Use of proton pump inhibitors and risk of adverse clinical outcomes from COVID‐19: a meta‐analysis
et al., Journal of Internal Medicine, doi:10.1111/joim.13183, Oct 2020
PPIs for COVID-19
1st treatment shown to increase risk in
September 2020, now with p = 0.000000048 from 40 studies.
6,400+ studies for
210+ treatments. c19early.org
|
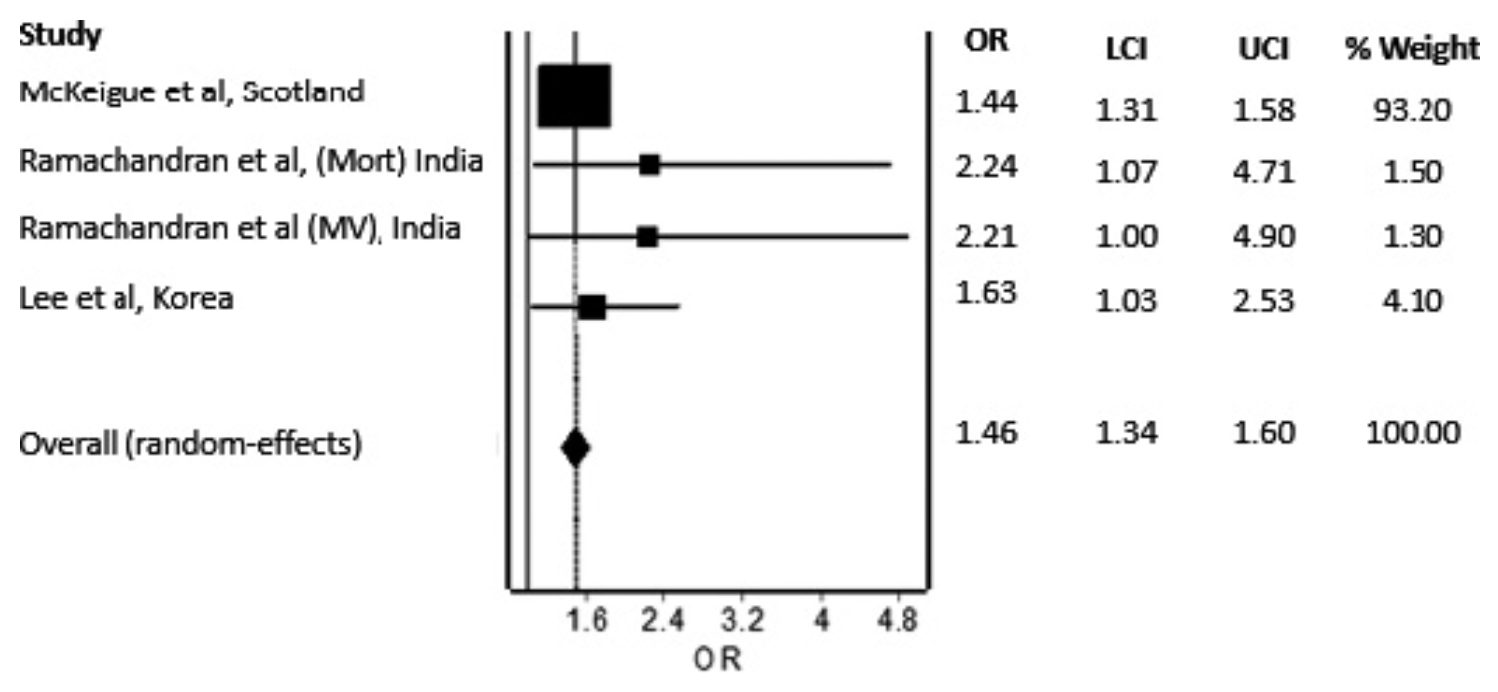
Meta analysis of 5 studies with 37,372 total patients showing significantly increased odds of severe/fatal COVID-19 and significantly higher risk of secondary infections with proton pump inhibitor (PPI) use.
8 meta-analyses show significant harm with proton pump inhibitors for mortality1-3,
severity1,2,4-8 , and
cases2.
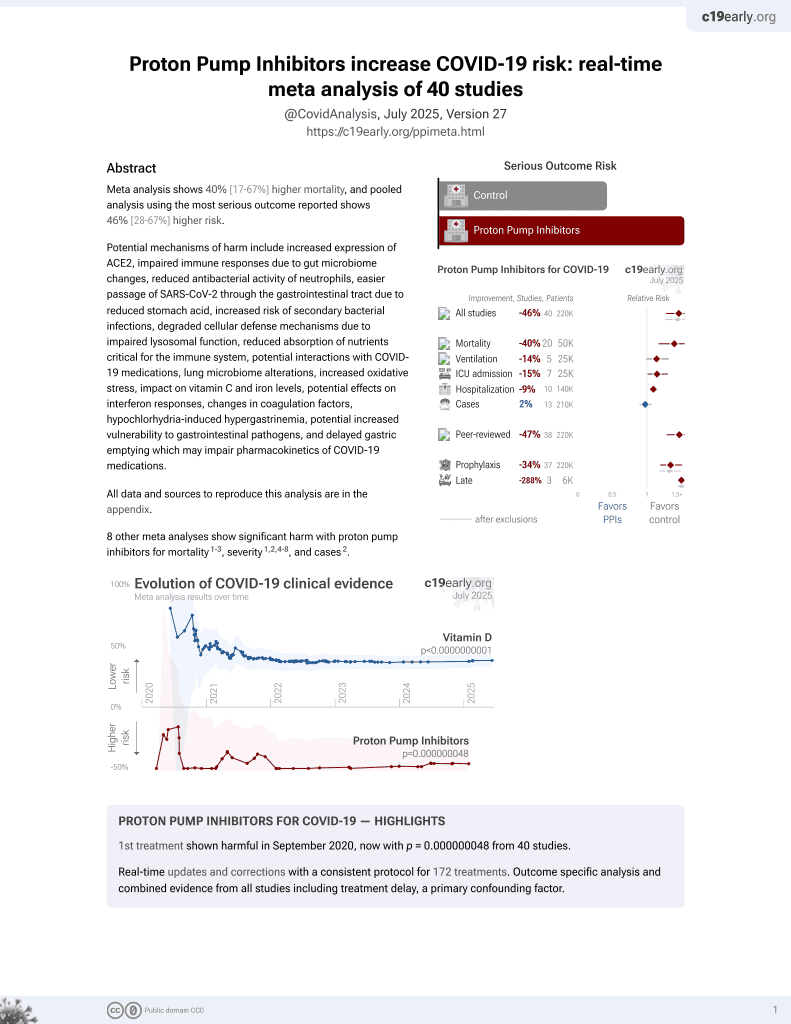
Currently there are 40 proton pump inhibitors for COVID-19 studies, showing 40% higher mortality [17‑67%], 14% higher ventilation [-1‑32%], 15% higher ICU admission [1‑30%], 9% higher hospitalization [3‑16%], and 2% fewer cases [-6‑10%].
|
risk of severe case, 46.0% higher, OR 1.46, p < 0.001, RR approximated with OR.
|
|
secondary infection, 191.0% higher, OR 2.91, p < 0.001, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Hariyanto et al., Proton pump inhibitor use is associated with increased risk of severity and mortality from coronavirus disease 2019 (COVID-19) infection, Digestive and Liver Disease, doi:10.1016/j.dld.2020.10.001.
2.
Fatima et al., The Use of Proton Pump Inhibitors and COVID-19: A Systematic Review and Meta-Analysis, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed7030037.
3.
Toubasi et al., Proton Pump Inhibitors: Current Use and the Risk of Coronavirus Infectious Disease 2019 Development and its Related Mortality. Meta-analysis, Archives of Medical Research, doi:10.1016/j.arcmed.2021.03.004.
4.
Wardhana et al., Different Outcome in COVID-19 Patients with or without PPI Use: A Systematic Review and Meta-analysis, International Journal of Biomedical Science and Travel Medicine, doi:10.22225/ijbstm.1.1.2024.19-23.
5.
Yan et al., Does Proton Pump Inhibitor Use Lead to a Higher Risk of Coronavirus Disease 2019 Infection and Progression to Severe Disease? a Meta-analysis, Japanese Journal of Infectious Diseases, doi:10.7883/yoken.JJID.2021.074.
6.
Li et al., Do proton pump inhibitors influence SARS-CoV-2 related outcomes? A meta-analysis, Gut, doi:10.1136/gutjnl-2020-323366.
Kow et al., 20 Oct 2020, peer-reviewed, 2 authors.
Abstract: Letter to the Editor
doi: 10.1111/joim.13183
Use of proton pump inhibitors and risk of adverse clinical
outcomes from COVID-19: a meta-analysis
Dear Editor,
We read with interest the study by Luxenburger
et al. [1] which reported that patients with coronavirus disease 2019 (COVID-19) receiving proton
pump inhibitors (PPIs) were at increased risk for
the development of secondary infection and acute
respiratory distress syndrome. Understandably,
the use of PPIs may lead to excessive suppression
of gastric acid, and thus leading to impaired
eradication of ingested pathogens, which results
in the increased risk of secondary infection
reported in the study. However, the association
between the use of PPIs and adverse clinical
outcomes such as acute respiratory syndrome in
patients with COVID-19 is not expected, since
previous in vitro study has demonstrated the ability
for PPIs to inhibit the production of pro-inflammatory cytokines, which is suggestive of their potential to dampen cytokine storm associated with
COVID-19 [2]. Since there have been few studies
addressing the same issue, we aimed to perform a
meta-analysis to summarize the overall effect of PPI
on the COVID-19 associated adverse clinical outcomes.
We performed literature searches in PubMed,
Google Scholar and medRxiv (preprint repository)
databases, up to 5 September 2020, for studies
evaluating the risk of adverse clinical outcomes
among COVID-19 patients with PPI use compared
to nonuse of PPI, with the following keywords and
their MeSH terms: ‘COVID-19’, ‘proton pump
inhibitor’ and ‘PPI’ without language restrictions.
The inclusion criteria were studies that investigated the use of PPIs on the risk of adverse clinical
outcomes in patients with COVID-19 with reported
adjusted measures of association. Each included
article was independently evaluated by two authors
(CSK and SSH) who extracted the study characteristics and measures of effect. The quality of
included studies was evaluated with the Newcastle-Ottawa Scale [3]. The outcome of interest
was the development of any COVID-19 associated
adverse clinical outcomes. Adjusted odds ratios
(ORs) and adjusted relative risks and their
corresponding 95% confidence intervals (CIs) from
each study were pooled in a random-effects model
of meta-analysis using Meta XL, version 5.3
(EpiGear International, Queensland, Australia).
The I2 statistic was performed to estimate the
heterogeneity.
Five studies that corresponded to inclusion criteria
with a total of 37 372 patients were included for
our meta-analysis [1,4-6]. Study characteristics
are depicted in Table 1. All studies included are
deemed good quality with a Newcastle-Ottawa
Scale of 8. There was nonuniformity in the definition of the adverse clinical outcomes utilized across
the five included studies. In the study by McKeigue
et al. [5], the adverse clinical outcome was defined
as an entry to critical care, death within 28 days,
or a death certificate with COVID-19 as an underlying cause. In the study by Ramachandran et al.
[6], the adverse clinical outcome was defined as inhospital mortality or the requirement for mechanical ventilation. In the study by Lee et al. [4], we
utilized the composite endpoint of requirement of
oxygen therapy, intensive care unit admission,
administration of invasive ventilation, or death to
define the adverse clinical outcome. In both the
studies by Luxenburger et al. [1] and by Li et al. [7],
the adverse clinical outcome was defined as..
DOI record:
{
"DOI": "10.1111/joim.13183",
"ISSN": [
"0954-6820",
"1365-2796"
],
"URL": "http://dx.doi.org/10.1111/joim.13183",
"alternative-id": [
"10.1111/joim.13183"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-09-22"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-09-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2020-10-20"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8186-2926",
"affiliation": [
{
"name": "School of Postgraduate Studies International Medical University Kuala Lumpur Malaysia"
}
],
"authenticated-orcid": false,
"family": "Kow",
"given": "C. S.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-4058-2215",
"affiliation": [
{
"name": "Department of Pharmacy University of Huddersfield Huddersfield United Kingdom"
},
{
"name": "School of Biomedical Sciences & Pharmacy University of Newcastle Callaghan NSW Australia"
}
],
"authenticated-orcid": false,
"family": "Hasan",
"given": "S. S.",
"sequence": "additional"
}
],
"container-title": "Journal of Internal Medicine",
"container-title-short": "J. Intern. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
10,
20
]
],
"date-time": "2020-10-20T09:31:11Z",
"timestamp": 1603186271000
},
"deposited": {
"date-parts": [
[
2023,
9,
3
]
],
"date-time": "2023-09-03T12:27:15Z",
"timestamp": 1693744035000
},
"indexed": {
"date-parts": [
[
2024,
7,
19
]
],
"date-time": "2024-07-19T14:26:37Z",
"timestamp": 1721399197026
},
"is-referenced-by-count": 29,
"issue": "1",
"issued": {
"date-parts": [
[
2020,
10,
20
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
20
]
],
"date-time": "2020-10-20T00:00:00Z",
"timestamp": 1603152000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/joim.13183",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/joim.13183",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/joim.13183",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "125-128",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2020,
10,
20
]
]
},
"published-online": {
"date-parts": [
[
2020,
10,
20
]
]
},
"published-print": {
"date-parts": [
[
2021,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"article-title": "Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID‐19: coincidence or underestimated risk factor?",
"author": "Luxenburger H",
"journal-title": "J Intern Med",
"key": "e_1_2_1_5_2_1",
"year": "2020"
},
{
"DOI": "10.1038/cddis.2016.218",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_5_3_1"
},
{
"author": "Wells G",
"key": "e_1_2_1_5_4_1",
"volume-title": "The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses",
"year": "2013"
},
{
"DOI": "10.1136/gutjnl-2020-323672",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_5_5_1"
},
{
"DOI": "10.1101/2020.07.23.20160747",
"author": "McKeigue PM",
"doi-asserted-by": "crossref",
"key": "e_1_2_1_5_6_1",
"volume-title": "Associations of severe COVID‐19 with polypharmacy in the REACT‐SCOT case‐control study",
"year": "2020"
},
{
"DOI": "10.1101/2020.07.12.20151084",
"author": "Ramachandran P",
"doi-asserted-by": "crossref",
"key": "e_1_2_1_5_7_1",
"volume-title": "Prehospitalization Proton Pump Inhibitor (PPI) use and Clinical Outcomes in COVID‐19",
"year": "2020"
},
{
"author": "Li J",
"key": "e_1_2_1_5_8_1",
"volume-title": "Risk Factors of Secondary Infections in Severe and Critical Patients Hospitalized with COVID‐19: A Case‐Control Study",
"year": "2020"
}
],
"reference-count": 7,
"references-count": 7,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/joim.13183"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Use of proton pump inhibitors and risk of adverse clinical outcomes from COVID‐19: a meta‐analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "289"
}