with meropenem against Staphylococcus aureus, Pharm Res, doi:10.1007/s11095-015-1632-3
Yadav, Landersdorfer, Nation, Boyce, Bulitta, Novel approach to optimize synergistic carbapenem-aminoglycoside combinations against carbapenem-resistant Acinetobacter baumannii, Antimicrob Agents Chemother, doi:10.1128/AAC.04379-14
Yadav, Wennerberg, Aittokallio, Tang, Searching for drug synergy in complex dose-response landscapes using an interaction potency model, Comput Struct Biotechnol J, doi:10.1016/j.csbj.2015.09.001
Zheng, Sun, Simeonov, Drug repurposing screens and synergistic drug-combinations for infectious diseases, Br J Pharmacol
DOI record:
{
"DOI": "10.1128/aac.01015-23",
"ISSN": [
"0066-4804",
"1098-6596"
],
"URL": "http://dx.doi.org/10.1128/aac.01015-23",
"abstract": "<jats:title>ABSTRACT</jats:title>\n <jats:sec>\n <jats:title />\n <jats:p>\n Existing pharmacodynamic (PD) mathematical models for drug combinations discriminate antagonistic, additive, multiplicative, and synergistic effects, but fail to consider how concentration-dependent drug interaction effects may vary across an entire dose-response matrix. We developed a two-way pharmacodynamic (TWPD) model to capture the PD of two-drug combinations. TWPD captures interactions between upstream and downstream drugs that act on different stages of viral replication, by quantifying upstream drug efficacy and concentration-dependent effects on downstream drug pharmacodynamic parameters. We applied TWPD to previously published\n <jats:italic>in vitro</jats:italic>\n drug matrixes for repurposed potential anti-Ebola and anti-SARS-CoV-2 drug pairs. Depending on the drug pairing, the model recapitulated combined efficacies as or more accurately than existing models and can be used to infer efficacy at untested drug concentrations. TWPD fits the data slightly better in one direction for all drug pairs, meaning that we can tentatively infer the upstream drug. Based on its high accuracy, TWPD could be used in concert with PK models to estimate the therapeutic effects of drug pairs\n <jats:italic>in vivo</jats:italic>\n .\n </jats:p>\n </jats:sec>",
"alternative-id": [
"10.1128/aac.01015-23"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-08-16"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2024-02-20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-03-12"
}
],
"author": [
{
"affiliation": [
{
"name": "Fred Hutchinson Cancer Research Center, Vaccine and Infectious Diseases Division, Seattle, Washington, USA"
}
],
"family": "Xu",
"given": "Shuang",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Fred Hutchinson Cancer Research Center, Vaccine and Infectious Diseases Division, Seattle, Washington, USA"
}
],
"family": "Esmaeili",
"given": "Shadisadat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fred Hutchinson Cancer Research Center, Vaccine and Infectious Diseases Division, Seattle, Washington, USA"
}
],
"family": "Cardozo-Ojeda",
"given": "E. Fabian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fred Hutchinson Cancer Research Center, Vaccine and Infectious Diseases Division, Seattle, Washington, USA"
}
],
"family": "Goyal",
"given": "Ashish",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0532-996X",
"affiliation": [
{
"name": "Department of Microbiology, University of Virginia, Charlottesville, Virginia, USA"
},
{
"name": "Department of Cell Biology, University of Virginia, Charlottesville, Virginia, USA"
}
],
"authenticated-orcid": true,
"family": "White",
"given": "Judith M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7475-9827",
"affiliation": [
{
"name": "Virology Division, Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA"
},
{
"name": "Department of Global Health, University of Washington, Seattle, Washington, USA"
},
{
"name": "Department of Microbiology, University of Washington, Seattle, Washington, USA"
}
],
"authenticated-orcid": true,
"family": "Polyak",
"given": "Stephen J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2598-1621",
"affiliation": [
{
"name": "Fred Hutchinson Cancer Research Center, Vaccine and Infectious Diseases Division, Seattle, Washington, USA"
},
{
"name": "Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA"
}
],
"authenticated-orcid": true,
"family": "Schiffer",
"given": "Joshua T.",
"sequence": "additional"
}
],
"container-title": "Antimicrobial Agents and Chemotherapy",
"container-title-short": "Antimicrob Agents Chemother",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.asm.org"
]
},
"created": {
"date-parts": [
[
2024,
3,
12
]
],
"date-time": "2024-03-12T13:01:08Z",
"timestamp": 1710248468000
},
"deposited": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T13:07:38Z",
"timestamp": 1712149658000
},
"editor": [
{
"affiliation": [],
"family": "Leggett",
"given": "James E.",
"sequence": "additional"
}
],
"funder": [
{
"award": [
"1 R01 AI178604-01"
],
"name": "HHS | National Institutes of Health"
},
{
"award": [
"1R01AI177512-01"
],
"name": "HHS | National Institutes of Health"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
4
]
],
"date-time": "2024-04-04T00:48:57Z",
"timestamp": 1712191737855
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2024,
4,
3
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2024,
4,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T00:00:00Z",
"timestamp": 1712102400000
}
},
{
"URL": "https://journals.asm.org/non-commercial-tdm-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T00:00:00Z",
"timestamp": 1712102400000
}
}
],
"link": [
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/aac.01015-23",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/aac.01015-23",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "235",
"original-title": [],
"prefix": "10.1128",
"published": {
"date-parts": [
[
2024,
4,
3
]
]
},
"published-print": {
"date-parts": [
[
2024,
4,
3
]
]
},
"publisher": "American Society for Microbiology",
"reference": [
{
"DOI": "10.1016/j.antiviral.2018.05.005",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_2_2"
},
{
"DOI": "10.1016/j.ijid.2020.02.018",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_3_2"
},
{
"DOI": "10.1016/j.drudis.2019.04.006",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_4_2"
},
{
"DOI": "10.1093/infdis/jiy304",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_5_2"
},
{
"DOI": "10.3389/fimmu.2019.00531",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_6_2"
},
{
"DOI": "10.1002/prp2.149",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_7_2"
},
{
"DOI": "10.1016/j.drudis.2016.05.015",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_8_2"
},
{
"DOI": "10.1111/bph.13895",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_9_2"
},
{
"DOI": "10.3389/fimmu.2019.02186",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_10_2"
},
{
"DOI": "10.1016/j.coph.2019.07.006",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_11_2"
},
{
"DOI": "10.2174/0929867327666200812215852",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_12_2"
},
{
"DOI": "10.1128/AAC.01983-17",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_13_2"
},
{
"DOI": "10.1002/jmv.24932",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_14_2"
},
{
"DOI": "10.1128/AAC.01146-20",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_15_2"
},
{
"DOI": "10.1016/j.antiviral.2016.11.017",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_16_2"
},
{
"DOI": "10.1016/j.ymthe.2020.12.016",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_17_2"
},
{
"DOI": "10.1016/j.antiviral.2020.104878",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_18_2"
},
{
"DOI": "10.3390/v12101178",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_19_2"
},
{
"DOI": "10.1128/spectrum.03331-22",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_20_2"
},
{
"DOI": "10.1093/nar/gkaa216",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_21_2"
},
{
"DOI": "10.1093/bioinformatics/btx162",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_22_2"
},
{
"DOI": "10.1093/nar/gkac382",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_23_2"
},
{
"DOI": "10.1016/0166-3542(90)90001-n",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_24_2"
},
{
"author": "Prichard M",
"key": "e_1_3_2_25_2",
"unstructured": "Prichard M, Aseltine K, Shipman JC. 1992. MacSynergyTM II. University of Michigan, Ann Arbor.",
"volume-title": "MacSynergyTM II",
"year": "1992"
},
{
"article-title": "What is synergy?",
"author": "Berenbaum MC",
"first-page": "93",
"journal-title": "Pharmacol Rev",
"key": "e_1_3_2_26_2",
"unstructured": "Berenbaum MC. 1989. What is synergy? Pharmacol Rev 41:93–141.",
"volume": "41",
"year": "1989"
},
{
"article-title": "The problem of synergism and antagonism of combined drugs",
"author": "Loewe S",
"first-page": "285",
"journal-title": "Arzneim-Forsch",
"key": "e_1_3_2_27_2",
"unstructured": "Loewe S. 1953. The problem of synergism and antagonism of combined drugs. Arzneim-Forsch 3:285–290.",
"volume": "3",
"year": "1953"
},
{
"DOI": "10.1111/j.1744-7348.1939.tb06990.x",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_28_2"
},
{
"DOI": "10.1016/j.csbj.2015.09.001",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_29_2"
},
{
"DOI": "10.1038/nm.2649",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_30_2"
},
{
"DOI": "10.1038/s41467-017-01929-y",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_31_2"
},
{
"DOI": "10.1023/a:1011907920641",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_32_2"
},
{
"DOI": "10.1016/j.drudis.2008.01.003",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_33_2"
},
{
"DOI": "10.3349/ymj.2017.58.1.1",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_34_2"
},
{
"DOI": "10.1080/03602532.2020.1726944",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_35_2"
},
{
"article-title": "The search for synergy: a critical review from a response surface perspective",
"author": "Greco WR",
"first-page": "331",
"journal-title": "Pharmacol Rev",
"key": "e_1_3_2_36_2",
"unstructured": "Greco WR, Bravo G, Parsons JC. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev 47:331–385.",
"volume": "47",
"year": "1995"
},
{
"DOI": "10.1128/AAC.44.4.1051-1058.2000",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_37_2"
},
{
"DOI": "10.1128/AAC.44.6.1655-1659.2000",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_38_2"
},
{
"DOI": "10.1128/AAC.01007-06",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_39_2"
},
{
"DOI": "10.1007/s11095-015-1632-3",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_40_2"
},
{
"author": "Box GE",
"key": "e_1_3_2_41_2",
"unstructured": "Box GE, Draper NR. 1987. Empirical model-building and response surfaces. John Wiley & Sons.",
"volume-title": "Empirical model-building and response surfaces",
"year": "1987"
},
{
"DOI": "10.3390/microorganisms9030566",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_42_2"
},
{
"DOI": "10.1128/AAC.04379-14",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_43_2"
},
{
"DOI": "10.1128/AAC.00092-13",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_44_2"
},
{
"DOI": "10.2174/138920111798808428",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_45_2"
},
{
"DOI": "10.1080/19466315.2018.1437071",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_46_2"
},
{
"DOI": "10.1126/scitranslmed.3005471",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_47_2"
},
{
"DOI": "10.1126/scitranslmed.aaa5597",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_48_2"
},
{
"DOI": "10.1128/JVI.02077-15",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_49_2"
},
{
"DOI": "10.1128/JVI.02185-18",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_50_2"
},
{
"DOI": "10.1038/nature17180",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_51_2"
},
{
"DOI": "10.1038/srep43395",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_52_2"
},
{
"DOI": "10.1126/scitranslmed.aal3653",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_53_2"
},
{
"DOI": "10.1016/j.antiviral.2014.07.014",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_54_2"
},
{
"DOI": "10.1371/journal.pone.0101311",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_55_2"
},
{
"DOI": "10.1016/j.antiviral.2017.09.001",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_56_2"
},
{
"DOI": "10.3390/microorganisms9020307",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_57_2"
},
{
"DOI": "10.1126/scitranslmed.aad6654",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_58_2"
},
{
"DOI": "10.1038/s41467-023-43384-y",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_59_2"
},
{
"DOI": "10.3390/microorganisms9030566",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_60_2"
},
{
"DOI": "10.1128/AAC.01114-13",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_61_2"
},
{
"DOI": "10.1101/2023.08.23.23294505",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_62_2"
},
{
"article-title": "Bepridil in the elderly. A pharmacokinetic and clinical monitoring study",
"author": "Hakamaki T",
"first-page": "752",
"journal-title": "Curr Ther Res",
"key": "e_1_3_2_63_2",
"unstructured": "Hakamaki T, Apoil E, Arstila M, Timmer C, Lehtonen A. 1988. Bepridil in the elderly. A pharmacokinetic and clinical monitoring study. Curr Ther Res 44:752–758.",
"volume": "44",
"year": "1988"
},
{
"DOI": "10.2165/00003088-199700321-00004",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_64_2"
},
{
"DOI": "10.1007/s002280000176",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_65_2"
},
{
"DOI": "10.5414/CP201843",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_66_2"
},
{
"DOI": "10.1016/S1473-3099(14)70717-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_67_2"
},
{
"DOI": "10.1111/cts.12840",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_68_2"
}
],
"reference-count": 67,
"references-count": 67,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.asm.org/doi/10.1128/aac.01015-23"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Two-way pharmacodynamic modeling of drug combinations and its application to pairs of repurposed Ebola and SARS-CoV-2 agents",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1128/asmj-crossmark-policy-page",
"volume": "68"
}