Dose prediction for repurposing nitazoxanide in SARS‐CoV‐2 treatment or chemoprophylaxis
et al., British Journal of Clinical Pharmacology, doi:10.1111/bcp.14619, Dec 2020
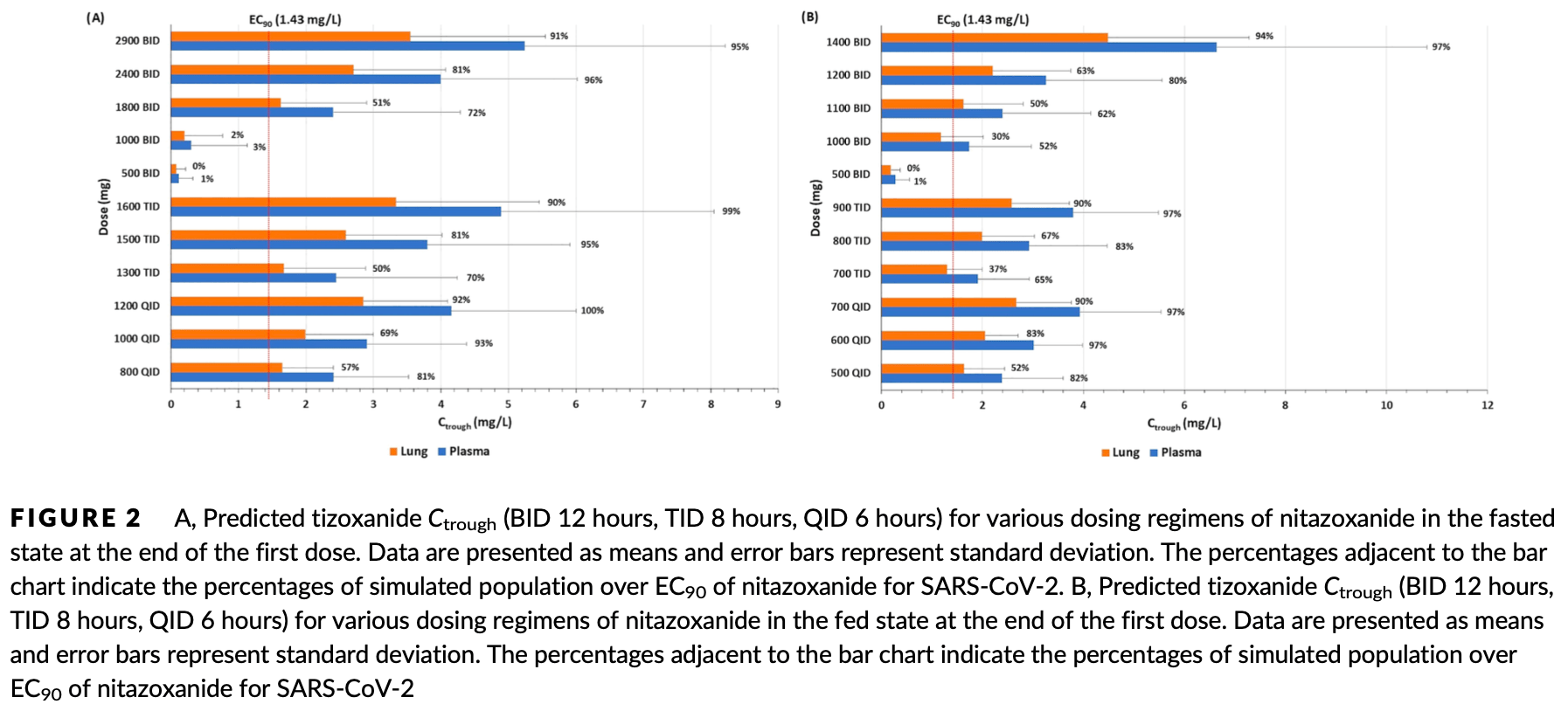
In silico physiologically based pharmacokinetic (PBPK) modeling study predicting optimal doses of nitazoxanide to maintain plasma and lung concentrations of the active metabolite tizoxanide above the SARS-CoV-2 EC90 in >90% of patients. Authors developed and validated a PBPK model against available pharmacokinetic data. Model simulations predicted optimal nitazoxanide doses of 700mg QID, 900mg TID or 1400mg BID with food to maintain target concentrations. Lower doses used in previous influenza trials were predicted to achieve target concentrations for only part of the dosing interval.
6 preclinical studies support the efficacy of nitazoxanide for COVID-19:
1.
Xu et al., Two-way pharmacodynamic modeling of drug combinations and its application to pairs of repurposed Ebola and SARS-CoV-2 agents, Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01015-23.
2.
Rajoli et al., Dose prediction for repurposing nitazoxanide in SARS‐CoV‐2 treatment or chemoprophylaxis, British Journal of Clinical Pharmacology, doi:10.1111/bcp.14619.
Rajoli et al., 31 Dec 2020, peer-reviewed, 23 authors.
Contact: aowen@liverpool.ac.uk.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Dose prediction for repurposing nitazoxanide in SARS‐CoV‐2 treatment or chemoprophylaxis
British Journal of Clinical Pharmacology, doi:10.1111/bcp.14619
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been declared a global pandemic and urgent treatment and prevention strategies are needed. Nitazoxanide, an anthelmintic drug, has been shown to exhibit in vitro activity against SARS-CoV-2. The present study used physiologically based pharmacokinetic (PBPK) modelling to inform optimal doses of nitazoxanide capable of maintaining plasma and lung tizoxanide exposures above the reported SARS-CoV-2 EC 90 . Methods: A whole-body PBPK model was validated against available pharmacokinetic data for healthy individuals receiving single and multiple doses between 500 and 4000 mg with and without food. The validated model was used to predict doses expected to maintain tizoxanide plasma and lung concentrations above the EC 90 in >90% of the simulated population. PopDes was used to estimate an optimal sparse sampling strategy for future clinical trials. Results: The PBPK model was successfully validated against the reported human pharmacokinetics. The model predicted optimal doses of 1200 mg QID, 1600 mg TID and 2900 mg BID in the fasted state and 700 mg QID, 900 mg TID and 1400 mg BID when given with food. For BID regimens an optimal sparse sampling strategy of 0.25, 1, 3 and 12 hours post dose was estimated.
Conclusion: The PBPK model predicted tizoxanide concentrations within doses of nitazoxanide already given to humans previously. The reported dosing strategies provide a rational basis for design of clinical trials with nitazoxanide for the treatment or prevention of SARS-CoV-2 infection. A concordant higher dose of nitazoxanide is now planned for investigation in the seamless phase I/IIa AGILE trial. The authors confirm that the PI for this paper is Andrew Owen and the study informs dosing optimisation using a mathematical model without any involvement of actual patients.
References
Adhikari, Meng, Wu, Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review, Infect Dis Poverty
Alexander, Armstrong, Davenport, A rational roadmap for SARS-CoV-2/COVID-19 pharmacotherapeutic research and development: IUPHAR review 29, Br J Pharmacol, doi:10.1111/bph.15094
Arshad, Pertinez, Box, Prioritization of anti-SARS-CoV-2 drug repurposing opportunities based on plasma and target site concentrations derived from their established human pharmacokinetics, Clin Pharmacol Ther, doi:10.1002/cpt.1909
Belardo, Cenciarelli, Carta, Rossignol, Gabriella, Nitazoxanide, a novel potential anti-influenza drug, acting in synergism with neuraminidase inhibitors
Belardo, Cenciarelli, La Frazia, Rossignol, Santoro, Synergistic effect of nitazoxanide with neuraminidase inhibitors against influenza a viruses In Vitro, Antimicrob Agents Chemother
Bobrowski, Chen, Eastman, Discovery of synergistic and antagonistic drug combinations against SARS-CoV-2 in vitro. bioRxiv: the preprint server for biology, doi:10.1101/2020.06.29.178889
Boffito, Back, Flexner, Towards consensus on correct interpretation of protein binding in plasma and other biological matrices for COVID-19 therapeutic development, Clin Pharmacol Ther, doi:10.1002/cpt.2099
Bosgra, Van Eijkeren, Bos, Zeilmaker, Slob, An improved model to predict physiologically based model parameters and their inter-individual variability from anthropometry, Crit Rev Toxicol
Broekhuysen, Stockis, Lins, De Graeve, Rossignol, Nitazoxanide: pharmacokinetics and metabolism in man, Int J Clin Pharmacol Ther
Broliden, Innate molecular and anatomic mucosal barriers against HIV infection in the genital tract of HIV-exposed seronegative individuals, J Infect Dis
Cao, Forrest, Zhang, A screen of the NIH clinical collection small molecule library identifies potential anti-coronavirus drugs, Antiviral Res
Charlton, Rossi-Bergmann, Denny, Steel, Repurposing as a strategy for the discovery of new anti-leishmanials: the-state-ofthe-art, Parasitology
Chenel, Bouzom, Aarons, Ogungbenro, Drug-drug interaction predictions with PBPK models and optimal multiresponse sampling time designs: application to midazolam and a phase I compound. Part 1: Comparison of uniresponse and multiresponse designs using PopDes, J Pharmacokinet Pharmacodyn
Clerici, Trabattoni, Pacei, Biasin, Rossignol, The antiinfective nitazoxanide shows strong immumodulating effects (155
Clinicaltrials, Gov, None, Clinical Trials
Cottrell, Srinivas, Kashuba, Pharmacokinetics of antiretrovirals in mucosal tissue, Expert Opin Drug Metab Toxicol
Dang, Xu, Ma, Nitazoxanide inhibits human norovirus replication and synergizes with ribavirin by activation of cellular antiviral response, Antimicrob Agents Chemother
Fryar, Kruszon-Moran, Gu, Ogden, Mean body weight, height, waist circumference, and body mass index among adults: United States, 1999-2000 through 2015-2016
Gertz, Harrison, Houston, Galetin, Prediction of human intestinal first-pass metabolism of 25 CYP3A substrates from in vitro clearance and permeability data, Drug Metab Dispos
Gns, Gr, Murahari, Krishnamurthy, An update on drug repurposing: re-written saga of the drug's fate, Biomed Pharmacother
Haffizulla, Hartman, Hoppers, Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a doubleblind, randomised, placebo-controlled, phase 2b/3 trial, Lancet Infect Dis
Keiser, Utzinger, Chapter 8 -The Drugs We Have and the Drugs We Need Against Major Helminth Infections
Korba, Elazar, Lui, Rossignol, Glenn, Potential for hepatitis C virus resistance to nitazoxanide or tizoxanide, Antimicrob Agents Chemother
Korba, Montero, Farrar, Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication, Antiviral Res
Koszalka, Tilmanis, Hurt, Influenza antivirals currently in late-phase clinical trial, Influenza Other Respi Viruses
Machhi, Herskovitz, Senan, The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections, J Neuroimmune Pharmacol
Marcellin, Boyer, Protopopescu, Determinants of unplanned antiretroviral treatment interruptions among people living with HIV in Yaoundé, Cameroon, J Trop Med Int Health
Marcelín-Jiménez, Contreras-Zavala, Castellanos, Moreno, García-González, Development of a method by UPLC-MS/MS for the quantification of tizoxanide in human plasma and its pharmacokinetic application, Bioanalysis
Mccreary, Pogue, Coronavirus disease 2019 treatment: a review of early and emerging options, Open Forum Infect Dis, doi:10.1093/ofid/ofaa105
Miner, Labitzke, Liu, Drug repurposing: the anthelmintics niclosamide and nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways, Front Pharmacol
Nepogodiev, Bhangu, Glasbey, Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study, The Lancet
Nyberg, Bazzoli, Ogungbenro, Methods and software tools for design evaluation in population pharmacokinetics-pharmacodynamics studies, Br J Clin Pharmacol
Pepperrell, Pilkington, Owen, Wang, Hill, Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19, J Virus Erad
Peters, Evaluation of a generic physiologically based pharmacokinetic model for Lineshape analysis, Clin Pharmacokinet
Pizzorno, Padey, Terrier, Rosa-Calatrava, Drug repurposing approaches for the treatment of influenza viral infection: reviving old drugs to fight against a Long-lived enemy, Front Immunol, doi:10.3389/fimmu.2019.00531
Rajoli, Back, Rannard, Physiologically based pharmacokinetic modelling to inform development of intramuscular longacting nanoformulations for HIV, Clin Pharmacokinet
Rajoli, Curley, Chiong, Predicting drug-drug interactions between rifampicin and long-acting cabotegravir and rilpivirine using physiologically based pharmacokinetic modeling, J Infect Dis
Ranjbar, Haridas, Nambu, Cytoplasmic RNA sensor pathways and nitazoxanide broadly inhibit intracellular Mycobacterium tuberculosis growth, iScience
Rodgers, Leahy, Rowland, Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-tostrong bases, J Pharm Sci
Rodgers, Rowland, Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions, J Pharm Sci
Rossignol, El-Gohary, Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial, Aliment Pharmacol Ther
Rossignol, Nitazoxanide: a first-in-class broad-spectrum antiviral agent, Antiviral Res
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review, JAMA
Schaefer, Padera, Solomon, In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19, Mod Pathol, doi:10.1038/s41379-020-0595
Senanayake, Drug repurposing strategies for COVID-19, Future Drug Discovery, doi:10.4155/fdd-2020-0010
Shereen, Khan, Kazmi, Bashir, Siddique, COVID-19 infection: origin, transmission, and characteristics of human coronaviruses, J Adv Res
Stockis, Allemon, De Bruyn, Gengler, Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses, Int J Clin Pharmacol Ther
Stockis, De Bruyn, Gengler, Rosillon, Nitazoxanide pharmacokinetics and tolerability in man during 7 days dosing with 0.5 g and 1 g b.i.d, Int J Clin Pharmacol Ther
Sun, Lennernas, Welage, Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs, Pharm Res
Taubenberger, Morens, The pathology of influenza virus infections, Annu Rev Pathol
Telleria, Drug repurposing for cancer therapy, J Cancer Sci Ther
Tilmanis, Van Baalen, Oh, Rossignol, Hurt, The susceptibility of circulating human influenza viruses to tizoxanide, the active metabolite of nitazoxanide, Antiviral Res
Toljan, Letter to the editor regarding the viewpoint "evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanism, ACS Chem Nerosci
Trezza, Kashuba, Pharmacokinetics of antiretrovirals in genital secretions and anatomic sites of HIV transmission: implications for HIV prevention, Clin Pharmacokinet
Venisse, Peytavin, Bouchet, Concerns about pharmacokinetic (PK) and pharmacokinetic-pharmacodynamic (PK-PD) studies in the new therapeutic area of COVID-19 infection, Antiviral Res, doi:10.1016/j.antiviral.2020.104866
Walls, Park, Tortorici, Wall, Mcguire et al., Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein, Cell
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Williams, Reference values for total blood volume and cardiac output in humans
Wilson, 296 -Antiparasitic Agents
Xu, Zhong, Deng, High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa, Int J Oral Sci, doi:10.1038/s41368-020-0074-x
Yao, Ye, Zhang, In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clin Infect Dis
Yu, Amidon, A compartmental absorption and transit model for estimating oral drug absorption, Int J Pharm
Zhang, Zhao, Zhang, The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China, Clin Immunol
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, The Lancet
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, N Engl J Med
DOI record:
{
"DOI": "10.1111/bcp.14619",
"ISSN": [
"0306-5251",
"1365-2125"
],
"URL": "http://dx.doi.org/10.1111/bcp.14619",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has been declared a global pandemic and urgent treatment and prevention strategies are needed. Nitazoxanide, an anthelmintic drug, has been shown to exhibit in vitro activity against SARS‐CoV‐2. The present study used physiologically based pharmacokinetic (PBPK) modelling to inform optimal doses of nitazoxanide capable of maintaining plasma and lung tizoxanide exposures above the reported SARS‐CoV‐2 EC<jats:sub>90</jats:sub>.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>A whole‐body PBPK model was validated against available pharmacokinetic data for healthy individuals receiving single and multiple doses between 500 and 4000 mg with and without food. The validated model was used to predict doses expected to maintain tizoxanide plasma and lung concentrations above the EC<jats:sub>90</jats:sub> in >90% of the simulated population. PopDes was used to estimate an optimal sparse sampling strategy for future clinical trials.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The PBPK model was successfully validated against the reported human pharmacokinetics. The model predicted optimal doses of 1200 mg QID, 1600 mg TID and 2900 mg BID in the fasted state and 700 mg QID, 900 mg TID and 1400 mg BID when given with food. For BID regimens an optimal sparse sampling strategy of 0.25, 1, 3 and 12 hours post dose was estimated.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>The PBPK model predicted tizoxanide concentrations within doses of nitazoxanide already given to humans previously. The reported dosing strategies provide a rational basis for design of clinical trials with nitazoxanide for the treatment or prevention of SARS‐CoV‐2 infection. A concordant higher dose of nitazoxanide is now planned for investigation in the seamless phase I/IIa AGILE trial.</jats:p></jats:sec>",
"alternative-id": [
"10.1111/bcp.14619"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6015-5712",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Rajoli",
"given": "Rajith K. R.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"family": "Pertinez",
"given": "Henry",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1586-1885",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Arshad",
"given": "Usman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"family": "Box",
"given": "Helen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9448-8876",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Tatham",
"given": "Lee",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4596-2708",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Curley",
"given": "Paul",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4960-2139",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Neary",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"family": "Sharp",
"given": "Joanne",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5980-8966",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Liptrott",
"given": "Neill J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"family": "Valentijn",
"given": "Anthony",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8504-2354",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "David",
"given": "Christopher",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6946-1097",
"affiliation": [
{
"name": "Department of Chemistry University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Rannard",
"given": "Steven P.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5288-4501",
"affiliation": [
{
"name": "Centre for Drugs and Diagnostics, and Department of Tropical Disease Biology Liverpool School of Tropical Medicine Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Aljayyoussi",
"given": "Ghaith",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7160-6275",
"affiliation": [
{
"name": "Centre for Drugs and Diagnostics, and Department of Tropical Disease Biology Liverpool School of Tropical Medicine Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Pennington",
"given": "Shaun H.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2875-0546",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Hill",
"given": "Andrew",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2213-1103",
"affiliation": [
{
"name": "Chelsea and Westminster NHS Foundation Trust and St Stephen's AIDS Trust 4th Floor Chelsea and Westminster Hospital London UK"
},
{
"name": "Jefferiss Research Trust Laboratories, Department of Medicine Imperial College London UK"
}
],
"authenticated-orcid": false,
"family": "Boffito",
"given": "Marta",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2331-3192",
"affiliation": [
{
"name": "Centre for Drugs and Diagnostics, and Department of Tropical Disease Biology Liverpool School of Tropical Medicine Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Ward",
"given": "Steve A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2769-0967",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Khoo",
"given": "Saye H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pat Bray Electrical Orrell Wigan UK"
}
],
"family": "Bray",
"given": "Patrick G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Chemistry University of Liverpool Liverpool UK"
}
],
"family": "O'Neill",
"given": "Paul M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Chemistry University of Liverpool Liverpool UK"
}
],
"family": "Hong",
"given": "W. David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Drugs and Diagnostics, and Department of Tropical Disease Biology Liverpool School of Tropical Medicine Liverpool UK"
}
],
"family": "Biagini",
"given": "Giancarlo A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9819-7651",
"affiliation": [
{
"name": "Department of Molecular and Clinical Pharmacology, Materials Innovation Factory University of Liverpool Liverpool UK"
}
],
"authenticated-orcid": false,
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
}
],
"container-title": "British Journal of Clinical Pharmacology",
"container-title-short": "Brit J Clinical Pharma",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
10,
21
]
],
"date-time": "2020-10-21T19:25:29Z",
"timestamp": 1603308329000
},
"deposited": {
"date-parts": [
[
2023,
8,
29
]
],
"date-time": "2023-08-29T05:54:26Z",
"timestamp": 1693288466000
},
"funder": [
{
"DOI": "10.13039/501100007155",
"award": [
"MR/S00467X/1"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100007155",
"id-type": "DOI"
}
],
"name": "Medical Research Council Canada"
},
{
"DOI": "10.13039/501100000780",
"award": [
"761104"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000780",
"id-type": "DOI"
}
],
"name": "European Commission"
},
{
"DOI": "10.13039/100000002",
"award": [
"R24AI118397",
"R01AI134091"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000002",
"id-type": "DOI"
}
],
"name": "National Institutes of Health"
},
{
"DOI": "10.13039/501100000266",
"award": [
"EP/S012265/1",
"EP/R024804/1"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000266",
"id-type": "DOI"
}
],
"name": "Engineering and Physical Sciences Research Council"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
6
]
],
"date-time": "2024-08-06T23:37:17Z",
"timestamp": 1722987437128
},
"is-referenced-by-count": 37,
"issue": "4",
"issued": {
"date-parts": [
[
2020,
12
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2021,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
1
]
],
"date-time": "2020-12-01T00:00:00Z",
"timestamp": 1606780800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/bcp.14619",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/bcp.14619",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://bpspubs.onlinelibrary.wiley.com/doi/pdf/10.1111/bcp.14619",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "2078-2088",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2020,
12
]
]
},
"published-online": {
"date-parts": [
[
2020,
12
]
]
},
"published-print": {
"date-parts": [
[
2021,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1007/s11481-020-09944-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"key": "e_1_2_10_3_1",
"unstructured": "Johns Hopkins University of Medicine.Coronavirus Resource Center. 2020 [cited 2020 17/04/2020]; Available from:https://coronavirus.jhu.edu/map.html"
},
{
"DOI": "10.1016/j.clim.2020.108393",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"DOI": "10.1016/j.biopha.2018.11.127",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1017/S0031182017000993",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.4172/1948-5956.1000e108",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.4155/fdd-2020-0010",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1016/j.jare.2020.03.005",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1186/s40249-020-00646-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1038/s41368-020-0074-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1021/acschemneuro.0c00174",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review",
"author": "Sanders JM",
"first-page": "1824",
"issue": "18",
"journal-title": "JAMA",
"key": "e_1_2_10_14_1",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1909",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1016/B978-1-4377-2702-9.00298-1",
"author": "Wilson CM",
"doi-asserted-by": "crossref",
"first-page": "1518",
"key": "e_1_2_10_16_1",
"volume-title": "296 ‐ Antiparasitic Agents, in Principles and Practice of Pediatric Infectious Diseases",
"year": "2012"
},
{
"key": "e_1_2_10_17_1",
"unstructured": "DrugBank.Nitazoxanide.2020[cited 202017/04/2020]; Available from:https://www.drugbank.ca/drugs/DB00507"
},
{
"author": "Keiser J",
"first-page": "197",
"key": "e_1_2_10_18_1",
"volume-title": "Advances in Parasitology",
"year": "2010"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1016/j.antiviral.2014.11.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"key": "e_1_2_10_21_1",
"unstructured": "OpenData Portal.Nitazoxanide. 2020 [10/09/2020]; Available from:https://opendata.ncats.nih.gov/covid19/databrowser?q=Nitazoxanide"
},
{
"article-title": "Discovery of synergistic and antagonistic drug combinations against SARS‐CoV‐2 in vitro",
"author": "Bobrowski T",
"journal-title": "bioRxiv",
"key": "e_1_2_10_22_1",
"year": "2020"
},
{
"DOI": "10.1111/j.1365-2036.2006.03128.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"DOI": "10.1128/AAC.00078-08",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"DOI": "10.1016/j.antiviral.2007.08.005",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"DOI": "10.1128/AAC.00707-18",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1111/irv.12446",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1016/j.antiviral.2014.07.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"key": "e_1_2_10_29_1",
"unstructured": "OpenData Portal.Tizoxanide. 2020 [10/09/2020]; Available from:https://opendata.ncats.nih.gov/covid19/databrowser?q=Tizoxanide"
},
{
"article-title": "Drug repurposing approaches for the treatment of influenza viral infection: reviving old drugs to fight against a Long‐lived enemy",
"author": "Pizzorno A",
"first-page": "1",
"issue": "531",
"journal-title": "Front Immunol",
"key": "e_1_2_10_30_1",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/S1473-3099(14)70717-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_31_1"
},
{
"DOI": "10.4049/jimmunol.186.Supp.155.21",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_32_1",
"unstructured": "ClericiM TrabattoniD PaceiM BiasinM RossignolJ‐F.The anti‐infective nitazoxanide shows strong immumodulating effects (155.21).2011;186(1 Supplement):155.21‐155.21.https://www.jimmunol.org/content/186/1_Supplement/155.21/tab-article-info"
},
{
"DOI": "10.3389/fphar.2019.00051",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_33_1"
},
{
"key": "e_1_2_10_34_1",
"unstructured": "ClinicalTrials.gov.Clinical Trials.2020[cited 2020 10/09/2020]; Available from:https://clinicaltrials.gov/"
},
{
"DOI": "10.1016/S2055-6640(20)30017-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_35_1"
},
{
"DOI": "10.5414/CPP40213",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_36_1"
},
{
"DOI": "10.1038/s41379-020-0595-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_37_1"
},
{
"DOI": "10.1016/S0140-6736(20)31182-X",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_38_1"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_39_1"
},
{
"DOI": "10.1007/s40262-014-0148-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_40_1"
},
{
"DOI": "10.1517/17425255.2015.1027682",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_41_1"
},
{
"DOI": "10.1086/655964",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_42_1"
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_43_1"
},
{
"DOI": "10.1111/bph.15094",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_44_1"
},
{
"DOI": "10.1016/j.antiviral.2020.104866",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_45_1"
},
{
"DOI": "10.1007/s40262-014-0227-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_46_1"
},
{
"DOI": "10.1093/infdis/jiy726",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_47_1"
},
{
"key": "e_1_2_10_48_1",
"unstructured": "FryarCD Kruszon–MoranD GuQ OgdenCL.Mean body weight height waist circumference and body mass index among adults: United States 1999–2000 through 2015–2016. National Health Statistics Reports; no 122.Hyattsville MD:National Center for Health Statistics;2018.https://www.cdc.gov/nchs/data/nhsr/nhsr122-508.pdf"
},
{
"DOI": "10.2172/10186900",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_49_1",
"unstructured": "Williams L. R.Reference values for total blood volume and cardiac output in humans. 1994 [cited Access 1994 Accessed: 18/01/2018]; Available from:https://www.osti.gov/biblio/10186900-reference-values-total-blood-volume-cardiac-output-humans"
},
{
"DOI": "10.3109/10408444.2012.709225",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_50_1"
},
{
"DOI": "10.1016/S0378-5173(99)00147-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_51_1"
},
{
"DOI": "10.2165/00003088-200847040-00004",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_52_1"
},
{
"DOI": "10.1002/jps.20322",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_53_1"
},
{
"DOI": "10.1002/jps.20502",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_54_1"
},
{
"DOI": "10.1023/A:1020483911355",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_55_1"
},
{
"DOI": "10.1124/dmd.110.032649",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_56_1"
},
{
"DOI": "10.1111/j.1365-3156.2008.02170.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_57_1"
},
{
"DOI": "10.5414/CPP38387",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_58_1"
},
{
"DOI": "10.1128/AAC.03947-14",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_59_1"
},
{
"key": "e_1_2_10_60_1",
"unstructured": "Giuseppe BelardoSLF CenciarelliO CartaS RossignolJ‐F GabriellaSM.Nitazoxanide a novel potential anti‐influenza drug acting in synergism with neuraminidase inhibitors. InIDSA Annual Meeting. 2011. Boston MA USA Available from:https://idsa.confex.com/idsa/2011/webprogram/Paper31075.html"
},
{
"DOI": "10.1007/s10928-008-9104-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_61_1"
},
{
"DOI": "10.1111/bcp.12352",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_62_1"
},
{
"DOI": "10.5414/CPP40221",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_63_1"
},
{
"DOI": "10.1016/j.antiviral.2017.10.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_64_1"
},
{
"DOI": "10.1093/ofid/ofaa105",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_65_1"
},
{
"DOI": "10.1016/j.cell.2020.02.058",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_66_1"
},
{
"DOI": "10.1146/annurev.pathmechdis.3.121806.154316",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_67_1"
},
{
"DOI": "10.1016/j.isci.2019.11.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_68_1"
},
{
"DOI": "10.1002/cpt.2099",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_69_1"
},
{
"key": "e_1_2_10_70_1",
"unstructured": "Drugs.com. Nitazoxanide.2020[cited 2020 18/04/2020]; Available from:https://www.drugs.com/ppa/nitazoxanide.html"
},
{
"DOI": "10.4155/bio.12.41",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_71_1"
}
],
"reference-count": 70,
"references-count": 70,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2020.05.01.20087130",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.14619"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Dose prediction for repurposing nitazoxanide in SARS‐CoV‐2 treatment or chemoprophylaxis",
"type": "journal-article",
"volume": "87"
}