Impairment of SARS-CoV-2 spike glycoprotein maturation and fusion activity by nitazoxanide: an effect independent of spike variants emergence
et al., Cellular and Molecular Life Sciences, doi:10.1007/s00018-022-04246-w, Apr 2022
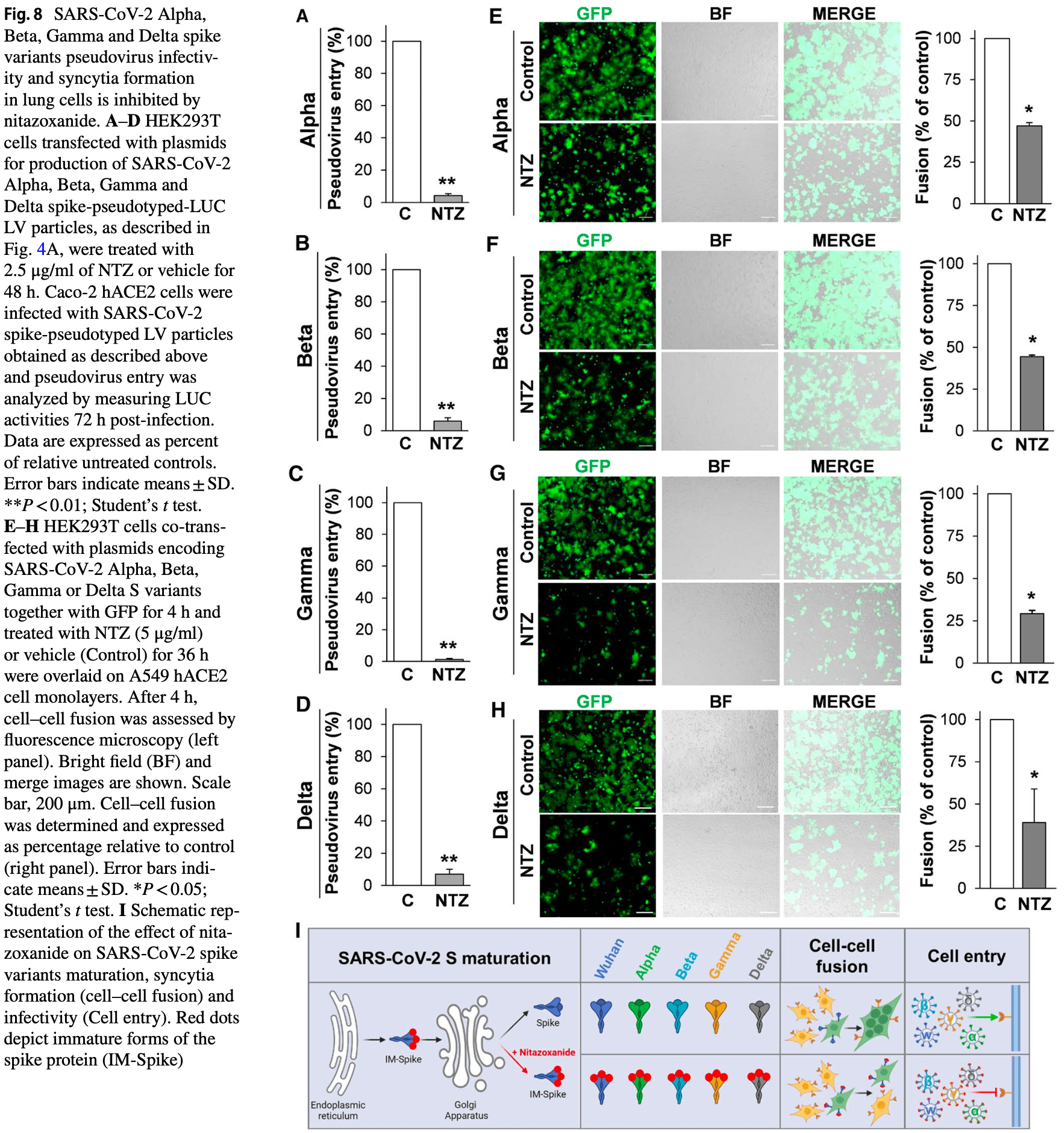
In vitro study showing that the host-directed broad-spectrum antiviral drug nitazoxanide may be effective for COVID-19 by hampering spike protein maturation and fusion activity. Authors note efficacy across alpha, beta, gamma and delta variants.
6 preclinical studies support the efficacy of nitazoxanide for COVID-19:
1.
Xu et al., Two-way pharmacodynamic modeling of drug combinations and its application to pairs of repurposed Ebola and SARS-CoV-2 agents, Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.01015-23.
2.
Rajoli et al., Dose prediction for repurposing nitazoxanide in SARS‐CoV‐2 treatment or chemoprophylaxis, British Journal of Clinical Pharmacology, doi:10.1111/bcp.14619.
Riccio et al., 7 Apr 2022, peer-reviewed, 6 authors.
Contact: santoro@uniroma2.it.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Impairment of SARS-CoV-2 spike glycoprotein maturation and fusion activity by nitazoxanide: an effect independent of spike variants emergence
Cellular and Molecular Life Sciences, doi:10.1007/s00018-022-04246-w
SARS-CoV-2, the causative agent of COVID-19, has caused an unprecedented global health crisis. The SARS-CoV-2 spike, a surface-anchored trimeric class-I fusion glycoprotein essential for viral entry, represents a key target for developing vaccines and therapeutics capable of blocking virus invasion. The emergence of SARS-CoV-2 spike variants that facilitate virus spread and may affect vaccine efficacy highlights the need to identify novel antiviral strategies for COVID-19 therapy. Here, we demonstrate that nitazoxanide, an antiprotozoal agent with recognized broad-spectrum antiviral activity, interferes with SARS-CoV-2 spike maturation, hampering its terminal glycosylation at an endoglycosidase H-sensitive stage. Engineering multiple SARS-CoV-2 variant-pseudoviruses and utilizing quantitative cell-cell fusion assays, we show that nitazoxanideinduced spike modifications hinder progeny virion infectivity as well as spike-driven pulmonary cell-cell fusion, a critical feature of COVID-19 pathology. Nitazoxanide, being equally effective against the ancestral SARS-CoV-2 Wuhan-spike and different emerging variants, including the Delta variant of concern, may represent a useful tool in the fight against COVID-19 infections.
Statistical analysis Statistical analysis was performed using the Student's t test for unpaired data or one-way ANOVA test (Prism 5.0 software, GraphPad). Data are expressed as the mean ± SD of samples derived from at least three biological repeats and P values ≤ 0.05 were considered significant. All the results shown are representative of at least three independent experiments, each in duplicate or triplicate.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s00018-022-04246-w. Author contributions AR, SS and SP performed the analysis of protein synthesis, maturation and intracellular localization; A. Rossi performed the pseudovirus studies; AR and SS conducted the cell-cell fusion study; MGS and JFR designed the study; MGS, AR and SS wrote the manuscript. All the authors contributed to the interpretation of the data and approve the content of the manuscript. Funding Open access funding provided by Università degli Studi di Roma Tor Vergata within the CRUI-CARE Agreement. This research was supported by Romark Laboratories LC, Tampa, Florida, and by a grant from the Italian Ministry of University and Scientific Research (PRIN project N 2010PHT9NF-006).
Declarations Conflict of interest Financial support for this study was in part provided by Romark Laboratories LC, the company that owns the intellectual property rights related to nitazoxanide. JF Rossignol is an employee and stockholder of Romark..
References
Alexander, Elder, Carbohydrate dramatically influences immune reactivity of antisera to viral glycoprotein antigens, Science
Belouzard, Chu, Whittaker, Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites, Proc Natl Acad Sci
Blum, Cimerman, Hunter, Tierno, Lacerda et al., Nitazoxanide superiority to placebo to treat moderate COVID-19 -A Pilot prove of concept randomized double-blind clinical trial, EClinicalMedicine
Boson, Legros, Zhou, Siret, Mathieu et al., The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles, J Biol Chem
Braakman, Van Anken, Folding of viral envelope glycoproteins in the endoplasmic reticulum, Traffic
Bracquemond, Muriaux, Betacoronavirus assembly: clues and perspectives for elucidating SARS-CoV-2 particle formation and egress, MBio
Buchrieser, Dufloo, Hubert, Monel, Planas et al., Syncytia formation by SARS-CoV-2-infected cells, EMBO J
Bussani, Schneider, Zentilin, Collesi, Ali et al., Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology, EBioMedicine
Cai, Zhang, Xiao, Peng, Sterling et al., Distinct conformational states of SARS-CoV-2 spike protein, Science
Campeau, Ruhl, Rodier, Smith, Rahmberg et al., A versatile viral system for expression and depletion of proteins in mammalian cells, PLoS ONE
Cao, Forrest, Zhang, A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs, Antiviral Res
Cattin-Ortolá, Welch, Maslen, Skehel, Papa et al., Sequences in the cytoplasmic tail of SARS-CoV-2 spike facilitate expression at the cell surface and syncytia formation, Nat Commun
Coccia, Rossi, Riccio, Trotta, Santoro, Human NF-κB repressing factor acts as a stress-regulated switch for ribosomal RNA processing and nucleolar homeostasis surveillance, Proc Natl Acad Sci
Compton, Schwartz, They might me giants: does syncytium formation sink or spread HIV infection?, PLoS Pathog
Cui, Li, Shi, Origin and evolution of pathogenic coronaviruses, Nat Rev Microbiol
De Wit, Van Doremalen, Falzarano, Munster, SARS and MERS: recent insights into emerging coronaviruses, Nat Rev Microbiol
Delmas, Laude, Carbohydrate-induced conformational changes strongly modulate the antigenicity of coronavirus TGEV glycoproteins S and M, Virus Res
Dong, Dai, Wang, Zhang, Zeng et al., The way of SARS-CoV-2 vaccine development: success and challenges, Signal Transduct Target Ther
Edridge, Kaczorowska, Hoste, Bakker, Klein et al., Seasonal coronavirus protective immunity is short-lasting, Nat Med
Ferren, Horvat, Mathieu, Measles encephalitis: towards new therapeutics, Viruses
Frazia, Ciucci, Arnoldi, Coira, Gianferretti et al., Thiazolides, a new class of antiviral agents effective against rotavirus infection, target viral morphogenesis, inhibiting viroplasm formation, J Virol
Fung, Liu, Human Coronavirus: host-pathogen interaction, Annu Rev Microbiol
Gong, Qin, Dai, Tian, The glycosylation in SARS-CoV-2 and its receptor ACE2, Signal Transduct Target Ther
Griffin, Measles virus persistence and its consequences, Curr Opin Virol
Haffizulla, Hartman, Hoppers, Resnick, Samudrala et al., Clinical Study Group (2014) Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial, Lancet Infect Dis
Harvey, Carabelli, Jackson, Gupta, Thomson et al., SARS-CoV-2 variants, spike mutations and immune escape, Nat Rev Microbiol
Hiscox, Khoo, Stewart, Owen, Shutting the gate before the horse has bolted: is it time for a conversation about SARS-CoV-2 and antiviral drug resistance?, J Antimicrob Chemother
Hoffmann, Kleine-Weber, Pöhlmann, A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells, Mol Cell
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Horimoto, Kawaoka, Influenza: lessons from past pandemics, warnings from current incidents, Nat Rev Microbiol
Hu, Guo, Zhou, Shi, Characteristics of SARS-CoV-2 and COVID-19, Nat Rev Microbiol
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat Rev Mol Cell Biol
Kim, Lee, Yang, Kim, Kim et al., The architecture of SARS-CoV-2 transcriptome, Cell
Korba, Montero, Farrar, Gaye, Mukerjee et al., Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication, Antiviral Res
Korber, Fischer, Gnanakaran, Yoon, Theiler et al., Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus, Cell
Krause, Fleming, Longini, Peto, Briand et al., SARS-CoV-2 variants and vaccines, N Engl J Med
Li, Clercq, Therapeutic options for the 2019 novel coronavirus (2019-nCoV), Nat Rev Drug Discov
Li, Zheng, Yu, Wu, Xue et al., Characterization of the SARS-CoV-2 spike in an early prefusion conformation, bioRxiv, doi:10.1101/2020.03.16.994152
Manasanch, Orlowski, Proteasome inhibitors in cancer therapy, Nat Rev Clin Oncol
Millet, Whittaker, Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein, Proc Natl Acad Sci
Millet, Whittaker, Murine leukemia virus (MLV)-based coronavirus spike-pseudotyped particle production and infection, Bio Protoc
Mishin, Novikov, Hayden, Gubareva, Effect of hemagglutinin glycosylation on influenza virus susceptibility to neuraminidase inhibitors, J Virol
Morito, Nagata, Pathogenic hijacking of ER-associated degradation: is ERAD flexible?, Mol Cell
Ohuchi, Ohuchi, Garten, Klenk, Oligosaccharides in the stem region maintain the influenza virus hemagglutinin in the metastable form required for fusion activity, J Virol
Ou, Liu, Lei, Li, Mi et al., Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV, Nat Commun
Piacentini, Frazia, Riccio, Pedersen, Topai et al., Nitazoxanide inhibits paramyxovirus replication by targeting the Fusion protein folding: role of glycoprotein-specific thiol oxidoreductase ERp57, Sci Rep
Pizzato Scomazzon, Riccio, Santopolo, Lanzilli, Coccia et al., The zinc-finger AN1-type domain 2a gene acts as a regulator of cell survival in human melanoma: role of E3-ligase cIAP2, Mol Cancer Res
Planas, Bruel, Grzelak, Guivel-Benhassine, Staropoli et al., Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies, Nat Med
Plante, Liu, Liu, Xia, Johnson et al., Spike mutation D614G alters SARS-CoV-2 fitness, Nature
Plescia, David, Patra, Sengupta, Amiar et al., SARS-CoV-2 viral budding and entry can be modeled using BSL-2 level virus-like particles, J Biol Chem
Ren, Glende, Al-Falah, De Vries, Schwegmann-Wessels et al., Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndromeassociated coronavirus, J Gen Virol
Robson, Khan, Le, Paris, Demirbag et al., Coronavirus RNA proofreading: molecular basis and therapeutic targeting, Mol Cell
Rocco, Silva, Cruz, Mac, Tierno et al., Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial, Eur Respir J
Rockx, Kuiken, Herfst, Bestebroer, Lamers et al., Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model, Science
Rossignol, Abu-Zekry, Hussein, Santoro, Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial, Lancet
Rossignol, Ayoub, Ayers, Treatment of diarrhea caused by Giardia intestinalis and Entamoeba histolytica or E. dispar: a randomized, double-blind, placebo-controlled study of nitazoxanide, J Infect Dis
Rossignol, Bardin, Fulgencio, Mogelnicki, Bréchot et al., Group (2022) A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19, eClinicalMedicine
Rossignol, Elfert, El-Gohary, Keeffe, Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin, Gastroenterology
Rossignol, Frazia, Chiappa, Ciucci, Santoro, Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level, J Biol Chem
Rossignol, Kabil, El-Gohary, Younis, Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species, Clin Gastroenterol Hepatol
Rossignol, Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus, J Infect Public Health
Rossignol, Nitazoxanide: a first-in-class broad-spectrum antiviral agent, Antiviral Res
Sanders, Jumper, Ackerman, Bracha, Donlic et al., SARS-CoV-2 requires cholesterol for viral entry and pathological syncytia formation, Elife
Santopolo, Riccio, Rossi, Santoro, The proteostasis guardian HSF1 directs the transcription of its paralog and interactor HSF2 during proteasome dysfunction, Cell Mol Life Sci
Santopolo, Riccio, Santoro, The biogenesis of SARS-CoV-2 spike glycoprotein: multiple targets for host-directed antiviral therapy, Biochem Biophys Res Commun
Santoro, Amici, Elia, Benedetto, Garaci, Inhibition of virus protein glycosylation as the mechanism of the antiviral action of prostaglandin A in Sendai virus-infected cells, J Gen Virol
Santoro, Carafoli, Remdesivir: from Ebola to COVID-19, Biochem Biophys Res Commun
Santoro, Jaffe, Prostaglandin A inhibits the replication of vesicular stomatitis virus: effect on virus glycoprotein, J Gen Virol
Sattentau, Avoiding the void: cell-to-cell spread of human viruses, Nat Rev Microbiol
Shang, Wan, Luo, Ye, Geng et al., Cell entry mechanisms of SARS-CoV-2, Proc Natl Acad Sci
Sicari, Chatziioannou, Koutsandreas, Sitia, Chevet, Role of the early secretory pathway in SARS-CoV-2 infection, J Cell Biol
Silva, Espejo, Pereyra, Lynch, Thompson et al., Efficacy of Nitazoxanide in reducing the viral load in COVID-19 patients. Randomized, placebo-controlled, single-blinded, parallel group, doi:10.1101/2021.03.03.21252509
Son, Huang, Zeng, Bricker, Case et al., 2 replication and coronavirus pathogenesis, doi:10.1101/2020.09.24.312165
Theuerkauf, Michels, Riechert, Maier, Cichutek et al., Quantitative assays reveal cell fusion at minimal levels of SARS-CoV-2 spike protein and fusionfrom-without, iScience
Tian, Hu, Niu, Liu, Xu et al., Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer, J Thorac Oncol
Tregoning, Flight, Higham, Wang, Pierce, Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape, Nat Rev Immunol
Wahl, Gralinski, Johnson, Yao, Kovarova et al., SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature
Walls, Park, Tortorici, Wall, Mcguire et al., Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein, Cell
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Weisblum, Schmidt, Zhang, Dasilva, Poston et al., Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants, Elife
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review, JAMA
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science
Xu, Ng, Glycosylation-directed quality control of protein folding, Nat Rev Mol Cell Biol
Yang, Hughes, Kelkar, Yu, Cheng et al., Inhibition of SARS-CoV-2 viral entry upon blocking N-and O-glycan elaboration, Elife
Zhang, Jackson, Mou, Ojha, Peng et al., SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity, Nat Commun
DOI record:
{
"DOI": "10.1007/s00018-022-04246-w",
"ISSN": [
"1420-682X",
"1420-9071"
],
"URL": "http://dx.doi.org/10.1007/s00018-022-04246-w",
"abstract": "<jats:title>Abstract</jats:title><jats:p>SARS-CoV-2, the causative agent of COVID-19, has caused an unprecedented global health crisis. The SARS-CoV-2 spike, a surface-anchored trimeric class-I fusion glycoprotein essential for viral entry, represents a key target for developing vaccines and therapeutics capable of blocking virus invasion. The emergence of SARS-CoV-2 spike variants that facilitate virus spread and may affect vaccine efficacy highlights the need to identify novel antiviral strategies for COVID-19 therapy. Here, we demonstrate that nitazoxanide, an antiprotozoal agent with recognized broad-spectrum antiviral activity, interferes with SARS-CoV-2 spike maturation, hampering its terminal glycosylation at an endoglycosidase H-sensitive stage. Engineering multiple SARS-CoV-2 variant-pseudoviruses and utilizing quantitative cell–cell fusion assays, we show that nitazoxanide-induced spike modifications hinder progeny virion infectivity as well as spike-driven pulmonary cell–cell fusion, a critical feature of COVID-19 pathology. Nitazoxanide, being equally effective against the ancestral SARS-CoV-2 Wuhan-spike and different emerging variants, including the Delta variant of concern, may represent a useful tool in the fight against COVID-19 infections.\n</jats:p>",
"alternative-id": [
"4246"
],
"article-number": "227",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "5 January 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Revised",
"name": "revised",
"order": 2,
"value": "3 March 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 3,
"value": "11 March 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 4,
"value": "7 April 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Date",
"name": "change_date",
"order": 5,
"value": "30 July 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Type",
"name": "change_type",
"order": 6,
"value": "Update"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Details",
"name": "change_details",
"order": 7,
"value": "Missing Open Access funding information has been added in the Funding Note"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Financial support for this study was in part provided by Romark Laboratories LC, the company that owns the intellectual property rights related to nitazoxanide. JF Rossignol is an employee and stockholder of Romark Laboratories, LC."
},
{
"group": {
"label": "Ethics approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "Not applicable."
},
{
"group": {
"label": "Consent to publish",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "Not applicable."
}
],
"author": [
{
"affiliation": [],
"family": "Riccio",
"given": "Anna",
"sequence": "first"
},
{
"affiliation": [],
"family": "Santopolo",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rossi",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piacentini",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rossignol",
"given": "Jean-Francois",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1432-4949",
"affiliation": [],
"authenticated-orcid": false,
"family": "Santoro",
"given": "M. Gabriella",
"sequence": "additional"
}
],
"container-title": "Cellular and Molecular Life Sciences",
"container-title-short": "Cell. Mol. Life Sci.",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
4,
7
]
],
"date-time": "2022-04-07T17:02:58Z",
"timestamp": 1649350978000
},
"deposited": {
"date-parts": [
[
2022,
8,
2
]
],
"date-time": "2022-08-02T05:04:08Z",
"timestamp": 1659416648000
},
"funder": [
{
"DOI": "10.13039/501100003407",
"award": [
"PRIN project N 2010PHT9NF-006"
],
"doi-asserted-by": "publisher",
"name": "Ministero dell’Istruzione, dell’Università e della Ricerca"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
26
]
],
"date-time": "2022-12-26T15:09:42Z",
"timestamp": 1672067382985
},
"is-referenced-by-count": 13,
"issue": "5",
"issued": {
"date-parts": [
[
2022,
4,
7
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2022,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
7
]
],
"date-time": "2022-04-07T00:00:00Z",
"timestamp": 1649289600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
7
]
],
"date-time": "2022-04-07T00:00:00Z",
"timestamp": 1649289600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s00018-022-04246-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s00018-022-04246-w/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s00018-022-04246-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
4,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
7
]
]
},
"published-print": {
"date-parts": [
[
2022,
5
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41579-018-0118-9",
"author": "J Cui",
"doi-asserted-by": "publisher",
"first-page": "181",
"journal-title": "Nat Rev Microbiol",
"key": "4246_CR1",
"unstructured": "Cui J, Li F, Shi ZL (2019) Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17:181–192",
"volume": "17",
"year": "2019"
},
{
"DOI": "10.1016/j.cell.2020.04.011",
"author": "D Kim",
"doi-asserted-by": "publisher",
"first-page": "914",
"journal-title": "Cell",
"key": "4246_CR2",
"unstructured": "Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H (2020) The architecture of SARS-CoV-2 transcriptome. Cell 181:914-921.e10",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1146/annurev-micro-020518-115759",
"author": "TS Fung",
"doi-asserted-by": "publisher",
"first-page": "529",
"journal-title": "Annu Rev Microbiol",
"key": "4246_CR3",
"unstructured": "Fung TS, Liu DX (2019) Human Coronavirus: host-pathogen interaction. Annu Rev Microbiol 73:529–557",
"volume": "73",
"year": "2019"
},
{
"DOI": "10.1038/nrmicro.2016.81",
"author": "E de Wit",
"doi-asserted-by": "publisher",
"first-page": "523",
"journal-title": "Nat Rev Microbiol",
"key": "4246_CR4",
"unstructured": "de Wit E, van Doremalen N, Falzarano D, Munster VJ (2016) SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 14:523–534",
"volume": "14",
"year": "2016"
},
{
"DOI": "10.1038/s41579-020-00459-7",
"author": "B Hu",
"doi-asserted-by": "publisher",
"first-page": "141",
"journal-title": "Nat Rev Microbiol",
"key": "4246_CR5",
"unstructured": "Hu B, Guo H, Zhou P, Shi ZL (2021) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19:141–154",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.12839",
"author": "WJ Wiersinga",
"doi-asserted-by": "publisher",
"first-page": "782",
"journal-title": "JAMA",
"key": "4246_CR6",
"unstructured": "Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC (2020) Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA 324:782–793",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.058",
"author": "AC Walls",
"doi-asserted-by": "publisher",
"first-page": "281",
"journal-title": "Cell",
"key": "4246_CR7",
"unstructured": "Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181:281-292.e6",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "Cell",
"key": "4246_CR8",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271-280.e8",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1126/science.abd4251",
"author": "Y Cai",
"doi-asserted-by": "publisher",
"first-page": "1586",
"journal-title": "Science",
"key": "4246_CR9",
"unstructured": "Cai Y, Zhang J, Xiao T, Peng H, Sterling SM, Walsh RM Jr, Rawson S, Rits-Volloch S, Chen B (2020) Distinct conformational states of SARS-CoV-2 spike protein. Science 369:1586–1592",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/j.bbrc.2020.10.080",
"author": "S Santopolo",
"doi-asserted-by": "publisher",
"first-page": "80",
"journal-title": "Biochem Biophys Res Commun",
"key": "4246_CR10",
"unstructured": "Santopolo S, Riccio A, Santoro MG (2020) The biogenesis of SARS-CoV-2 spike glycoprotein: multiple targets for host-directed antiviral therapy. Biochem Biophys Res Commun 538:80–87",
"volume": "538",
"year": "2020"
},
{
"DOI": "10.1038/s41392-021-00809-8",
"author": "Y Gong",
"doi-asserted-by": "publisher",
"first-page": "396",
"journal-title": "Signal Transduct Target Ther",
"key": "4246_CR11",
"unstructured": "Gong Y, Qin S, Dai L, Tian Z (2021) The glycosylation in SARS-CoV-2 and its receptor ACE2. Signal Transduct Target Ther 6:396",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1083/jcb.202006005",
"author": "D Sicari",
"doi-asserted-by": "publisher",
"first-page": "e202006005",
"journal-title": "J Cell Biol",
"key": "4246_CR12",
"unstructured": "Sicari D, Chatziioannou A, Koutsandreas T, Sitia R, Chevet E (2020) Role of the early secretory pathway in SARS-CoV-2 infection. J Cell Biol 219:e202006005",
"volume": "219",
"year": "2020"
},
{
"DOI": "10.1126/science.6505693",
"author": "S Alexander",
"doi-asserted-by": "publisher",
"first-page": "1328",
"journal-title": "Science",
"key": "4246_CR13",
"unstructured": "Alexander S, Elder JH (1984) Carbohydrate dramatically influences immune reactivity of antisera to viral glycoprotein antigens. Science 226:1328–1330",
"volume": "226",
"year": "1984"
},
{
"DOI": "10.1034/j.1600-0854.2000.010702.x",
"author": "I Braakman",
"doi-asserted-by": "publisher",
"first-page": "533",
"journal-title": "Traffic",
"key": "4246_CR14",
"unstructured": "Braakman I, van Anken E (2000) Folding of viral envelope glycoproteins in the endoplasmic reticulum. Traffic 1:533–539",
"volume": "1",
"year": "2000"
},
{
"DOI": "10.1016/j.molcel.2020.04.022",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "779",
"journal-title": "Mol Cell",
"key": "4246_CR15",
"unstructured": "Hoffmann M, Kleine-Weber H, Pöhlmann S (2020) A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell 78:779-784.e5",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"author": "CB Jackson",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "Nat Rev Mol Cell Biol",
"key": "4246_CR16",
"unstructured": "Jackson CB, Farzan M, Chen B, Choe H (2022) Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23:3–20",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1038/nrmicro1208",
"author": "T Horimoto",
"doi-asserted-by": "publisher",
"first-page": "591",
"journal-title": "Nat Rev Microbiol",
"key": "4246_CR17",
"unstructured": "Horimoto T, Kawaoka Y (2005) Influenza: lessons from past pandemics, warnings from current incidents. Nat Rev Microbiol 3:591–600",
"volume": "3",
"year": "2005"
},
{
"DOI": "10.1056/NEJMsr2105280",
"author": "PR Krause",
"doi-asserted-by": "publisher",
"first-page": "179",
"journal-title": "N Engl J Med",
"key": "4246_CR18",
"unstructured": "Krause PR, Fleming TR, Longini IM, Peto R, Briand S, Heymann DL, Beral V, Snape MD, Rees H, Ropero AM et al (2021) SARS-CoV-2 variants and vaccines. N Engl J Med 385:179–186",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1038/s41577-021-00592-1",
"author": "JS Tregoning",
"doi-asserted-by": "publisher",
"first-page": "626",
"journal-title": "Nat Rev Immunol",
"key": "4246_CR19",
"unstructured": "Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF (2021) Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol 21:626–636",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1038/s41392-021-00796-w",
"author": "Y Dong",
"doi-asserted-by": "publisher",
"first-page": "387",
"journal-title": "Signal Transduct Target Ther",
"key": "4246_CR20",
"unstructured": "Dong Y, Dai T, Wang B, Zhang L, Zeng LH, Huang J, Yan H, Zhang L, Zhou F (2021) The way of SARS-CoV-2 vaccine development: success and challenges. Signal Transduct Target Ther 6:387",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.7554/eLife.61312",
"author": "Y Weisblum",
"doi-asserted-by": "publisher",
"first-page": "e61312",
"journal-title": "Elife",
"key": "4246_CR21",
"unstructured": "Weisblum Y, Schmidt F, Zhang F, DaSilva J, Poston D, Lorenzi JC, Muecksch F, Rutkowska M, Hoffmann HH, Michailidis E et al (2020) Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. Elife 9:e61312",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1038/s41591-021-01318-5",
"author": "D Planas",
"doi-asserted-by": "publisher",
"first-page": "917",
"journal-title": "Nat Med",
"key": "4246_CR22",
"unstructured": "Planas D, Bruel T, Grzelak L, Guivel-Benhassine F, Staropoli I, Porrot F, Planchais C, Buchrieser J, Rajah MM, Bishop E et al (2021) Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med 27:917–924",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1038/s41579-021-00573-0",
"author": "WT Harvey",
"doi-asserted-by": "publisher",
"first-page": "409",
"journal-title": "Nat Rev Microbiol",
"key": "4246_CR23",
"unstructured": "Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A et al (2021) SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 19:409–424",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1086/322038",
"author": "JF Rossignol",
"doi-asserted-by": "publisher",
"first-page": "381",
"journal-title": "J Infect Dis",
"key": "4246_CR24",
"unstructured": "Rossignol JF, Ayoub A, Ayers MS (2001) Treatment of diarrhea caused by Giardia intestinalis and Entamoeba histolytica or E. dispar: a randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis 184:381–384",
"volume": "184",
"year": "2001"
},
{
"DOI": "10.1016/j.cgh.2005.12.020",
"author": "JF Rossignol",
"doi-asserted-by": "publisher",
"first-page": "320",
"journal-title": "Clin Gastroenterol Hepatol",
"key": "4246_CR25",
"unstructured": "Rossignol JF, Kabil SM, el-Gohary Y, Younis AM (2006) Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species. Clin Gastroenterol Hepatol 4:320–324",
"volume": "4",
"year": "2006"
},
{
"DOI": "10.1016/j.antiviral.2014.07.014",
"author": "JF Rossignol",
"doi-asserted-by": "publisher",
"first-page": "94",
"journal-title": "Antiviral Res",
"key": "4246_CR26",
"unstructured": "Rossignol JF (2014) Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res 110:94–103",
"volume": "110",
"year": "2014"
},
{
"DOI": "10.1038/d41573-020-00016-0",
"author": "G Li",
"doi-asserted-by": "publisher",
"first-page": "149",
"journal-title": "Nat Rev Drug Discov",
"key": "4246_CR27",
"unstructured": "Li G, De Clercq E (2020) Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 19:149–150",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1074/jbc.M109.029470",
"author": "JF Rossignol",
"doi-asserted-by": "publisher",
"first-page": "29798",
"journal-title": "J Biol Chem",
"key": "4246_CR28",
"unstructured": "Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG (2009) Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem 284:29798–29808",
"volume": "284",
"year": "2009"
},
{
"DOI": "10.1038/s41598-018-28172-9",
"author": "S Piacentini",
"doi-asserted-by": "publisher",
"first-page": "10425",
"journal-title": "Sci Rep",
"key": "4246_CR29",
"unstructured": "Piacentini S, La Frazia S, Riccio A, Pedersen JZ, Topai A, Nicolotti O, Rossignol JF, Santoro MG (2018) Nitazoxanide inhibits paramyxovirus replication by targeting the Fusion protein folding: role of glycoprotein-specific thiol oxidoreductase ERp57. Sci Rep 8:10425",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.1016/j.antiviral.2007.08.005",
"author": "BE Korba",
"doi-asserted-by": "publisher",
"first-page": "56",
"journal-title": "Antiviral Res",
"key": "4246_CR30",
"unstructured": "Korba BE, Montero AB, Farrar K, Gaye K, Mukerjee S, Ayers MS, Rossignol JF (2008) Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res 77:56–63",
"volume": "77",
"year": "2008"
},
{
"DOI": "10.1128/JVI.01213-13",
"author": "S La Frazia",
"doi-asserted-by": "publisher",
"first-page": "11096",
"journal-title": "J Virol",
"key": "4246_CR31",
"unstructured": "La Frazia S, Ciucci A, Arnoldi F, Coira M, Gianferretti P, Angelini M, Belardo G, Burrone OR, Rossignol JF, Santoro MG (2013) Thiazolides, a new class of antiviral agents effective against rotavirus infection, target viral morphogenesis, inhibiting viroplasm formation. J Virol 87:11096–11106",
"volume": "87",
"year": "2013"
},
{
"DOI": "10.1016/S0140-6736(06)68852-1",
"author": "JF Rossignol",
"doi-asserted-by": "publisher",
"first-page": "124",
"journal-title": "Lancet",
"key": "4246_CR32",
"unstructured": "Rossignol JF, Abu-Zekry M, Hussein A, Santoro MG (2006) Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet 368:124–129",
"volume": "368",
"year": "2006"
},
{
"DOI": "10.1016/S1473-3099(14)70717-0",
"author": "J Haffizulla",
"doi-asserted-by": "publisher",
"first-page": "609",
"journal-title": "Lancet Infect Dis",
"key": "4246_CR33",
"unstructured": "Haffizulla J, Hartman A, Hoppers M, Resnick H, Samudrala S, Ginocchio C, Bardin M, Rossignol JF, US Nitazoxanide Influenza Clinical Study Group (2014) Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis 14:609–618",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.1053/j.gastro.2008.11.037",
"author": "JF Rossignol",
"doi-asserted-by": "publisher",
"first-page": "856",
"journal-title": "Gastroenterology",
"key": "4246_CR34",
"unstructured": "Rossignol JF, Elfert A, El-Gohary Y, Keeffe EB (2009) Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin. Gastroenterology 136:856–862",
"volume": "136",
"year": "2009"
},
{
"DOI": "10.1016/j.antiviral.2014.11.010",
"author": "J Cao",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Antiviral Res",
"key": "4246_CR35",
"unstructured": "Cao J, Forrest JC, Zhang X (2015) A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs. Antiviral Res 114:1–10",
"volume": "114",
"year": "2015"
},
{
"DOI": "10.1016/j.jiph.2016.04.001",
"author": "JF Rossignol",
"doi-asserted-by": "publisher",
"first-page": "227",
"journal-title": "J Infect Public Health",
"key": "4246_CR36",
"unstructured": "Rossignol JF (2016) Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J Infect Public Health 9:227–230",
"volume": "9",
"year": "2016"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"author": "M Wang",
"doi-asserted-by": "publisher",
"first-page": "269",
"journal-title": "Cell Res",
"key": "4246_CR37",
"unstructured": "Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1101/2020.09.24.312165",
"author": "J Son",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "4246_CR38",
"unstructured": "Son J, Huang S, Zeng Q, Bricker TL, Case JB, Zhou J, Zang R, Liu Z, Chang X, Harastani HH et al (2021) JIB-04 has broad-spectrum antiviral activity and inhibits SARS-CoV-2 replication and coronavirus pathogenesis. bioRxiv. https://doi.org/10.1101/2020.09.24.312165",
"year": "2021"
},
{
"DOI": "10.1183/13993003.03725-2020",
"author": "PRM Rocco",
"doi-asserted-by": "publisher",
"first-page": "2003725",
"journal-title": "Eur Respir J",
"key": "4246_CR39",
"unstructured": "Rocco PRM, Silva PL, Cruz FF, Melo-Junior MAC, Tierno PFGMM, Moura MA, De Oliveira LFG, Lima CC, Dos Santos EA, Junior WF et al (2021) Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial. Eur Respir J 58:2003725",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.1101/2021.03.03.21252509",
"author": "M Silva",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "4246_CR40",
"unstructured": "Silva M, Espejo A, Pereyra ML, Lynch M, Thompson M, Taconelli H, Baré P, Pereson MJ, Garbini M, Crucci P et al (2021) Efficacy of Nitazoxanide in reducing the viral load in COVID-19 patients. Randomized, placebo-controlled, single-blinded, parallel group, pilot study. medRxiv. https://doi.org/10.1101/2021.03.03.21252509",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100981",
"author": "VF Blum",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "4246_CR41",
"unstructured": "Blum VF, Cimerman S, Hunter JR, Tierno P, Lacerda A, Soeiro A, Cardoso F, Bellei NC, Maricato J, Mantovani N et al (2021) Nitazoxanide superiority to placebo to treat moderate COVID-19 - A Pilot prove of concept randomized double-blind clinical trial. EClinicalMedicine 37:100981",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2022.101310",
"author": "JF Rossignol",
"doi-asserted-by": "publisher",
"first-page": "101310",
"journal-title": "eClinicalMedicine",
"key": "4246_CR42",
"unstructured": "Rossignol JF, Bardin MC, Fulgencio J, Mogelnicki D, Bréchot C, for the Vanguard Study Group (2022) A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. eClinicalMedicine 45:101310",
"volume": "45",
"year": "2022"
},
{
"DOI": "10.1126/science.abb2507",
"author": "D Wrapp",
"doi-asserted-by": "publisher",
"first-page": "1260",
"journal-title": "Science",
"key": "4246_CR43",
"unstructured": "Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367:1260–1263",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1101/2020.03.16.994152",
"author": "T Li",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "4246_CR44",
"unstructured": "Li T, Zheng Q, Yu H, Wu D, Xue W, Zhang Y, Huang X, Zhou L, Zhang Z, Zha Z et al (2020) Characterization of the SARS-CoV-2 spike in an early prefusion conformation. bioRxiv. https://doi.org/10.1101/2020.03.16.994152",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-15562-9",
"author": "X Ou",
"doi-asserted-by": "publisher",
"first-page": "1620",
"journal-title": "Nat Commun",
"key": "4246_CR45",
"unstructured": "Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J et al (2020) Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 11:1620",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1073/pnas.1407087111",
"author": "JK Millet",
"doi-asserted-by": "publisher",
"first-page": "15214",
"journal-title": "Proc Natl Acad Sci USA",
"key": "4246_CR46",
"unstructured": "Millet JK, Whittaker GR (2014) Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci USA 111:15214–15219",
"volume": "111",
"year": "2014"
},
{
"DOI": "10.1038/s41586-020-2895-3",
"author": "JA Plante",
"doi-asserted-by": "publisher",
"first-page": "116",
"journal-title": "Nature",
"key": "4246_CR47",
"unstructured": "Plante JA, Liu Y, Liu J, Xia H, Johnson BA, Lokugamage KG, Zhang X, Muruato AE, Zou J, Fontes-Garfias CR et al (2020) Spike mutation D614G alters SARS-CoV-2 fitness. Nature 592:116–121",
"volume": "592",
"year": "2020"
},
{
"DOI": "10.1038/nrclinonc.2016.206",
"author": "EE Manasanch",
"doi-asserted-by": "publisher",
"first-page": "417",
"journal-title": "Nat Rev Clin Oncol",
"key": "4246_CR48",
"unstructured": "Manasanch EE, Orlowski RZ (2017) Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol 14:417–433",
"volume": "14",
"year": "2017"
},
{
"DOI": "10.1016/j.molcel.2015.06.010",
"author": "D Morito",
"doi-asserted-by": "publisher",
"first-page": "335",
"journal-title": "Mol Cell",
"key": "4246_CR49",
"unstructured": "Morito D, Nagata K (2015) Pathogenic hijacking of ER-associated degradation: is ERAD flexible? Mol Cell 59:335–344",
"volume": "59",
"year": "2015"
},
{
"DOI": "10.1016/0168-1702(91)90103-3",
"author": "B Delmas",
"doi-asserted-by": "publisher",
"first-page": "107",
"journal-title": "Virus Res",
"key": "4246_CR50",
"unstructured": "Delmas B, Laude H (1991) Carbohydrate-induced conformational changes strongly modulate the antigenicity of coronavirus TGEV glycoproteins S and M. Virus Res 20:107–120",
"volume": "20",
"year": "1991"
},
{
"DOI": "10.1038/nrm4073",
"author": "C Xu",
"doi-asserted-by": "publisher",
"first-page": "742",
"journal-title": "Nat Rev Mol Cell Biol",
"key": "4246_CR51",
"unstructured": "Xu C, Ng DTW (2015) Glycosylation-directed quality control of protein folding. Nat Rev Mol Cell Biol 16:742–752",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1128/jvi.71.5.3719-3725.1997",
"author": "R Ohuchi",
"doi-asserted-by": "publisher",
"first-page": "3719",
"journal-title": "J Virol",
"key": "4246_CR52",
"unstructured": "Ohuchi R, Ohuchi M, Garten W, Klenk HD (1997) Oligosaccharides in the stem region maintain the influenza virus hemagglutinin in the metastable form required for fusion activity. J Virol 71:3719–3725",
"volume": "71",
"year": "1997"
},
{
"DOI": "10.1128/JVI.79.19.12416-12424.2005",
"author": "VP Mishin",
"doi-asserted-by": "publisher",
"first-page": "12416",
"journal-title": "J Virol",
"key": "4246_CR53",
"unstructured": "Mishin VP, Novikov D, Hayden FG, Gubareva LV (2005) Effect of hemagglutinin glycosylation on influenza virus susceptibility to neuraminidase inhibitors. J Virol 79:12416–12424",
"volume": "79",
"year": "2005"
},
{
"DOI": "10.1074/jbc.RA120.016175",
"author": "B Boson",
"doi-asserted-by": "publisher",
"first-page": "100111",
"journal-title": "J Biol Chem",
"key": "4246_CR54",
"unstructured": "Boson B, Legros V, Zhou B, Siret E, Mathieu C, Cosset FL, Lavillette D, Denolly S (2021) The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J Biol Chem 296:100111",
"volume": "296",
"year": "2021"
},
{
"DOI": "10.7554/eLife.61552",
"author": "Q Yang",
"doi-asserted-by": "publisher",
"first-page": "e61552",
"journal-title": "Elife",
"key": "4246_CR55",
"unstructured": "Yang Q, Hughes TA, Kelkar A, Yu X, Cheng K, Park S, Huang WC, Lovell JF, Neelamegham S (2020) Inhibition of SARS-CoV-2 viral entry upon blocking N- and O-glycan elaboration. Elife 9:e61552",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1099/vir.0.81749-0",
"author": "X Ren",
"doi-asserted-by": "publisher",
"first-page": "1691",
"journal-title": "J Gen Virol",
"key": "4246_CR56",
"unstructured": "Ren X, Glende J, Al-Falah M, de Vries V, Schwegmann-Wessels C, Qu X, Tan L, Tschernig T, Deng H, Naim HY et al (2006) Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J Gen Virol 87:1691–1695",
"volume": "87",
"year": "2006"
},
{
"DOI": "10.1073/pnas.0809524106",
"author": "S Belouzard",
"doi-asserted-by": "publisher",
"first-page": "5871",
"journal-title": "Proc Natl Acad Sci USA",
"key": "4246_CR57",
"unstructured": "Belouzard S, Chu VC, Whittaker GR (2009) Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci USA 106:5871–5876",
"volume": "106",
"year": "2009"
},
{
"DOI": "10.1016/j.ebiom.2020.103104",
"author": "R Bussani",
"doi-asserted-by": "publisher",
"first-page": "103104",
"journal-title": "EBioMedicine",
"key": "4246_CR58",
"unstructured": "Bussani R, Schneider E, Zentilin L, Collesi C, Ali H, Braga L, Volpe MC, Colliva A, Zanconati F, Berlot G et al (2020) Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine 61:103104",
"volume": "61",
"year": "2020"
},
{
"DOI": "10.15252/embj.2020106267",
"author": "J Buchrieser",
"doi-asserted-by": "publisher",
"first-page": "e106267",
"journal-title": "EMBO J",
"key": "4246_CR59",
"unstructured": "Buchrieser J, Dufloo J, Hubert M, Monel B, Planas D, Rajah MM, Planchais C, Porrot F, Guivel-Benhassine F, Van der Werf S et al (2020) Syncytia formation by SARS-CoV-2-infected cells. EMBO J 39:e106267",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1074/jbc.RA120.016148",
"author": "CB Plescia",
"doi-asserted-by": "publisher",
"first-page": "100103",
"journal-title": "J Biol Chem",
"key": "4246_CR60",
"unstructured": "Plescia CB, David EA, Patra D, Sengupta R, Amiar S, Su Y, Stahelin RV (2021) SARS-CoV-2 viral budding and entry can be modeled using BSL-2 level virus-like particles. J Biol Chem 296:100103",
"volume": "296",
"year": "2021"
},
{
"DOI": "10.1128/mBio.02371-21",
"author": "D Bracquemond",
"doi-asserted-by": "publisher",
"first-page": "e0237121",
"journal-title": "MBio",
"key": "4246_CR61",
"unstructured": "Bracquemond D, Muriaux D (2021) Betacoronavirus assembly: clues and perspectives for elucidating SARS-CoV-2 particle formation and egress. MBio 12:e0237121",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.molcel.2020.07.027",
"author": "F Robson",
"doi-asserted-by": "publisher",
"first-page": "710",
"journal-title": "Mol Cell",
"key": "4246_CR62",
"unstructured": "Robson F, Khan KS, Le TK, Paris C, Demirbag S, Barfuss P, Rocchi P, Ng WL (2020) Coronavirus RNA proofreading: molecular basis and therapeutic targeting. Mol Cell 79:710–727",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.1093/jac/dkab189",
"author": "JA Hiscox",
"doi-asserted-by": "publisher",
"first-page": "2230",
"journal-title": "J Antimicrob Chemother",
"key": "4246_CR63",
"unstructured": "Hiscox JA, Khoo SH, Stewart JP, Owen A (2021) Shutting the gate before the horse has bolted: is it time for a conversation about SARS-CoV-2 and antiviral drug resistance? J Antimicrob Chemother 76:2230–2233",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.06.043",
"author": "B Korber",
"doi-asserted-by": "publisher",
"first-page": "812",
"journal-title": "Cell",
"key": "4246_CR64",
"unstructured": "Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, Hengartner N, Giorgi EE, Bhattacharya T, Foley B et al (2020) Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 182:812-827.e19",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-1083-1",
"author": "AWD Edridge",
"doi-asserted-by": "publisher",
"first-page": "1691",
"journal-title": "Nat Med",
"key": "4246_CR65",
"unstructured": "Edridge AWD, Kaczorowska J, Hoste ACR, Bakker M, Klein M, Loens K, Jebbink MF, Matser A, Kinsella CM, Rueda P et al (2020) Seasonal coronavirus protective immunity is short-lasting. Nat Med 26:1691–1693",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.bbrc.2020.11.043",
"author": "MG Santoro",
"doi-asserted-by": "publisher",
"first-page": "145",
"journal-title": "Biochem Biophys Res Commun",
"key": "4246_CR66",
"unstructured": "Santoro MG, Carafoli E (2020) Remdesivir: from Ebola to COVID-19. Biochem Biophys Res Commun 538:145–150",
"volume": "538",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03312-w",
"author": "A Wahl",
"doi-asserted-by": "publisher",
"first-page": "451",
"journal-title": "Nature",
"key": "4246_CR67",
"unstructured": "Wahl A, Gralinski LE, Johnson CE, Yao W, Kovarova M, Dinnon KH 3rd, Liu H, Madden VJ, Krzystek HM, De C et al (2021) SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature 591:451–457",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1016/j.isci.2021.102170",
"author": "SA Theuerkauf",
"doi-asserted-by": "publisher",
"first-page": "102170",
"journal-title": "iScience",
"key": "4246_CR68",
"unstructured": "Theuerkauf SA, Michels A, Riechert V, Maier TJ, Flory E, Cichutek K, Buchholz CJ (2021) Quantitative assays reveal cell fusion at minimal levels of SARS-CoV-2 spike protein and fusion-from-without. iScience 24:102170",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1016/j.coviro.2020.03.003",
"author": "DE Griffin",
"doi-asserted-by": "publisher",
"first-page": "46",
"journal-title": "Curr Opin Virol",
"key": "4246_CR69",
"unstructured": "Griffin DE (2020) Measles virus persistence and its consequences. Curr Opin Virol 41:46–51",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1006099",
"author": "AA Compton",
"doi-asserted-by": "publisher",
"first-page": "e1006099",
"journal-title": "PLoS Pathog",
"key": "4246_CR70",
"unstructured": "Compton AA, Schwartz O (2017) They might me giants: does syncytium formation sink or spread HIV infection? PLoS Pathog 13:e1006099",
"volume": "13",
"year": "2017"
},
{
"DOI": "10.1038/nrmicro1972",
"author": "Q Sattentau",
"doi-asserted-by": "publisher",
"first-page": "815",
"journal-title": "Nat Rev Microbiol",
"key": "4246_CR71",
"unstructured": "Sattentau Q (2008) Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol 6:815–826",
"volume": "6",
"year": "2008"
},
{
"DOI": "10.3390/v11111017",
"author": "M Ferren",
"doi-asserted-by": "publisher",
"first-page": "1017",
"journal-title": "Viruses",
"key": "4246_CR72",
"unstructured": "Ferren M, Horvat B, Mathieu C (2019) Measles encephalitis: towards new therapeutics. Viruses 11:1017",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1016/j.jtho.2020.02.010",
"author": "S Tian",
"doi-asserted-by": "publisher",
"first-page": "700",
"journal-title": "J Thorac Oncol",
"key": "4246_CR73",
"unstructured": "Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY (2020) Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol 15:700–704",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1126/science.abb7314",
"author": "B Rockx",
"doi-asserted-by": "publisher",
"first-page": "1012",
"journal-title": "Science",
"key": "4246_CR74",
"unstructured": "Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, de Meulder D, van Amerongen G, van den Brand J, Okba NMA et al (2020) Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 368:1012–1015",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-25589-1",
"author": "J Cattin-Ortolá",
"doi-asserted-by": "publisher",
"first-page": "5333",
"journal-title": "Nat Commun",
"key": "4246_CR75",
"unstructured": "Cattin-Ortolá J, Welch L, Maslen SL, Skehel JM, Papa G, James LC, Munro S (2021) Sequences in the cytoplasmic tail of SARS-CoV-2 spike facilitate expression at the cell surface and syncytia formation. Nat Commun 12:5333",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.7554/eLife.65962",
"author": "DW Sanders",
"doi-asserted-by": "publisher",
"first-page": "e65962",
"journal-title": "Elife",
"key": "4246_CR76",
"unstructured": "Sanders DW, Jumper CC, Ackerman PJ, Bracha D, Donlic A, Kim H, Kenney D, Castello-Serrano I, Suzuki S, Tamura T et al (2021) SARS-CoV-2 requires cholesterol for viral entry and pathological syncytia formation. Elife 10:e65962",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-19808-4",
"author": "L Zhang",
"doi-asserted-by": "publisher",
"first-page": "6013",
"journal-title": "Nat Commun",
"key": "4246_CR77",
"unstructured": "Zhang L, Jackson CB, Mou H, Ojha A, Peng H, Quinlan BD, Rangarajan ES, Pan A, Vanderheiden A, Suthar MS et al (2020) SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat Commun 11:6013",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.21769/BioProtoc.2035",
"author": "JK Millet",
"doi-asserted-by": "publisher",
"first-page": "e2035",
"journal-title": "Bio Protoc",
"key": "4246_CR78",
"unstructured": "Millet JK, Whittaker GR (2016) Murine leukemia virus (MLV)-based coronavirus spike-pseudotyped particle production and infection. Bio Protoc 6:e2035",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1073/pnas.1616112114",
"author": "M Coccia",
"doi-asserted-by": "publisher",
"first-page": "1045",
"journal-title": "Proc Natl Acad Sci USA",
"key": "4246_CR79",
"unstructured": "Coccia M, Rossi A, Riccio A, Trotta E, Santoro MG (2017) Human NF-κB repressing factor acts as a stress-regulated switch for ribosomal RNA processing and nucleolar homeostasis surveillance. Proc Natl Acad Sci USA 114:1045–1050",
"volume": "114",
"year": "2017"
},
{
"DOI": "10.1099/0022-1317-64-12-2797",
"author": "MG Santoro",
"doi-asserted-by": "publisher",
"first-page": "2797",
"journal-title": "J Gen Virol",
"key": "4246_CR80",
"unstructured": "Santoro MG, Jaffe BM, Esteban M (1983) Prostaglandin A inhibits the replication of vesicular stomatitis virus: effect on virus glycoprotein. J Gen Virol 64:2797–2801",
"volume": "64",
"year": "1983"
},
{
"DOI": "10.1099/0022-1317-70-4-789",
"author": "MG Santoro",
"doi-asserted-by": "publisher",
"first-page": "789",
"journal-title": "J Gen Virol",
"key": "4246_CR81",
"unstructured": "Santoro MG, Amici C, Elia G, Benedetto A, Garaci E (1989) Inhibition of virus protein glycosylation as the mechanism of the antiviral action of prostaglandin A in Sendai virus-infected cells. J Gen Virol 70:789–800",
"volume": "70",
"year": "1989"
},
{
"DOI": "10.1158/1541-7786.MCR-19-0243",
"author": "S Pizzato Scomazzon",
"doi-asserted-by": "publisher",
"first-page": "2444",
"journal-title": "Mol Cancer Res",
"key": "4246_CR82",
"unstructured": "Pizzato Scomazzon S, Riccio A, Santopolo S, Lanzilli G, Coccia M, Rossi A, Santoro MG (2019) The zinc-finger AN1-type domain 2a gene acts as a regulator of cell survival in human melanoma: role of E3-ligase cIAP2. Mol Cancer Res 17:2444–2456",
"volume": "17",
"year": "2019"
},
{
"DOI": "10.1007/s00018-020-03568-x",
"author": "S Santopolo",
"doi-asserted-by": "publisher",
"first-page": "1113",
"journal-title": "Cell Mol Life Sci",
"key": "4246_CR83",
"unstructured": "Santopolo S, Riccio A, Rossi A, Santoro MG (2021) The proteostasis guardian HSF1 directs the transcription of its paralog and interactor HSF2 during proteasome dysfunction. Cell Mol Life Sci 78:1113–1129",
"volume": "78",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0006529",
"author": "E Campeau",
"doi-asserted-by": "publisher",
"first-page": "e6529",
"journal-title": "PLoS ONE",
"key": "4246_CR84",
"unstructured": "Campeau E, Ruhl VE, Rodier F, Smith CL, Rahmberg BL, Fuss JO, Campisi J, Yaswen P, Cooper PK, Kaufman PD (2009) A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS ONE 4:e6529",
"volume": "4",
"year": "2009"
},
{
"DOI": "10.1073/pnas.2003138117",
"author": "J Shang",
"doi-asserted-by": "publisher",
"first-page": "11727",
"journal-title": "Proc Natl Acad Sci USA",
"key": "4246_CR85",
"unstructured": "Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F (2020) Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci USA 117:11727–11734",
"volume": "117",
"year": "2020"
}
],
"reference-count": 85,
"references-count": 85,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.04.12.439201",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s00018-022-04246-w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cell Biology",
"Cellular and Molecular Neuroscience",
"Pharmacology",
"Molecular Biology",
"Molecular Medicine"
],
"subtitle": [],
"title": "Impairment of SARS-CoV-2 spike glycoprotein maturation and fusion activity by nitazoxanide: an effect independent of spike variants emergence",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "79"
}