Different Outcome in COVID-19 Patients with or without PPI Use: A Systematic Review and Meta-analysis
et al., International Journal of Biomedical Science and Travel Medicine, doi:10.22225/ijbstm.1.1.2024.19-23, Mar 2024
PPIs for COVID-19
1st treatment shown to increase risk in
September 2020, now with p = 0.000000048 from 40 studies.
6,400+ studies for
210+ treatments. c19early.org
|
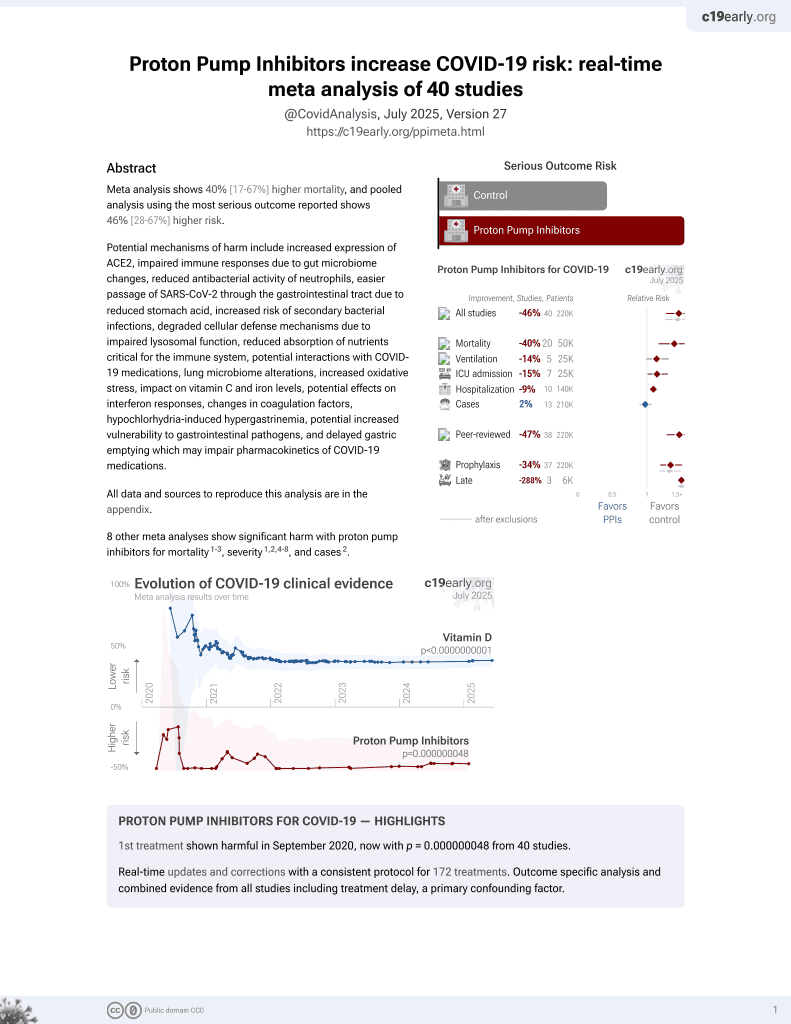
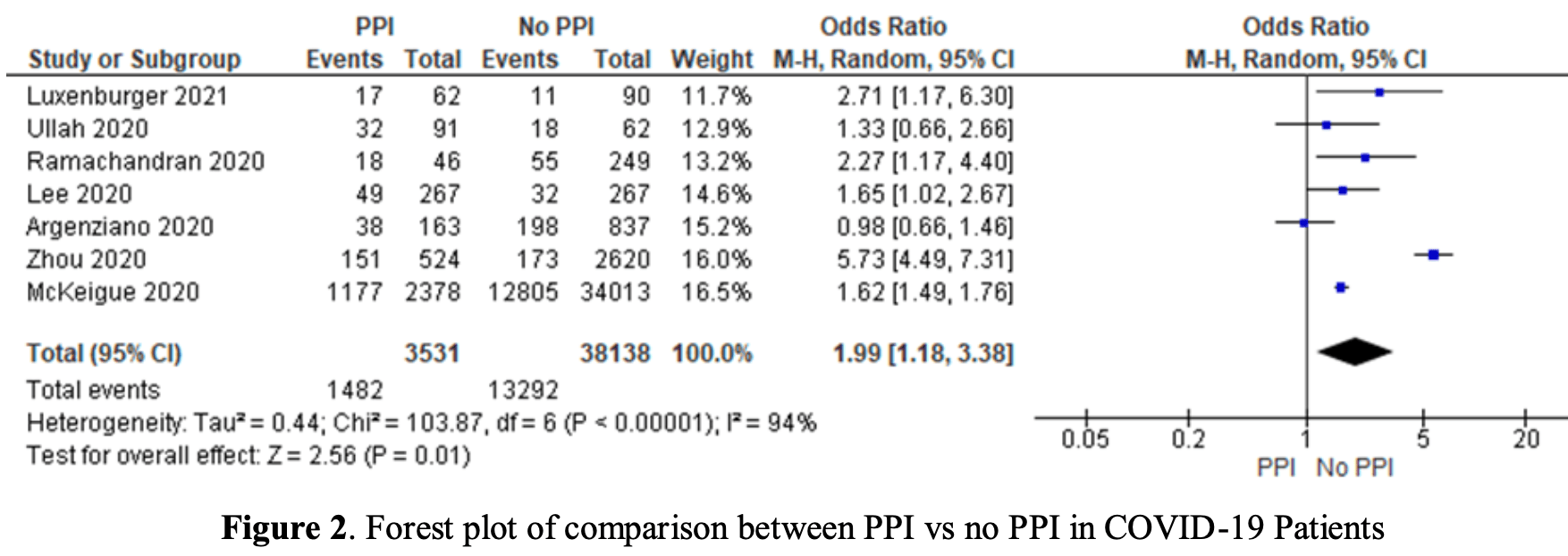
Meta analysis of 7 studies with over 30,000 COVID-19 patients showing 2 times higher risk of worse outcomes with proton pump inhibitor (PPI) use.
8 meta-analyses show significant harm with proton pump inhibitors for mortality1-3,
severity1,2,4-8 , and
cases2.
Currently there are 40 proton pump inhibitors for COVID-19 studies, showing 40% higher mortality [17‑67%], 14% higher ventilation [-1‑32%], 15% higher ICU admission [1‑30%], 9% higher hospitalization [3‑16%], and 2% fewer cases [-6‑10%].
|
risk of severe case, 99.0% higher, OR 1.99, p = 0.01, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Hariyanto et al., Proton pump inhibitor use is associated with increased risk of severity and mortality from coronavirus disease 2019 (COVID-19) infection, Digestive and Liver Disease, doi:10.1016/j.dld.2020.10.001.
2.
Fatima et al., The Use of Proton Pump Inhibitors and COVID-19: A Systematic Review and Meta-Analysis, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed7030037.
3.
Toubasi et al., Proton Pump Inhibitors: Current Use and the Risk of Coronavirus Infectious Disease 2019 Development and its Related Mortality. Meta-analysis, Archives of Medical Research, doi:10.1016/j.arcmed.2021.03.004.
4.
Wardhana et al., Different Outcome in COVID-19 Patients with or without PPI Use: A Systematic Review and Meta-analysis, International Journal of Biomedical Science and Travel Medicine, doi:10.22225/ijbstm.1.1.2024.19-23.
5.
Yan et al., Does Proton Pump Inhibitor Use Lead to a Higher Risk of Coronavirus Disease 2019 Infection and Progression to Severe Disease? a Meta-analysis, Japanese Journal of Infectious Diseases, doi:10.7883/yoken.JJID.2021.074.
6.
Li et al., Do proton pump inhibitors influence SARS-CoV-2 related outcomes? A meta-analysis, Gut, doi:10.1136/gutjnl-2020-323366.
Wardhana et al., 30 Mar 2024, Indonesia, peer-reviewed, 2 authors.
Contact: saraswatilaksmi@yahoo.com (corresponding author).
Different Outcome in COVID-19 Patients with or without PPI Use: A Systematic Review and Meta-analysis
Background The coronavirus disease (COVID-19) pandemic still happening and when it's going to be resolved is not known. In this COVID-19 era, physicians need to better understand the risk and purpose of giving drugs that patients do not need. Proton pump inhibitors (PPI) are sometimes easily prescribed and misused by physicians. The study objective is to find out whether PPI use is associated with better or worse outcomes in patients with COVID-19.
Method We searched retrospective studies in various publication libraries like PubMed, Embase, and CENTRAL from 2020 to 2022. Inclusion criteria were studied which differentiated patients with COVID-19 who regularly used PPI and control which is COVID-19 patients who did not use PPI. That study also needs to report the outcomes. The outcome was then divided into two categories which are good outcomes and worse outcomes consisting of severe COVID-19 needing oxygen therapy, admission to intensive care unit (ICU), acute respiratory distress syndrome (ARDS), shock or mortality, to get each study and total odd ratio (OR), 95% confidence interval, and weight. Studies that did not report the outcomes were excluded. We also analyze the data using a fixed or random effect model accordingly and asses the possibility of publication bias using Egger's test. Case Seven of 11 studies with more than 30.000 COVID-19 patients were analyzed in this study. These patients were divided into 2 groups: patients with COVID-19 who were using PPI up to 30 days before being infected and COVID-19 patients who didn't use PPI before. The total number of patients in the first group is 3531 patients and the second group is 38138 patients. After statistical analysis, we found that the data is heterogenous with p <0,05, I2 94,22% (95%CI 90,44-96,51%) suggesting the OR needs to be determined in the random effect model. We found that pooled OR is 1.99 (p 0.01, 95% CI, 1.18-3.38). Egger's test for the possibility of publication bias is 0,64 (95%CI -7,24-4,93). Conclusion COVID-19 patients who use PPI are twice as likely to have a worse outcome than COVID-19 patients who don't use PPI. This study is statistically significant with a low possibility of publication bias.
Author contributions IMWW write the full text, IMWW and SLD search the online publication library and review all related articles, IMWW and SLD make the metaanalysis. All authors review the full text.
Conflict of interest None to be disclosed
References
Argenziano, Bruce, Slater, Tiao, Baldwin et al., Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series, BMJ
Buendgens, Bruensing, Matthes, Dückers, Luedde et al., The administration of proton pump inhibitors in critically ill medical patients is associated with an increased risk of developing Clostridium difficile-associated diarrhoea, J Crit Care
Dial, Proton pump inhibitor use and enteric infections, Am J Gastroenterol
Hariyanto, Prasetya, Kurniawan, Proton pump inhibitor use is associated with increased risk of severity and mortality from coronavirus disease 2019 (COVID-19) infection, Digestive and liver disease
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Israelsen, Ernst, Lundh, Lundbo, Sandholdt et al., Proton Pump Inhibitor Use Is Not Strongly Associated With SARS -CoV-2 Related Outcomes: A Nationwide Study and Meta-analysis, Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc
Johnson, Harris, Cain, Hummer, Goyal et al., pulmonary and extra-pulmonary clinical manifestations of COVID-19, Front Med
Laheij, Sturkenboom, Hassing, Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs, JAMA
Lee, Ha, Yeniova, Moon, Kim, Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching, Gut, doi:10.1136/gutjnl-2020-322248
Lee, Ha, Yeniova, Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching, GUT
Liberati, Altman, Tetzlaff, The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration, Ann Intern Med
Luxenburger, Sturm, Biever, Rieg, Duerschmied, Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: coincidence or underestimated risk factor, JIM
Mckeigue, Kennedy, Weir, Bishop, Mcgurnaghan et al., Associations of severe COVID-19 with polypharmacy in the REACT-SCOT case-control study, medRxiv, doi:.org/10.1101/2020.07.23.20160747
Nguyen, Islam, Galvin, Chang, Sy et al., Meta-analysis of proton pump inhibitors induced risk of community-acquired pneumonia, International Journal for Quality in Health Care, doi:10.1093/intqhc/mzaa041
Pranata, Huang, Lawrensia, Henrina, Lim et al., Proton pump inhibitor on susceptibility to COVID-19 and its severity: A systematic review and meta-analysis, Pharmacol. Rep, doi:10.1007/s43440-021-00263-x
Ramachandran, Perisetti, Gajendran, Jean-Loius, Bansal, Pre-hospitalization proton pump inhibitor use and clinical outcomes in COVID-19, medRxiv, doi:10.1101/2020.07.12.20151084
Stroup, Berlin, Morton, Metaanalysis of observational studies in epidemiology: a proposal for reporting, JAMA
Ullah, Sivapalan, Kocher, Chelala, COVID-19 in patients with hepatobiliary and pancreatic diseases: A single-centre cross-sectional study in East London, Pancreatology
Wells, Shea, 'connell, The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses
Xie, Bowe, Yan, Estimates of all-cause mortality and cause-specific mortality associated with proton pump inhibitors among US veterans: cohort study, BMJ
Zhou, Wang, Lee, Proton pump inhibitor or famotidine use and severe COVID-19 disease: a propensity score-matched territory-wide study, Gut Epub ahead of print, doi:10.1136/gutjnl-2020-323668
DOI record:
{
"DOI": "10.22225/ijbstm.1.1.2024.19-23",
"ISSN": [
"3047-7433",
"3047-7441"
],
"URL": "http://dx.doi.org/10.22225/ijbstm.1.1.2024.19-23",
"abstract": "<jats:p>Background The coronavirus disease (COVID-19) pandemic still happening and when it’s going to be resolved is not known. In this COVID-19 era, physicians need to better understand the risk and purpose of giving drugs that patients do not need. Proton pump inhibitors (PPI) are sometimes easily prescribed and misused by physicians. The study objective is to find out whether PPI use is associated with better or worse outcomes in patients with COVID-19.\r\nMethod We searched retrospective studies in various publication libraries like PubMed, Embase, and CENTRAL from 2020 to 2022. Inclusion criteria were studied which differentiated patients with COVID-19 who regularly used PPI and control which is COVID-19 patients who did not use PPI. That study also needs to report the outcomes. The outcome was then divided into two categories which are good outcomes and worse outcomes consisting of severe COVID-19 needing oxygen therapy, admission to intensive care unit (ICU), acute respiratory distress syndrome (ARDS), shock or mortality, to get each study and total odd ratio (OR), 95% confidence interval, and weight. Studies that did not report the outcomes were excluded. We also analyze the data using a fixed or random effect model accordingly and asses the possibility of publication bias using Egger’s test.\r\nCase Seven of 11 studies with more than 30.000 COVID-19 patients were analyzed in this study. These patients were divided into 2 groups: patients with COVID-19 who were using PPI up to 30 days before being infected and COVID-19 patients who didn’t use PPI before. The total number of patients in the first group is 3531 patients and the second group is 38138 patients. After statistical analysis, we found that the data is heterogenous with p <0,05, I2 94,22% (95%CI 90,44-96,51%) suggesting the OR needs to be determined in the random effect model. We found that pooled OR is 1.99 (p 0.01, 95% CI, 1.18-3.38). Egger’s test for the possibility of publication bias is 0,64 (95%CI -7,24-4,93).\r\nConclusion COVID-19 patients who use PPI are twice as likely to have a worse outcome than COVID-19 patients who don’t use PPI. This study is statistically significant with a low possibility of publication bias.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Wardhana",
"given": "I Made Wisnu",
"sequence": "first"
},
{
"affiliation": [],
"family": "Dewi",
"given": "Saraswati Laksmi",
"sequence": "additional"
}
],
"container-title": "International Journal of Biomedical Science and Travel Medicine",
"container-title-short": "ijbstm",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
6,
20
]
],
"date-time": "2024-06-20T06:42:16Z",
"timestamp": 1718865736000
},
"deposited": {
"date-parts": [
[
2024,
6,
20
]
],
"date-time": "2024-06-20T06:42:19Z",
"timestamp": 1718865739000
},
"indexed": {
"date-parts": [
[
2024,
6,
21
]
],
"date-time": "2024-06-21T00:25:18Z",
"timestamp": 1718929518958
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
3,
30
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
3,
30
]
]
}
},
"link": [
{
"URL": "https://www.ejournal.warmadewa.ac.id/index.php/ijbstm/article/download/9176/5450",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.ejournal.warmadewa.ac.id/index.php/ijbstm/article/download/9176/5450",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "9632",
"original-title": [],
"page": "19-23",
"prefix": "10.22225",
"published": {
"date-parts": [
[
2024,
3,
30
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
30
]
]
},
"publisher": "Universitas Warmadewa",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ejournal.warmadewa.ac.id/index.php/ijbstm/article/view/9176"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Different Outcome in COVID-19 Patients with or without PPI Use: A Systematic Review and Meta-analysis",
"type": "journal-article",
"volume": "1"
}