Structural Basis and Inhibitor Development of SARS-CoV-2 Papain-like Protease
et al., Molecules, doi:10.3390/molecules31030474, Jan 2026
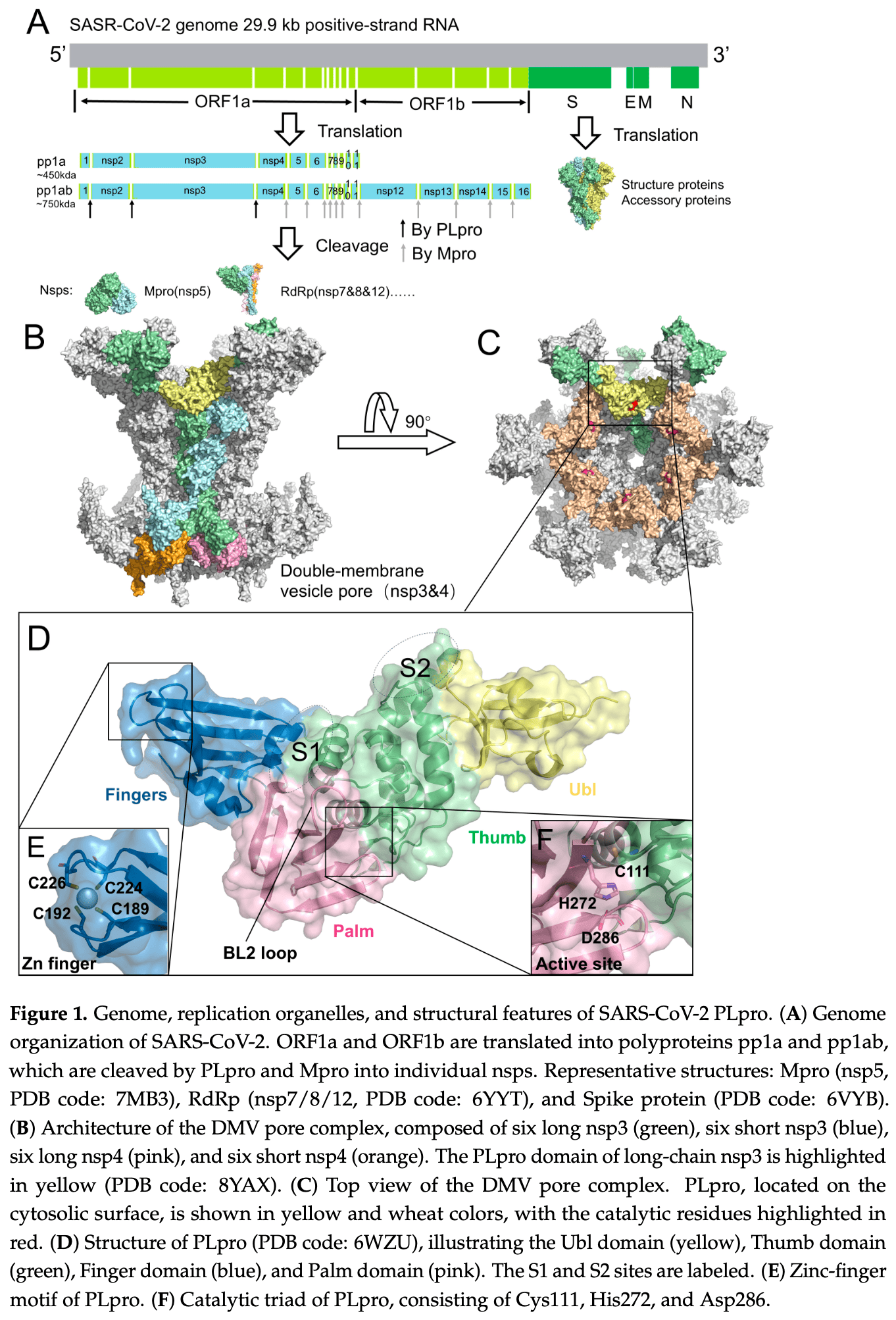
Review of structural insights and inhibitor development for SARS-CoV-2 papain-like protease (PLpro).
Wang et al., 29 Jan 2026, China, peer-reviewed, 13 authors.
Contact: 11212@zzrvtc.edu.cn (corresponding author), wjunshuai@emails.bjut.edu.cn, xuyuancong@bjut.edu.cn, yishu-y@bjut.edu.cn, zhangbotao@emails.bjut.edu.cn, sixuchen@emails.bjut.edu.cn, zhaoyangli@emails.bjut.edu.cn, zhj2023@emails.bjut.edu.cn, yanghuai@emails.bjut.edu.cn, zhouyubai@bjut.edu.cn, pengcao@bjut.edu.cn, 10554@zzrvtc.edu.cn, yonggong@ihep.ac.cn.
Structural Basis and Inhibitor Development of SARS-CoV-2 Papain-like Protease
Molecules, doi:10.3390/molecules31030474
Papain-like protease (PLpro), a crucial functional domain of the SARS-CoV-2 non-structural protein 3 (nsp3), plays a dual role in both hydrolyzing viral polyprotein precursors and modulating host immune responses. These critical functions position PLpro as a key target in the ongoing development of antiviral therapies for SARS-CoV-2. This review analyzes more than 100 PLpro-ligand co-crystal structures and summarizes the major binding modes between these ligands and PLpro. Most of these ligands bind to sites analogous to those targeted by the classical non-covalent inhibitor GRL0617, primarily involving the P3 and P4 subsites and the BL2 loop. Based on these structural insights, optimized inhibitors have expanded targeting beyond the canonical binding site to auxiliary regions such as the BL2 groove and the Val70 site, and in some cases toward the catalytic Cys111 buried within a narrow pocket. Certain ligands identified through various screening approaches bind to non-canonical or allosteric regions, such as the S1 and S2 sites or the zinc-finger domain, engaging PLpro through distinct interaction modes and thereby offering additional opportunities for PLpro inhibitor design. The review also discusses potential strategies for future PLpro inhibitor development informed by recent structural advances. Taken together, these structural and functional insights support ongoing efforts in the structure-guided design and optimization of PLpro inhibitors.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Adams, Lefkowitz, King, Harrach, Harrison et al., Ratification Vote on Taxonomic Proposals to the International Committee on Taxonomy of Viruses, Arch. Virol, doi:10.1007/s00705-016-2977-6
Agarwal, Hunt, Stegemann, Rochwerg, Lamontagne et al., A Living WHO Guideline on Drugs for COVID-19, BMJ, doi:10.1136/bmj.m3379
Alugubelli, Xiao, Khatua, Kumar, Sun et al., Discovery of First-in-Class PROTAC Degraders of SARS-CoV-2 Main Protease, J. Med. Chem, doi:10.1021/acs.jmedchem.3c02416
Amporndanai, Meng, Shang, Jin, Rogers et al., Inhibition Mechanism of SARS-CoV-2 Main Protease by Ebselen and Its Derivatives, Nat. Commun, doi:10.1038/s41467-021-23313-7
Anand, Ziebuhr, Wadhwani, Mesters, Hilgenfeld, Coronavirus Main Proteinase (3CL pro ) Structure: Basis for Design of Anti-SARS Drugs, Science, doi:10.1126/science.1085658
Arya, Prashar, Kumar, Identification and Characterization of Aurintricarboxylic Acid as a Potential Inhibitor of SARS-CoV-2 PLpro, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2023.123347
Atatreh, Mahgoub, Ghattas, Evaluating the Potential of PLpro as a Drug Target in SARS-CoV-2: MD Simulations and Druggability Analysis, Results Chem, doi:10.1016/j.rechem.2025.102565
Ayala-Torres, Liu, Dantuma, Masucci, Regulation of N-Degron Recognin-Mediated Autophagy by the SARS-CoV-2 PLpro Ubiquitin Deconjugase, Autophagy, doi:10.1080/15548627.2024.2442849
Ayoup, Elshafey, Abdel-Hamid, Ghareeb, Abu-Serie et al., Repurposing 1,2,4-Oxadiazoles as SARS-CoV-2 PLpro Inhibitors and Investigation of Their Possible Viral Entry Blockade Potential, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2023.115272
Bader, Calleja, Devine, Kuchel, Lu et al., A Novel PLpro Inhibitor Improves Outcomes in a Pre-Clinical Model of Long COVID, Nat. Commun, doi:10.1038/s41467-025-57905-4
Bajaj, Wehri, Suryawanshi, King, Pardeshi et al., Mercapto-Pyrimidines Are Reversible Covalent Inhibitors of the Papain-like Protease (PLpro) and Inhibit SARS-CoV-2 (SCoV-2) Replication, RSC Adv, doi:10.1039/D3RA01915B
Bayega, Reiling, Liu, Dubuc, Gravel et al., A Benchmark of Methods for SARS-CoV-2 Whole Genome Sequencing and Development of a More Sensitive Method, Front. Genet, doi:10.3389/fgene.2025.1516791
Bayoumy, Simsek, Seinen, Mulder, Ansari et al., The Continuous Rediscovery and the Benefit-Risk Ratio of Thioguanine, a Comprehensive Review, Expert Opin. Drug Metab. Toxicol, doi:10.1080/17425255.2020.1719996
Bissaro, Bolcato, Pavan, Bassani, Sturlese et al., Inspecting the Mechanism of Fragment Hits Binding on SARS-CoV-2 M pro by Using Supervised Molecular Dynamics (SuMD) Simulations, ChemMedChem
Boakye, Obirikorang, Afum-Adjei Awuah, Adu, Winter et al., Genetic Association of ACE2 Rs2285666 (C>T) and Rs2106809 (A>G) and Susceptibility to SARS-CoV-2 Infection among the Ghanaian Population, Front. Genet, doi:10.3389/fgene.2025.1555515
Calleja, Kuchel, Lu, Birkinshaw, Klemm et al., Insights into Drug Repurposing, as Well as Specificity and Compound Properties of Piperidine-Based SARS-CoV-2 PLpro Inhibitors, Front. Chem, doi:10.3389/fchem.2022.861209
Calleja, Lessene, Komander, Inhibitors of SARS-CoV-2 PLpro, Front. Chem, doi:10.3389/fchem.2022.876212
Cannalire, Cerchia, Beccari, Di Leva, Summa, Targeting SARS-CoV-2 Proteases and Polymerase for COVID-19 Treatment: State of the Art and Future Opportunities, J. Med. Chem, doi:10.1021/acs.jmedchem.0c01140
Cao, Coventry, Goreshnik, Huang, Sheffler et al., Design of Protein-Binding Proteins from the Target Structure Alone, Nature, doi:10.1038/s41586-022-04654-9
Cao, Duan, Huang, Xiong, Zhang et al., The SARS-CoV-2 Papain-like Protease Suppresses Type I Interferon Responses by Deubiquitinating STING, Sci. Signal, doi:10.1126/scisignal.add0082
Chan, Kok, Zhu, Chu, To et al., Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan, Emerg. Microbes Infect, doi:10.1080/22221751.2020.1719902
Chen, Huang, Ma, Kuzmič, Zhou et al., Preclinical Evaluation of the SARS-CoV-2 Mpro Inhibitor RAY1216 Shows Improved Pharmacokinetics Compared with Nirmatrelvir, Nat. Microbiol, doi:10.1038/s41564-024-01618-9
Chen, Tian, He, Tian, Han et al., Overview of Lethal Human Coronaviruses, Signal Transduct. Target. Ther
Cheng, Cheng, Chen, Lin, Chuang et al., Thiopurine Analogs and Mycophenolic Acid Synergistically Inhibit the Papain-like Protease of Middle East Respiratory Syndrome Coronavirus, Antivir. Res, doi:10.1016/j.antiviral.2014.12.011
Chou, Chien, Han, Prebanda, Hsieh et al., Thiopurine Analogues Inhibit Papain-like Protease of Severe Acute Respiratory Syndrome Coronavirus, Biochem. Pharmacol, doi:10.1016/j.bcp.2008.01.005
Costacurta, Dodaro, Bante, Schöppe, Peng et al., A Comprehensive Study of SARS-CoV-2 Main Protease (Mpro) Inhibitor-Resistant Mutants Selected in a VSV-Based System, PLoS Pathog, doi:10.1371/journal.ppat.1012522
Dana, Prusty, Dhayal, Gupta, Dar et al., Potent Antimalarial Activity of Acriflavine In Vitro and In Vivo, ACS Chem. Biol, doi:10.1021/cb500476q
Duan, Zhou, Liu, Iketani, Lin et al., Molecular Mechanisms of SARS-CoV-2 Resistance to Nirmatrelvir, Nature, doi:10.1038/s41586-023-06609-0
Dömling, Gao, Chemistry and Biology of SARS-CoV-2, Chem, doi:10.1016/j.chempr.2020.04.023
Ewert, Günther, Miglioli, Falke, Reinke et al., Hydrazones and Thiosemicarbazones Targeting Protein-Protein-Interactions of SARS-CoV-2 Papain-like Protease, Front. Chem, doi:10.3389/fchem.2022.832431
Fu, Huang, Tang, Liu, Liu et al., The Complex Structure of GRL0617 and SARS-CoV-2 PLpro Reveals a Hot Spot for Antiviral Drug Discovery, Nat. Commun, doi:10.1038/s41467-020-20718-8
Gao, Qin, Chen, Zhu, Hou et al., Crystal Structure of SARS-CoV-2 Papain-like Protease, Acta Pharm. Sin. B, doi:10.1016/j.apsb.2020.08.014
Garnsey, Robinson, Nguyen, Cardin, Tillotson et al., Discovery of SARS-CoV-2 Papain-like Protease (PL pro ) Inhibitors with Efficacy in a Murine Infection Model, Sci. Adv, doi:10.1126/sciadv.ado4288
Gil-Moles, O'beirne, Esarev, Lippmann, Tacke et al., Silver N-Heterocyclic Carbene Complexes Are Potent Uncompetitive Inhibitors of the Papain-like Protease with Antiviral Activity against SARS-CoV-2, RSC Med. Chem, doi:10.1039/D3MD00067B
Gil-Moles, Türck, Basu, Pettenuzzo, Bhattacharya et al., Metallodrug Profiling against SARS-CoV-2 Target Proteins Identifies Highly Potent Inhibitors of the S/ACE2 Interaction and the Papain-like Protease PL pro, Chem. A Eur. J, doi:10.1002/chem.202103258
Gorbalenya, Baker, Baric, De Groot, Drosten et al., The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-nCoV and Naming It SARS-CoV-2, Nat. Microbiol, doi:10.1038/s41564-020-0695-z
Han, Gracia, Røise, Boike, Leon et al., A Covalent Inhibitor Targeting the Papain-like Protease from SARS-CoV-2 Inhibits Viral Replication, RSC Adv, doi:10.1039/D3RA00426K
Hersi, Sebastian, Tarazi, Srinivasulu, Mostafa et al., Discovery of Novel Papain-like Protease Inhibitors for Potential Treatment of COVID-19, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2023.115380
Herzog, Göbel, Marongiu, Ruetalo, Alonso et al., Compounds Derived from Humulus Lupulus Inhibit SARS-CoV-2 Papain-like Protease and Virus Replication, Phytomedicine, doi:10.1016/j.phymed.2023.155176
Hilgenfeld, Peiris, From SARS to MERS: 10 Years of Research on Highly Pathogenic Human Coronaviruses, Antivir. Res, doi:10.1016/j.antiviral.2013.08.015
Hu, Guo, Zhou, Shi, Author, Correction: Characteristics of SARS-CoV-2 and COVID-19, Nat. Rev. Microbiol, doi:10.1038/s41579-022-00711-2
Huang, Wang, Zhong, Zhang, Zhang et al., Molecular Architecture of Coronavirus Double-Membrane Vesicle Pore Complex, Nature, doi:10.1038/s41586-024-07817-y
Iketani, Mohri, Culbertson, Hong, Duan et al., Multiple Pathways for SARS-CoV-2 Resistance to Nirmatrelvir, Nature, doi:10.1038/s41586-022-05514-2
Ivanenkov, Polykovskiy, Bezrukov, Zagribelnyy, Aladinskiy et al., Chemistry42: An AI-Driven Platform for Molecular Design and Optimization, J. Chem. Inf. Model, doi:10.1021/acs.jcim.2c01191
Jabeen, Ahmad, Raza, Global Gene Expression and Docking Profiling of COVID-19 Infection, Front. Genet, doi:10.3389/fgene.2022.870836
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 Entry into Cells, Nat. Rev. Mol. Cell Biol, doi:10.1038/s41580-021-00418-x
Jadhav, Huang, Osipiuk, Zhang, Tan et al., Structure-Based Design of SARS-CoV-2 Papain-like Protease Inhibitors, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2023.116011
Jadhav, Liang, Ansari, Tan, Tan et al., Design of Quinoline SARS-CoV-2 Papain-like Protease Inhibitors as Oral Antiviral Drug Candidates, Nat. Commun, doi:10.1038/s41467-025-56902-x
Jeong, Song, Yoon, Kim, Kwon, Therapeutic Strategies Against COVID-19 and Structural Characterization of SARS-CoV-2: A Review, Front. Microbiol, doi:10.3389/fmicb.2020.01723
Jin, Du, Xu, Deng, Liu et al., Structure of Mpro from SARS-CoV-2 and Discovery of Its Inhibitors, Nature, doi:10.1038/s41586-020-2223-y
Kattula, Reddi, Jangam, Naik, Adimoolam et al., Development of 2-Chloroquinoline Based Heterocyclic Frameworks as Dual Inhibitors of SARS-CoV-2 MPro and PLPro, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2023.124772
Kim, Hauner, Laureanti, Agustin, Raugei et al., Mechanistic Investigation of SARS-CoV-2 Main Protease to Accelerate Design of Covalent Inhibitors, Sci. Rep, doi:10.1038/s41598-022-23570-6
Kladnik, Dolinar, Kljun, Perea, Grau-Expósito et al., Zinc Pyrithione Is a Potent Inhibitor of PL Pro and Cathepsin L Enzymes with Ex Vivo Inhibition of SARS-CoV-2 Entry and Replication, J. Enzym. Inhib. Med. Chem, doi:10.1080/14756366.2022.2108417
Klemm, Ebert, Calleja, Allison, Richardson et al., Mechanism and Inhibition of the Papain-like Protease, PLpro, of SARS-CoV-2, EMBO J, doi:10.15252/embj.2020106275
Lei, Kusov, Hilgenfeld, Nsp3 of Coronaviruses: Structures and Functions of a Large Multi-Domain Protein, Antivir. Res, doi:10.1016/j.antiviral.2017.11.001
Li, Song, Structure and Function of SARS-CoV and SARS-CoV-2 Main Proteases and Their Inhibition: A Comprehensive Review, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2023.115772
Li, Song, Targeting SARS-CoV-2 Nonstructural Protein 3: Function, Structure, Inhibition, and Perspective in Drug Discovery, Drug Discov. Today, doi:10.1016/j.drudis.2023.103832
Lima, Moreira, Coêlho, Cruz, Dhalia et al., Ovo-Designed Miniprotein Inhibits the Enzymatic Activity of the SARS-CoV-2 Main Protease, J. Chem. Inf. Model, doi:10.1021/acs.jcim.5c01708
Lindner, Fotouhi-Ardakani, Lytvyn, Lachance, Sulea et al., The Papain-Like Protease from the Severe Acute Respiratory Syndrome Coronavirus Is a Deubiquitinating Enzyme, J. Virol, doi:10.1128/JVI.79.24.15199-15208.2005
Liu, Wang, Wang, Yue, Hu et al., Discovery of New Non-Covalent and Covalent Inhibitors Targeting SARS-CoV-2 Papain-like Protease and Main Protease, Bioorg. Chem, doi:10.1016/j.bioorg.2023.106830
Liu, Zhang, Joo, Sun, Nf-Κb, Signaling in Inflammation, Signal Transduct. Target. Ther, doi:10.1038/sigtrans.2017.23
Liu, Zhang, Lei, Wang, Zhan et al., Design and Evaluation of a Novel Peptide-Drug Conjugate Covalently Targeting SARS-CoV-2 Papain-like Protease, J. Med. Chem, doi:10.1021/acs.jmedchem.1c02022
Lu, Yang, Ran, Zhang, Li et al., Discovery of Orally Bioavailable SARS-CoV-2 Papain-like Protease Inhibitor as a Potential Treatment for COVID-19, Nat. Commun, doi:10.1038/s41467-024-54462-0
Ma, Hu, Townsend, Lagarias, Marty et al., Disulfiram, Carmofur, PX-12, Tideglusib, and Shikonin Are Nonspecific Promiscuous SARS-CoV-2 Main Protease Inhibitors, ACS Pharmacol. Transl. Sci, doi:10.1021/acsptsci.0c00130
Ma, Hu, Wang, Choza, Wang, Drug-Repurposing Screening Identified Tropifexor as a SARS-CoV-2 Papain-like Protease Inhibitor, ACS Infect. Dis, doi:10.1021/acsinfecdis.1c00629
Ma, Sacco, Xia, Lambrinidis, Townsend et al., Discovery of SARS-CoV-2 Papain-like Protease Inhibitors through a Combination of High-Throughput Screening and a FlipGFP-Based Reporter Assay, ACS Cent. Sci, doi:10.1021/acscentsci.1c00519
Malone, Urakova, Snijder, Campbell, Structures and Functions of Coronavirus Replication-Transcription Complexes and Their Relevance for SARS-CoV-2 Drug Design, Nat. Rev. Mol. Cell Biol, doi:10.1038/s41580-021-00432-z
Mcclain, Vabret, SARS-CoV-2: The Many Pros of Targeting PLpro, Signal Transduct. Target. Ther, doi:10.1038/s41392-020-00335-z
Meewan, Kattoula, Kattoula, Skinner, Fajtová et al., Discovery of Triple Inhibitors of Both SARS-CoV-2 Proteases and Human Cathepsin L, Pharmaceuticals, doi:10.3390/ph15060744
Moghadasi, Heilmann, Khalil, Nnabuife, Kearns et al., Transmissible SARS-CoV-2 Variants with Resistance to Clinical Protease Inhibitors, Sci. Adv, doi:10.1126/sciadv.ade8778
Napolitano, Dabrowska, Schorpp, Mourão, Barreto-Duran et al., a Clinically Approved Drug, Inhibits SARS-CoV-2 and Other Betacoronaviruses, Cell Chem. Biol, doi:10.1016/j.chembiol.2021.11.006
Osipiuk, Azizi, Dvorkin, Endres, Jedrzejczak et al., Structure of Papain-like Protease from SARS-CoV-2 and Its Complexes with Non-Covalent Inhibitors, Nat. Commun, doi:10.1038/s41467-021-21060-3
Owen, Allerton, Anderson, Aschenbrenner, Avery et al., An Oral SARS-CoV-2 M pro Inhibitor Clinical Candidate for the Treatment of COVID-19, Science, doi:10.1126/science.abl4784
Pacesa, Nickel, Schellhaas, Schmidt, Pyatova et al., One-Shot Design of Functional Protein Binders with BindCraft, Nature, doi:10.1038/s41586-025-09429-6
Persinoti, De Aguiar Peres, Jacob, Rossi, Vêncio et al., RNA-Sequencing Analysis of Trichophyton Rubrumtranscriptome in Response to Sublethal Doses of Acriflavine, BMC Genom, doi:10.1186/1471-2164-15-S7-S1
Protić, Kaličanin, Sencanski, Prodanović, Milicevic et al., In Silico and In Vitro Inhibition of SARS-CoV-2 PLpro with Gramicidin D, Int. J. Mol. Sci, doi:10.3390/ijms24031955
Puhl, Godoy, Noske, Nakamura, Gawriljuk et al., Discovery of PL pro and M pro Inhibitors for SARS-CoV-2, ACS Omega, doi:10.1021/acsomega.3c01110
Pépin, Nejad, Thomas, Ferrand, Mcarthur et al., Activation of cGAS-Dependent Antiviral Responses by DNA Intercalating Agents, Nucleic Acids Res, doi:10.1093/nar/gkw878
Ratia, Saikatendu, Santarsiero, Barretto, Baker et al., Severe Acute Respiratory Syndrome Coronavirus Papain-like Protease: Structure of a Viral Deubiquitinating Enzyme, Proc. Natl. Acad. Sci
Reboud-Ravaux, El Amri, COVID-19 Therapies: Protease Inhibitions and Novel Degrader Strategies, Front. Drug Discov, doi:10.3389/fddsv.2022.892057
Ren, Aliper, Chen, Zhao, Rao et al., A Small-Molecule TNIK Inhibitor Targets Fibrosis in Preclinical and Clinical Models, Nat. Biotechnol, doi:10.1038/s41587-024-02143-0
Rut, Lv, Zmudzinski, Patchett, Nayak et al., Activity Profiling and Crystal Structures of Inhibitor-Bound SARS-CoV-2 Papain-like Protease: A Framework for Anti-COVID-19 Drug Design, Sci. Adv
Sanachai, Mahalapbutr, Sanghiran Lee, Rungrotmongkol, Hannongbua, In Silico Elucidation of Potent Inhibitors and Rational Drug Design against SARS-CoV-2 Papain-like Protease, J. Phys. Chem. B, doi:10.1021/acs.jpcb.1c07060
Sanders, Pokhrel, Labbe, Mathews, Cooper et al., Potent and Selective Covalent Inhibition of the Papain-like Protease from SARS-CoV-2, Nat. Commun, doi:10.1038/s41467-023-37254-w
Sargsyan, Lin, Chen, Grauffel, Chen et al., Multi-Targeting of Functional Cysteines in Multiple Conserved SARS-CoV-2 Domains by Clinically Safe Zn-Ejectors, Chem. Sci, doi:10.1039/D0SC02646H
Shan, Liu, Shen, Dai, Xu et al., Development of Potent and Selective Inhibitors Targeting the Papain-like Protease of SARS-CoV-2, Cell Chem. Biol, doi:10.1016/j.chembiol.2021.04.020
Sheikh, Dickensheets, Gamero, Vogel, Donnelly, An Essential Role for IFN-β in the Induction of IFN-Stimulated Gene Expression by LPS in Macrophages, J. Leukoc. Biol, doi:10.1189/jlb.2A0414-191R
Shen, Ratia, Cooper, Kong, Lee et al., Design of SARS-CoV-2 PLpro Inhibitors for COVID-19 Antiviral Therapy Leveraging Binding Cooperativity, J. Med. Chem, doi:10.1021/acs.jmedchem.1c01307
Shin, Mukherjee, Grewe, Bojkova, Baek et al., Papain-like Protease Regulates SARS-CoV-2 Viral Spread and Innate Immunity, Nature, doi:10.1038/s41586-020-2601-5
Snijder, Bredenbeek, Dobbe, Thiel, Ziebuhr et al., Unique and Conserved Features of Genome and Proteome of SARS-Coronavirus, an Early Split-off from the Coronavirus Group 2 Lineage, J. Mol. Biol, doi:10.1016/S0022-2836(03)00865-9
Srinivasan, Brognaro, Prabhu, De Souza, Günther et al., Antiviral Activity of Natural Phenolic Compounds in Complex at an Allosteric Site of SARS-CoV-2 Papain-like Protease, Commun. Biol, doi:10.1038/s42003-022-03737-7
Stetson, Medzhitov, Type I Interferons in Host Defense, Immunity, doi:10.1016/j.immuni.2006.08.007
Subissi, Imbert, Ferron, Collet, Coutard et al., SARS-CoV ORF1b-Encoded Nonstructural Proteins 12-16: Replicative Enzymes as Antiviral Targets, Antivir. Res, doi:10.1016/j.antiviral.2013.11.006
Swaim, Dwivedi, Perng, Zhao, Canadeo et al., 6-Thioguanine Blocks SARS-CoV-2 Replication by Inhibition of PLpro, iScience, doi:10.1016/j.isci.2021.103213
Tan, Liang, Ansari, Jadhav, Tan et al., Structure-Based Design of Covalent SARS-CoV-2 Papain-like Protease Inhibitors, J. Med. Chem, doi:10.1021/acs.jmedchem.4c01872
Tan, Ma, Wang, Invalidation of Dieckol and 1,2,3,4,6-Pentagalloylglucose (PGG) as SARS-CoV-2 Main Protease Inhibitors and the Discovery of PGG as a Papain-like Protease Inhibitor, Med. Chem. Res, doi:10.1007/s00044-022-02903-0
Tan, Zhang, Ansari, Jadhav, Tan et al., Design of a SARS-CoV-2 Papain-like Protease Inhibitor with Antiviral Efficacy in a Mouse Model, Science, doi:10.1126/science.adm9724
Taylor, Amporndanai, Rietz, Zhao, Thiruvaipati et al., Fragment-Based Screen of SARS-CoV-2 Papain-like Protease (PL pro ), ACS Med. Chem. Lett, doi:10.1021/acsmedchemlett.4c00238
Teng, Piñón, Weiss, Expression of Murine Coronavirus Recombinant Papain-Like Proteinase: Efficient Cleavage Is Dependent on the Lengths of Both the Substrate and the Proteinase Polypeptides, J. Virol, doi:10.1128/JVI.73.4.2658-2666.1999
Ton, Pandey, Smith, Ban, Fernandez et al., Targeting SARS-CoV-2 Papain-like Protease in the Postvaccine Era, Trends Pharmacol. Sci, doi:10.1016/j.tips.2022.08.008
Tully, Rucker, Chianelli, Williams, Vidal et al., Discovery of Tropifexor (LJN452), a Highly Potent Non-Bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH), J. Med. Chem, doi:10.1021/acs.jmedchem.7b00907
V'kovski, Kratzel, Steiner, Stalder, Thiel, Coronavirus Biology and Replication: Implications for SARS-CoV-2, Nat. Rev. Microbiol, doi:10.1038/s41579-020-00468-6
Van Vliet, Huynh, Palà, Patel, Singer et al., Ubiquitin Variants Potently Inhibit SARS-CoV-2 PLpro and Viral Replication via a Novel Site Distal to the Protease Active Site, PLoS Pathog, doi:10.1371/journal.ppat.1011065
Varghese, Liu, Liu, Guo, Dong et al., Analysis of Structures of SARS-CoV-2 Papain-like Protease Bound with Ligands Unveils Structural Features for Inhibiting the Enzyme, Molecules, doi:10.3390/molecules30030491
Velma, Shen, Holberg, Fu, Soleymani et al., Non-Covalent Inhibitors of SARS-CoV-2 Papain-Like Protease (PLpro): In Vitro and In Vivo Antiviral Activity, J. Med. Chem, doi:10.1021/acs.jmedchem.4c00378
Wang, Cheetham, Angacian, Su, Xie et al., Peptide-Drug Conjugates as Effective Prodrug Strategies for Targeted Delivery, Adv. Drug Deliv. Rev, doi:10.1016/j.addr.2016.06.015
Wang, Chen, He, Li, Xiong et al., Structure-Based Design of Potent Peptidomimetic Inhibitors Covalently Targeting SARS-CoV-2 Papain-like Protease, Int. J. Mol. Sci, doi:10.3390/ijms24108633
Wang, Xiong, Zhu, Liu, Zhao et al., Covalent DNA-Encoded Library Workflow Drives Discovery of SARS-CoV-2 Nonstructural Protein Inhibitors, J. Am. Chem. Soc, doi:10.1021/jacs.4c12992
Watson, Juergens, Bennett, Trippe, Yim et al., De Novo Design of Protein Structure and Function with RFdiffusion, Nature, doi:10.1038/s41586-023-06415-8
Wolff, Melia, Snijder, Bárcena, Double-Membrane Vesicles as Platforms for Viral Replication, Trends Microbiol, doi:10.1016/j.tim.2020.05.009
Wu, Go, Nguyen, Kuchel, Lu et al., Mutational Profiling of SARS-CoV-2 Papain-like Protease Reveals Requirements for Function, Structure, and Drug Escape, Nat. Commun, doi:10.1038/s41467-024-50566-9
Wydorski, Osipiuk, Lanham, Tesar, Endres et al., Dual Domain Recognition Determines SARS-CoV-2 PLpro Selectivity for Human ISG15 and K48-Linked Di-Ubiquitin, Nat. Commun, doi:10.1038/s41467-023-38031-5
Yan, Liu, Yan, Liu, Liu et al., A Robust High-Throughput Fluorescence Polarization Assay for Rapid Screening of SARS-CoV-2 Papain-like Protease Inhibitors, Virology, doi:10.1016/j.virol.2022.07.006
Yan, Wu, Potential 3-chymotrypsin-like Cysteine Protease Cleavage Sites in the Coronavirus Polyproteins Pp1a and Pp1ab and Their Possible Relevance to COVID-19 Vaccine and Drug Development, FASEB J, doi:10.1096/fj.202100280RR
Yan, Zheng, Zeng, He, Cheng, Structural Biology of SARS-CoV-2: Open the Door for Novel Therapies, Signal Transduct. Target. Ther, doi:10.1038/s41392-022-00884-5
Yi, Yu, Xue, Jin, Zhang et al., Chrysin 7-O-β-D-Glucuronide, a Dual Inhibitor of SARS-CoV-2 3CLpro and PLpro, for the Prevention and Treatment of COVID-19, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2023.107039
Yu, Zhao, Ye, Wu, Liao et al., Structure-Based Design of a Dual-Targeted Covalent Inhibitor Against Papain-like and Main Proteases of SARS-CoV-2, J. Med. Chem, doi:10.1021/acs.jmedchem.2c00954
Yuan, Gao, Tang, Cai, Hu et al., Targeting Papain-like Protease for Broad-Spectrum Coronavirus Inhibition, Protein Cell, doi:10.1007/s13238-022-00909-3
Zang, Su, Wang, Cheng, Zhang et al., High-Throughput Screening of SARS-CoV-2 Main and Papain-like Protease Inhibitors, Protein Cell, doi:10.1093/procel/pwac016
Zhang, Li, Yan, Huang, Wang et al., ISGylation in Innate Antiviral Immunity and Pathogen Defense Responses: A Review, Front. Cell Dev. Biol, doi:10.3389/fcell.2021.788410
Zhang, Zhang, Zhang, Zhang, Liu et al., Oridonin Inhibits SARS-CoV-2 Replication by Targeting Viral Proteinase and Polymerase, Virol. Sin, doi:10.1016/j.virs.2023.04.008
Zhao, Du, Duan, Pan, Sun et al., High-Throughput Screening Identifies Established Drugs as SARS-CoV-2 PLpro Inhibitors, Protein Cell, doi:10.1007/s13238-021-00836-9
Zhou, Yang, Wang, Hu, Zhang et al., A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin, Nature, doi:10.1038/s41586-020-2012-7
Ziebuhr, Thiel, Gorbalenya, The Autocatalytic Release of a Putative RNA Virus Transcription Factor from Its Polyprotein Precursor Involves Two Paralogous Papain-like Proteases That Cleave the Same Peptide Bond, J. Biol. Chem, doi:10.1074/jbc.M104097200
Zmudzinski, Rut, Olech, Granda, Giurg et al., Ebselen Derivatives Inhibit SARS-CoV-2 Replication by Inhibition of Its Essential Proteins: PLpro and Mpro Proteases, and Nsp14 Guanine N7-Methyltransferase, Sci. Rep, doi:10.1038/s41598-023-35907-w
DOI record:
{
"DOI": "10.3390/molecules31030474",
"ISSN": [
"1420-3049"
],
"URL": "http://dx.doi.org/10.3390/molecules31030474",
"abstract": "<jats:p>Papain-like protease (PLpro), a crucial functional domain of the SARS-CoV-2 non-structural protein 3 (nsp3), plays a dual role in both hydrolyzing viral polyprotein precursors and modulating host immune responses. These critical functions position PLpro as a key target in the ongoing development of antiviral therapies for SARS-CoV-2. This review analyzes more than 100 PLpro-ligand co-crystal structures and summarizes the major binding modes between these ligands and PLpro. Most of these ligands bind to sites analogous to those targeted by the classical non-covalent inhibitor GRL0617, primarily involving the P3 and P4 subsites and the BL2 loop. Based on these structural insights, optimized inhibitors have expanded targeting beyond the canonical binding site to auxiliary regions such as the BL2 groove and the Val70 site, and in some cases toward the catalytic Cys111 buried within a narrow pocket. Certain ligands identified through various screening approaches bind to non-canonical or allosteric regions, such as the S1 and S2 sites or the zinc-finger domain, engaging PLpro through distinct interaction modes and thereby offering additional opportunities for PLpro inhibitor design. The review also discusses potential strategies for future PLpro inhibitor development informed by recent structural advances. Taken together, these structural and functional insights support ongoing efforts in the structure-guided design and optimization of PLpro inhibitors.</jats:p>",
"alternative-id": [
"molecules31030474"
],
"author": [
{
"affiliation": [
{
"name": "College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China"
},
{
"name": "Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China"
}
],
"family": "Wang",
"given": "Junshuai",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-1671-6156",
"affiliation": [
{
"name": "College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China"
}
],
"authenticated-orcid": false,
"family": "Xu",
"given": "Yuancong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China"
}
],
"family": "Yang",
"given": "Yishu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China"
},
{
"name": "Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China"
}
],
"family": "Zhang",
"given": "Botao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China"
},
{
"name": "Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China"
}
],
"family": "Chen",
"given": "Sixu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China"
},
{
"name": "Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China"
}
],
"family": "Li",
"given": "Zhaoyang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China"
}
],
"family": "Zhu",
"given": "Haojia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China"
},
{
"name": "Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China"
}
],
"family": "Yang",
"given": "Huai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Railway Food Safety Management Engineering Technology Research Center, Zhengzhou Railway Vocational & Technology College, Zhengzhou 451400, China"
}
],
"family": "Wang",
"given": "Hongtao",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1066-7071",
"affiliation": [
{
"name": "College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China"
}
],
"authenticated-orcid": false,
"family": "Zhou",
"given": "Yubai",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4616-5914",
"affiliation": [
{
"name": "College of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China"
},
{
"name": "Institute of Matter Science, Beijing University of Technology, Beijing 100124, China"
}
],
"authenticated-orcid": false,
"family": "Cao",
"given": "Peng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Railway Food Safety Management Engineering Technology Research Center, Zhengzhou Railway Vocational & Technology College, Zhengzhou 451400, China"
}
],
"family": "Zhai",
"given": "Baiqiang",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4294-964X",
"affiliation": [
{
"name": "Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China"
}
],
"authenticated-orcid": false,
"family": "Gong",
"given": "Yong",
"sequence": "additional"
}
],
"container-title": "Molecules",
"container-title-short": "Molecules",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2026,
1,
29
]
],
"date-time": "2026-01-29T14:52:36Z",
"timestamp": 1769698356000
},
"deposited": {
"date-parts": [
[
2026,
1,
29
]
],
"date-time": "2026-01-29T14:52:54Z",
"timestamp": 1769698374000
},
"funder": [
{
"award": [
"KZ202210005001"
],
"award-info": [
{
"award-number": [
"KZ202210005001"
]
}
],
"name": "R&D Program of Beijing Municipal Education Commission"
},
{
"award": [
"242102311178"
],
"award-info": [
{
"award-number": [
"242102311178"
]
}
],
"name": "Science and technology project of Henan Province"
},
{
"award": [
"252102520079"
],
"award-info": [
{
"award-number": [
"252102520079"
]
}
],
"name": "Henan Provincial International Science and Technology Cooperation Project"
}
],
"indexed": {
"date-parts": [
[
2026,
1,
30
]
],
"date-time": "2026-01-30T05:57:55Z",
"timestamp": 1769752675695,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2026,
1,
29
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2026,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
29
]
],
"date-time": "2026-01-29T00:00:00Z",
"timestamp": 1769644800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1420-3049/31/3/474/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "474",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2026,
1,
29
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
29
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/j.antiviral.2013.08.015",
"article-title": "From SARS to MERS: 10 Years of Research on Highly Pathogenic Human Coronaviruses",
"author": "Hilgenfeld",
"doi-asserted-by": "crossref",
"first-page": "286",
"journal-title": "Antivir. Res.",
"key": "ref_1",
"volume": "100",
"year": "2013"
},
{
"DOI": "10.1038/s41392-020-0190-2",
"article-title": "Overview of Lethal Human Coronaviruses",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "89",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_2",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-0695-z",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, Gorbalenya, A.E., Baker, S.C., Baric, R.S., De Groot, R.J., Drosten, C., Gulyaeva, A.A., Haagmans, B.L., Lauber, C., and Leontovich, A.M. (2020). The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-nCoV and Naming It SARS-CoV-2. Nat. Microbiol., 5, 536–544."
},
{
"DOI": "10.1038/s41586-020-2012-7",
"article-title": "A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "270",
"journal-title": "Nature",
"key": "ref_4",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1007/s00705-016-2977-6",
"article-title": "Ratification Vote on Taxonomic Proposals to the International Committee on Taxonomy of Viruses (2016)",
"author": "Adams",
"doi-asserted-by": "crossref",
"first-page": "2921",
"journal-title": "Arch. Virol.",
"key": "ref_5",
"volume": "161",
"year": "2016"
},
{
"DOI": "10.1080/22221751.2020.1719902",
"article-title": "Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "221",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_6",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1038/s41579-022-00711-2",
"article-title": "Author Correction: Characteristics of SARS-CoV-2 and COVID-19",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "315",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_7",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2013.11.006",
"article-title": "SARS-CoV ORF1b-Encoded Nonstructural Proteins 12–16: Replicative Enzymes as Antiviral Targets",
"author": "Subissi",
"doi-asserted-by": "crossref",
"first-page": "122",
"journal-title": "Antivir. Res.",
"key": "ref_8",
"volume": "101",
"year": "2014"
},
{
"DOI": "10.1038/s41580-021-00432-z",
"article-title": "Structures and Functions of Coronavirus Replication–Transcription Complexes and Their Relevance for SARS-CoV-2 Drug Design",
"author": "Malone",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "ref_9",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.3389/fgene.2025.1555515",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Boakye, A.O., Obirikorang, C., Afum-Adjei Awuah, A., Adu, E.A., Winter, D., Boham, E.E., Alani, H., Newton, S.K., Almoustapha, N.S.T., and Deke, J. (2025). Genetic Association of ACE2 Rs2285666 (C>T) and Rs2106809 (A>G) and Susceptibility to SARS-CoV-2 Infection among the Ghanaian Population. Front. Genet., 16."
},
{
"DOI": "10.3389/fgene.2025.1516791",
"doi-asserted-by": "crossref",
"key": "ref_11",
"unstructured": "Bayega, A., Reiling, S.J., Liu, J.L., Dubuc, I., Gravel, A., Flamand, L., and Ragoussis, J. (2025). A Benchmark of Methods for SARS-CoV-2 Whole Genome Sequencing and Development of a More Sensitive Method. Front. Genet., 16."
},
{
"DOI": "10.1016/j.rechem.2025.102565",
"article-title": "Evaluating the Potential of PLpro as a Drug Target in SARS-CoV-2: MD Simulations and Druggability Analysis",
"author": "Atatreh",
"doi-asserted-by": "crossref",
"first-page": "102565",
"journal-title": "Results Chem.",
"key": "ref_12",
"volume": "17",
"year": "2025"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"article-title": "Mechanisms of SARS-CoV-2 Entry into Cells",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "ref_13",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.chempr.2020.04.023",
"article-title": "Chemistry and Biology of SARS-CoV-2",
"author": "Gao",
"doi-asserted-by": "crossref",
"first-page": "1283",
"journal-title": "Chem",
"key": "ref_14",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.ejmech.2023.115772",
"article-title": "Structure and Function of SARS-CoV and SARS-CoV-2 Main Proteases and Their Inhibition: A Comprehensive Review",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "115772",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_15",
"volume": "260",
"year": "2023"
},
{
"DOI": "10.1021/acs.jmedchem.0c01140",
"article-title": "Targeting SARS-CoV-2 Proteases and Polymerase for COVID-19 Treatment: State of the Art and Future Opportunities",
"author": "Cannalire",
"doi-asserted-by": "crossref",
"first-page": "2716",
"journal-title": "J. Med. Chem.",
"key": "ref_16",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.3389/fmicb.2020.01723",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Jeong, G.U., Song, H., Yoon, G.Y., Kim, D., and Kwon, Y.-C. (2020). Therapeutic Strategies Against COVID-19 and Structural Characterization of SARS-CoV-2: A Review. Front. Microbiol., 11."
},
{
"DOI": "10.1016/j.ijbiomac.2023.123347",
"article-title": "Identification and Characterization of Aurintricarboxylic Acid as a Potential Inhibitor of SARS-CoV-2 PLpro",
"author": "Arya",
"doi-asserted-by": "crossref",
"first-page": "123347",
"journal-title": "Int. J. Biol. Macromol.",
"key": "ref_18",
"volume": "230",
"year": "2023"
},
{
"DOI": "10.1038/s41579-020-00468-6",
"article-title": "Coronavirus Biology and Replication: Implications for SARS-CoV-2",
"author": "Kratzel",
"doi-asserted-by": "crossref",
"first-page": "155",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_19",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1096/fj.202100280RR",
"article-title": "Potential 3-chymotrypsin-like Cysteine Protease Cleavage Sites in the Coronavirus Polyproteins Pp1a and Pp1ab and Their Possible Relevance to COVID-19 Vaccine and Drug Development",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "e21573",
"journal-title": "FASEB J.",
"key": "ref_20",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1126/science.1085658",
"article-title": "Coronavirus Main Proteinase (3CLpro) Structure: Basis for Design of Anti-SARS Drugs",
"author": "Anand",
"doi-asserted-by": "crossref",
"first-page": "1763",
"journal-title": "Science",
"key": "ref_21",
"volume": "300",
"year": "2003"
},
{
"DOI": "10.1038/s41598-022-23570-6",
"article-title": "Mechanistic Investigation of SARS-CoV-2 Main Protease to Accelerate Design of Covalent Inhibitors",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "21037",
"journal-title": "Sci. Rep.",
"key": "ref_22",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/S0022-2836(03)00865-9",
"article-title": "Unique and Conserved Features of Genome and Proteome of SARS-Coronavirus, an Early Split-off from the Coronavirus Group 2 Lineage",
"author": "Snijder",
"doi-asserted-by": "crossref",
"first-page": "991",
"journal-title": "J. Mol. Biol.",
"key": "ref_23",
"volume": "331",
"year": "2003"
},
{
"DOI": "10.1080/15548627.2024.2442849",
"article-title": "Regulation of N-Degron Recognin-Mediated Autophagy by the SARS-CoV-2 PLpro Ubiquitin Deconjugase",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "1019",
"journal-title": "Autophagy",
"key": "ref_24",
"volume": "21",
"year": "2025"
},
{
"DOI": "10.15252/embj.2020106275",
"article-title": "Mechanism and Inhibition of the Papain-like Protease, PLpro, of SARS-CoV-2",
"author": "Klemm",
"doi-asserted-by": "crossref",
"first-page": "e106275",
"journal-title": "EMBO J.",
"key": "ref_25",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1038/sigtrans.2017.23",
"article-title": "NF-κB Signaling in Inflammation",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "17023",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_26",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.1073/pnas.0510851103",
"article-title": "Severe Acute Respiratory Syndrome Coronavirus Papain-like Protease: Structure of a Viral Deubiquitinating Enzyme",
"author": "Ratia",
"doi-asserted-by": "crossref",
"first-page": "5717",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_27",
"volume": "103",
"year": "2006"
},
{
"DOI": "10.1189/jlb.2A0414-191R",
"article-title": "An Essential Role for IFN-β in the Induction of IFN-Stimulated Gene Expression by LPS in Macrophages",
"author": "Sheikh",
"doi-asserted-by": "crossref",
"first-page": "591",
"journal-title": "J. Leukoc. Biol.",
"key": "ref_28",
"volume": "96",
"year": "2014"
},
{
"DOI": "10.1016/j.immuni.2006.08.007",
"article-title": "Type I Interferons in Host Defense",
"author": "Stetson",
"doi-asserted-by": "crossref",
"first-page": "373",
"journal-title": "Immunity",
"key": "ref_29",
"volume": "25",
"year": "2006"
},
{
"DOI": "10.1038/s41392-020-00335-z",
"article-title": "SARS-CoV-2: The Many Pros of Targeting PLpro",
"author": "McClain",
"doi-asserted-by": "crossref",
"first-page": "223",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_30",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.3389/fgene.2022.870836",
"doi-asserted-by": "crossref",
"key": "ref_31",
"unstructured": "Jabeen, A., Ahmad, N., and Raza, K. (2022). Global Gene Expression and Docking Profiling of COVID-19 Infection. Front. Genet., 13."
},
{
"DOI": "10.1038/s41586-020-2601-5",
"article-title": "Papain-like Protease Regulates SARS-CoV-2 Viral Spread and Innate Immunity",
"author": "Shin",
"doi-asserted-by": "crossref",
"first-page": "657",
"journal-title": "Nature",
"key": "ref_32",
"volume": "587",
"year": "2020"
},
{
"DOI": "10.1016/j.tips.2022.08.008",
"article-title": "Targeting SARS-CoV-2 Papain-like Protease in the Postvaccine Era",
"author": "Ton",
"doi-asserted-by": "crossref",
"first-page": "906",
"journal-title": "Trends Pharmacol. Sci.",
"key": "ref_33",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.1038/s41467-024-50566-9",
"article-title": "Mutational Profiling of SARS-CoV-2 Papain-like Protease Reveals Requirements for Function, Structure, and Drug Escape",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "6219",
"journal-title": "Nat. Commun.",
"key": "ref_34",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1016/j.drudis.2023.103832",
"article-title": "Targeting SARS-CoV-2 Nonstructural Protein 3: Function, Structure, Inhibition, and Perspective in Drug Discovery",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "103832",
"journal-title": "Drug Discov. Today",
"key": "ref_35",
"volume": "29",
"year": "2024"
},
{
"article-title": "High-Throughput Screening of SARS-CoV-2 Main and Papain-like Protease Inhibitors",
"author": "Zang",
"first-page": "17",
"journal-title": "Protein Cell",
"key": "ref_36",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3389/fchem.2022.876212",
"doi-asserted-by": "crossref",
"key": "ref_37",
"unstructured": "Calleja, D.J., Lessene, G., and Komander, D. (2022). Inhibitors of SARS-CoV-2 PLpro. Front. Chem., 10."
},
{
"DOI": "10.1126/science.abl4784",
"article-title": "An Oral SARS-CoV-2 Mpro Inhibitor Clinical Candidate for the Treatment of COVID-19",
"author": "Owen",
"doi-asserted-by": "crossref",
"first-page": "1586",
"journal-title": "Science",
"key": "ref_38",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m3379",
"article-title": "A Living WHO Guideline on Drugs for COVID-19",
"author": "Agarwal",
"doi-asserted-by": "crossref",
"first-page": "m3379",
"journal-title": "BMJ",
"key": "ref_39",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1038/s41564-024-01618-9",
"article-title": "Preclinical Evaluation of the SARS-CoV-2 Mpro Inhibitor RAY1216 Shows Improved Pharmacokinetics Compared with Nirmatrelvir",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "1075",
"journal-title": "Nat. Microbiol.",
"key": "ref_40",
"volume": "9",
"year": "2024"
},
{
"DOI": "10.1038/s41586-023-06609-0",
"article-title": "Molecular Mechanisms of SARS-CoV-2 Resistance to Nirmatrelvir",
"author": "Duan",
"doi-asserted-by": "crossref",
"first-page": "376",
"journal-title": "Nature",
"key": "ref_41",
"volume": "622",
"year": "2023"
},
{
"DOI": "10.1038/s41586-022-05514-2",
"article-title": "Multiple Pathways for SARS-CoV-2 Resistance to Nirmatrelvir",
"author": "Iketani",
"doi-asserted-by": "crossref",
"first-page": "558",
"journal-title": "Nature",
"key": "ref_42",
"volume": "613",
"year": "2023"
},
{
"DOI": "10.1371/journal.ppat.1012522",
"doi-asserted-by": "crossref",
"key": "ref_43",
"unstructured": "Costacurta, F., Dodaro, A., Bante, D., Schöppe, H., Peng, J.-Y., Sprenger, B., He, X., Moghadasi, S.A., Egger, L.M., and Fleischmann, J. (2024). A Comprehensive Study of SARS-CoV-2 Main Protease (Mpro) Inhibitor-Resistant Mutants Selected in a VSV-Based System. PLoS Pathog., 20."
},
{
"DOI": "10.1126/sciadv.ade8778",
"article-title": "Transmissible SARS-CoV-2 Variants with Resistance to Clinical Protease Inhibitors",
"author": "Moghadasi",
"doi-asserted-by": "crossref",
"first-page": "eade8778",
"journal-title": "Sci. Adv.",
"key": "ref_44",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.1016/j.antiviral.2017.11.001",
"article-title": "Nsp3 of Coronaviruses: Structures and Functions of a Large Multi-Domain Protein",
"author": "Lei",
"doi-asserted-by": "crossref",
"first-page": "58",
"journal-title": "Antivir. Res.",
"key": "ref_45",
"volume": "149",
"year": "2018"
},
{
"DOI": "10.1016/j.tim.2020.05.009",
"article-title": "Double-Membrane Vesicles as Platforms for Viral Replication",
"author": "Wolff",
"doi-asserted-by": "crossref",
"first-page": "1022",
"journal-title": "Trends Microbiol.",
"key": "ref_46",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1038/s41392-022-00884-5",
"article-title": "Structural Biology of SARS-CoV-2: Open the Door for Novel Therapies",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "26",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_47",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1038/s41586-024-07817-y",
"article-title": "Molecular Architecture of Coronavirus Double-Membrane Vesicle Pore Complex",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "224",
"journal-title": "Nature",
"key": "ref_48",
"volume": "633",
"year": "2024"
},
{
"DOI": "10.1128/JVI.73.4.2658-2666.1999",
"article-title": "Expression of Murine Coronavirus Recombinant Papain-Like Proteinase: Efficient Cleavage Is Dependent on the Lengths of Both the Substrate and the Proteinase Polypeptides",
"author": "Teng",
"doi-asserted-by": "crossref",
"first-page": "2658",
"journal-title": "J. Virol.",
"key": "ref_49",
"volume": "73",
"year": "1999"
},
{
"DOI": "10.1074/jbc.M104097200",
"article-title": "The Autocatalytic Release of a Putative RNA Virus Transcription Factor from Its Polyprotein Precursor Involves Two Paralogous Papain-like Proteases That Cleave the Same Peptide Bond",
"author": "Ziebuhr",
"doi-asserted-by": "crossref",
"first-page": "33220",
"journal-title": "J. Biol. Chem.",
"key": "ref_50",
"volume": "276",
"year": "2001"
},
{
"DOI": "10.1126/scisignal.add0082",
"article-title": "The SARS-CoV-2 Papain-like Protease Suppresses Type I Interferon Responses by Deubiquitinating STING",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "eadd0082",
"journal-title": "Sci. Signal.",
"key": "ref_51",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1038/s41467-023-38031-5",
"article-title": "Dual Domain Recognition Determines SARS-CoV-2 PLpro Selectivity for Human ISG15 and K48-Linked Di-Ubiquitin",
"author": "Wydorski",
"doi-asserted-by": "crossref",
"first-page": "2366",
"journal-title": "Nat. Commun.",
"key": "ref_52",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.3389/fcell.2021.788410",
"doi-asserted-by": "crossref",
"key": "ref_53",
"unstructured": "Zhang, M., Li, J., Yan, H., Huang, J., Wang, F., Liu, T., Zeng, L., and Zhou, F. (2021). ISGylation in Innate Antiviral Immunity and Pathogen Defense Responses: A Review. Front. Cell Dev. Biol., 9."
},
{
"DOI": "10.1128/JVI.79.24.15199-15208.2005",
"article-title": "The Papain-Like Protease from the Severe Acute Respiratory Syndrome Coronavirus Is a Deubiquitinating Enzyme",
"author": "Lindner",
"doi-asserted-by": "crossref",
"first-page": "15199",
"journal-title": "J. Virol.",
"key": "ref_54",
"volume": "79",
"year": "2005"
},
{
"DOI": "10.3390/molecules30030491",
"doi-asserted-by": "crossref",
"key": "ref_55",
"unstructured": "Varghese, A., Liu, J., Liu, B., Guo, W., Dong, F., Patterson, T.A., and Hong, H. (2025). Analysis of Structures of SARS-CoV-2 Papain-like Protease Bound with Ligands Unveils Structural Features for Inhibiting the Enzyme. Molecules, 30."
},
{
"DOI": "10.1002/chem.202103258",
"article-title": "Metallodrug Profiling against SARS-CoV-2 Target Proteins Identifies Highly Potent Inhibitors of the S/ACE2 Interaction and the Papain-like Protease PLpro",
"author": "Basu",
"doi-asserted-by": "crossref",
"first-page": "17928",
"journal-title": "Chem. A Eur. J.",
"key": "ref_56",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1080/14756366.2022.2108417",
"article-title": "Zinc Pyrithione Is a Potent Inhibitor of PLPro and Cathepsin L Enzymes with Ex Vivo Inhibition of SARS-CoV-2 Entry and Replication",
"author": "Kladnik",
"doi-asserted-by": "crossref",
"first-page": "2158",
"journal-title": "J. Enzym. Inhib. Med. Chem.",
"key": "ref_57",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1039/D3MD00067B",
"article-title": "Silver N-Heterocyclic Carbene Complexes Are Potent Uncompetitive Inhibitors of the Papain-like Protease with Antiviral Activity against SARS-CoV-2",
"author": "Esarev",
"doi-asserted-by": "crossref",
"first-page": "1260",
"journal-title": "RSC Med. Chem.",
"key": "ref_58",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/s41467-020-20718-8",
"article-title": "The Complex Structure of GRL0617 and SARS-CoV-2 PLpro Reveals a Hot Spot for Antiviral Drug Discovery",
"author": "Fu",
"doi-asserted-by": "crossref",
"first-page": "488",
"journal-title": "Nat. Commun.",
"key": "ref_59",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.bioorg.2023.106830",
"article-title": "Discovery of New Non-Covalent and Covalent Inhibitors Targeting SARS-CoV-2 Papain-like Protease and Main Protease",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "106830",
"journal-title": "Bioorg. Chem.",
"key": "ref_60",
"volume": "140",
"year": "2023"
},
{
"DOI": "10.1016/j.apsb.2020.08.014",
"article-title": "Crystal Structure of SARS-CoV-2 Papain-like Protease",
"author": "Gao",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "Acta Pharm. Sin. B",
"key": "ref_61",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-21060-3",
"article-title": "Structure of Papain-like Protease from SARS-CoV-2 and Its Complexes with Non-Covalent Inhibitors",
"author": "Osipiuk",
"doi-asserted-by": "crossref",
"first-page": "743",
"journal-title": "Nat. Commun.",
"key": "ref_62",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.ejmech.2023.116011",
"article-title": "Structure-Based Design of SARS-CoV-2 Papain-like Protease Inhibitors",
"author": "Jadhav",
"doi-asserted-by": "crossref",
"first-page": "116011",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_63",
"volume": "264",
"year": "2024"
},
{
"DOI": "10.1021/acscentsci.1c00519",
"article-title": "Discovery of SARS-CoV-2 Papain-like Protease Inhibitors through a Combination of High-Throughput Screening and a FlipGFP-Based Reporter Assay",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "1245",
"journal-title": "ACS Cent. Sci.",
"key": "ref_64",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1016/j.chembiol.2021.04.020",
"article-title": "Development of Potent and Selective Inhibitors Targeting the Papain-like Protease of SARS-CoV-2",
"author": "Shan",
"doi-asserted-by": "crossref",
"first-page": "855",
"journal-title": "Cell Chem. Biol.",
"key": "ref_65",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.3389/fchem.2022.861209",
"doi-asserted-by": "crossref",
"key": "ref_66",
"unstructured": "Calleja, D.J., Kuchel, N., Lu, B.G.C., Birkinshaw, R.W., Klemm, T., Doerflinger, M., Cooney, J.P., Mackiewicz, L., Au, A.E., and Yap, Y.Q. (2022). Insights into Drug Repurposing, as Well as Specificity and Compound Properties of Piperidine-Based SARS-CoV-2 PLpro Inhibitors. Front. Chem., 10."
},
{
"DOI": "10.1021/acs.jmedchem.1c01307",
"article-title": "Design of SARS-CoV-2 PLpro Inhibitors for COVID-19 Antiviral Therapy Leveraging Binding Cooperativity",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "2940",
"journal-title": "J. Med. Chem.",
"key": "ref_67",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1021/acs.jmedchem.4c00378",
"article-title": "Non-Covalent Inhibitors of SARS-CoV-2 Papain-Like Protease (PLpro): In Vitro and In Vivo Antiviral Activity",
"author": "Velma",
"doi-asserted-by": "crossref",
"first-page": "13681",
"journal-title": "J. Med. Chem.",
"key": "ref_68",
"volume": "67",
"year": "2024"
},
{
"DOI": "10.1038/s41467-024-54462-0",
"article-title": "Discovery of Orally Bioavailable SARS-CoV-2 Papain-like Protease Inhibitor as a Potential Treatment for COVID-19",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "10169",
"journal-title": "Nat. Commun.",
"key": "ref_69",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1126/sciadv.ado4288",
"article-title": "Discovery of SARS-CoV-2 Papain-like Protease (PLpro) Inhibitors with Efficacy in a Murine Infection Model",
"author": "Garnsey",
"doi-asserted-by": "crossref",
"first-page": "eado4288",
"journal-title": "Sci. Adv.",
"key": "ref_70",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1126/science.adm9724",
"article-title": "Design of a SARS-CoV-2 Papain-like Protease Inhibitor with Antiviral Efficacy in a Mouse Model",
"author": "Tan",
"doi-asserted-by": "crossref",
"first-page": "1434",
"journal-title": "Science",
"key": "ref_71",
"volume": "383",
"year": "2024"
},
{
"DOI": "10.1038/s41467-025-57905-4",
"article-title": "A Novel PLpro Inhibitor Improves Outcomes in a Pre-Clinical Model of Long COVID",
"author": "Bader",
"doi-asserted-by": "crossref",
"first-page": "2900",
"journal-title": "Nat. Commun.",
"key": "ref_72",
"volume": "16",
"year": "2025"
},
{
"DOI": "10.1021/acsmedchemlett.4c00238",
"article-title": "Fragment-Based Screen of SARS-CoV-2 Papain-like Protease (PLpro)",
"author": "Taylor",
"doi-asserted-by": "crossref",
"first-page": "1351",
"journal-title": "ACS Med. Chem. Lett.",
"key": "ref_73",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1126/sciadv.abd4596",
"article-title": "Activity Profiling and Crystal Structures of Inhibitor-Bound SARS-CoV-2 Papain-like Protease: A Framework for Anti–COVID-19 Drug Design",
"author": "Rut",
"doi-asserted-by": "crossref",
"first-page": "eabd4596",
"journal-title": "Sci. Adv.",
"key": "ref_74",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1021/acs.jpcb.1c07060",
"article-title": "In Silico Elucidation of Potent Inhibitors and Rational Drug Design against SARS-CoV-2 Papain-like Protease",
"author": "Sanachai",
"doi-asserted-by": "crossref",
"first-page": "13644",
"journal-title": "J. Phys. Chem. B",
"key": "ref_75",
"volume": "125",
"year": "2021"
},
{
"DOI": "10.3390/ijms24108633",
"doi-asserted-by": "crossref",
"key": "ref_76",
"unstructured": "Wang, Q., Chen, G., He, J., Li, J., Xiong, M., Su, H., Li, M., Hu, H., and Xu, Y. (2023). Structure-Based Design of Potent Peptidomimetic Inhibitors Covalently Targeting SARS-CoV-2 Papain-like Protease. Int. J. Mol. Sci., 24."
},
{
"DOI": "10.1038/s41467-023-37254-w",
"article-title": "Potent and Selective Covalent Inhibition of the Papain-like Protease from SARS-CoV-2",
"author": "Sanders",
"doi-asserted-by": "crossref",
"first-page": "1733",
"journal-title": "Nat. Commun.",
"key": "ref_77",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1021/acs.jmedchem.4c01872",
"article-title": "Structure-Based Design of Covalent SARS-CoV-2 Papain-like Protease Inhibitors",
"author": "Tan",
"doi-asserted-by": "crossref",
"first-page": "20399",
"journal-title": "J. Med. Chem.",
"key": "ref_78",
"volume": "67",
"year": "2024"
},
{
"DOI": "10.1038/s41467-025-56902-x",
"article-title": "Design of Quinoline SARS-CoV-2 Papain-like Protease Inhibitors as Oral Antiviral Drug Candidates",
"author": "Jadhav",
"doi-asserted-by": "crossref",
"first-page": "1604",
"journal-title": "Nat. Commun.",
"key": "ref_79",
"volume": "16",
"year": "2025"
},
{
"DOI": "10.3390/ijms24031955",
"doi-asserted-by": "crossref",
"key": "ref_80",
"unstructured": "Protić, S., Kaličanin, N., Sencanski, M., Prodanović, O., Milicevic, J., Perovic, V., Paessler, S., Prodanović, R., and Glisic, S. (2023). In Silico and In Vitro Inhibition of SARS-CoV-2 PLpro with Gramicidin D. Int. J. Mol. Sci., 24."
},
{
"DOI": "10.1021/acs.jmedchem.1c02022",
"article-title": "Design and Evaluation of a Novel Peptide–Drug Conjugate Covalently Targeting SARS-CoV-2 Papain-like Protease",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "876",
"journal-title": "J. Med. Chem.",
"key": "ref_81",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1016/j.addr.2016.06.015",
"article-title": "Peptide–Drug Conjugates as Effective Prodrug Strategies for Targeted Delivery",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "112",
"journal-title": "Adv. Drug Deliv. Rev.",
"key": "ref_82",
"volume": "110–111",
"year": "2017"
},
{
"DOI": "10.1021/cb500476q",
"article-title": "Potent Antimalarial Activity of Acriflavine In Vitro and In Vivo",
"author": "Dana",
"doi-asserted-by": "crossref",
"first-page": "2366",
"journal-title": "ACS Chem. Biol.",
"key": "ref_83",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1093/nar/gkw878",
"article-title": "Activation of cGAS-Dependent Antiviral Responses by DNA Intercalating Agents",
"author": "Nejad",
"doi-asserted-by": "crossref",
"first-page": "198",
"journal-title": "Nucleic Acids Res.",
"key": "ref_84",
"volume": "45",
"year": "2017"
},
{
"DOI": "10.1186/1471-2164-15-S7-S1",
"doi-asserted-by": "crossref",
"key": "ref_85",
"unstructured": "Persinoti, G.F., De Aguiar Peres, N.T., Jacob, T.R., Rossi, A., Vêncio, R.Z., and Martinez-Rossi, N.M. (2014). RNA-Sequencing Analysis of Trichophyton Rubrumtranscriptome in Response to Sublethal Doses of Acriflavine. BMC Genom., 15."
},
{
"DOI": "10.1016/j.chembiol.2021.11.006",
"article-title": "Acriflavine, a Clinically Approved Drug, Inhibits SARS-CoV-2 and Other Betacoronaviruses",
"author": "Napolitano",
"doi-asserted-by": "crossref",
"first-page": "774",
"journal-title": "Cell Chem. Biol.",
"key": "ref_86",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1007/s13238-021-00836-9",
"article-title": "High-Throughput Screening Identifies Established Drugs as SARS-CoV-2 PLpro Inhibitors",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "877",
"journal-title": "Protein Cell",
"key": "ref_87",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fchem.2022.832431",
"doi-asserted-by": "crossref",
"key": "ref_88",
"unstructured": "Ewert, W., Günther, S., Miglioli, F., Falke, S., Reinke, P.Y.A., Niebling, S., Günther, C., Han, H., Srinivasan, V., and Brognaro, H. (2022). Hydrazones and Thiosemicarbazones Targeting Protein-Protein-Interactions of SARS-CoV-2 Papain-like Protease. Front. Chem., 10."
},
{
"DOI": "10.1038/s41586-020-2223-y",
"article-title": "Structure of Mpro from SARS-CoV-2 and Discovery of Its Inhibitors",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "289",
"journal-title": "Nature",
"key": "ref_89",
"volume": "582",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-23313-7",
"article-title": "Inhibition Mechanism of SARS-CoV-2 Main Protease by Ebselen and Its Derivatives",
"author": "Amporndanai",
"doi-asserted-by": "crossref",
"first-page": "3061",
"journal-title": "Nat. Commun.",
"key": "ref_90",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1021/acsptsci.0c00130",
"article-title": "Ebselen, Disulfiram, Carmofur, PX-12, Tideglusib, and Shikonin Are Nonspecific Promiscuous SARS-CoV-2 Main Protease Inhibitors",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "1265",
"journal-title": "ACS Pharmacol. Transl. Sci.",
"key": "ref_91",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1039/D0SC02646H",
"article-title": "Multi-Targeting of Functional Cysteines in Multiple Conserved SARS-CoV-2 Domains by Clinically Safe Zn-Ejectors",
"author": "Sargsyan",
"doi-asserted-by": "crossref",
"first-page": "9904",
"journal-title": "Chem. Sci.",
"key": "ref_92",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41598-023-35907-w",
"article-title": "Ebselen Derivatives Inhibit SARS-CoV-2 Replication by Inhibition of Its Essential Proteins: PLpro and Mpro Proteases, and Nsp14 Guanine N7-Methyltransferase",
"author": "Zmudzinski",
"doi-asserted-by": "crossref",
"first-page": "9161",
"journal-title": "Sci. Rep.",
"key": "ref_93",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1038/s42003-022-03737-7",
"article-title": "Antiviral Activity of Natural Phenolic Compounds in Complex at an Allosteric Site of SARS-CoV-2 Papain-like Protease",
"author": "Srinivasan",
"doi-asserted-by": "crossref",
"first-page": "805",
"journal-title": "Commun. Biol.",
"key": "ref_94",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1021/jacs.4c12992",
"article-title": "Covalent DNA-Encoded Library Workflow Drives Discovery of SARS-CoV-2 Nonstructural Protein Inhibitors",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "33983",
"journal-title": "J. Am. Chem. Soc.",
"key": "ref_95",
"volume": "146",
"year": "2024"
},
{
"DOI": "10.1371/journal.ppat.1011065",
"doi-asserted-by": "crossref",
"key": "ref_96",
"unstructured": "Van Vliet, V.J.E., Huynh, N., Palà, J., Patel, A., Singer, A., Slater, C., Chung, J., Van Huizen, M., Teyra, J., and Miersch, S. (2022). Ubiquitin Variants Potently Inhibit SARS-CoV-2 PLpro and Viral Replication via a Novel Site Distal to the Protease Active Site. PLoS Pathog., 18."
},
{
"DOI": "10.1021/acsomega.3c01110",
"article-title": "Discovery of PLpro and Mpro Inhibitors for SARS-CoV-2",
"author": "Puhl",
"doi-asserted-by": "crossref",
"first-page": "22603",
"journal-title": "ACS Omega",
"key": "ref_97",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1021/acs.jmedchem.7b00907",
"article-title": "Discovery of Tropifexor (LJN452), a Highly Potent Non-Bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH)",
"author": "Tully",
"doi-asserted-by": "crossref",
"first-page": "9960",
"journal-title": "J. Med. Chem.",
"key": "ref_98",
"volume": "60",
"year": "2017"
},
{
"DOI": "10.1021/acsinfecdis.1c00629",
"article-title": "Drug-Repurposing Screening Identified Tropifexor as a SARS-CoV-2 Papain-like Protease Inhibitor",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "1022",
"journal-title": "ACS Infect. Dis.",
"key": "ref_99",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2014.12.011",
"article-title": "Thiopurine Analogs and Mycophenolic Acid Synergistically Inhibit the Papain-like Protease of Middle East Respiratory Syndrome Coronavirus",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "Antivir. Res.",
"key": "ref_100",
"volume": "115",
"year": "2015"
},
{
"article-title": "The Continuous Rediscovery and the Benefit–Risk Ratio of Thioguanine, a Comprehensive Review",
"author": "Bayoumy",
"first-page": "111",
"journal-title": "Expert Opin. Drug Metab. Toxicol.",
"key": "ref_101",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1016/j.bcp.2008.01.005",
"article-title": "Thiopurine Analogues Inhibit Papain-like Protease of Severe Acute Respiratory Syndrome Coronavirus",
"author": "Chou",
"doi-asserted-by": "crossref",
"first-page": "1601",
"journal-title": "Biochem. Pharmacol.",
"key": "ref_102",
"volume": "75",
"year": "2008"
},
{
"DOI": "10.1016/j.isci.2021.103213",
"article-title": "6-Thioguanine Blocks SARS-CoV-2 Replication by Inhibition of PLpro",
"author": "Swaim",
"doi-asserted-by": "crossref",
"first-page": "103213",
"journal-title": "iScience",
"key": "ref_103",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1039/D3RA00426K",
"article-title": "A Covalent Inhibitor Targeting the Papain-like Protease from SARS-CoV-2 Inhibits Viral Replication",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "10636",
"journal-title": "RSC Adv.",
"key": "ref_104",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1021/acs.jmedchem.2c00954",
"article-title": "Structure-Based Design of a Dual-Targeted Covalent Inhibitor Against Papain-like and Main Proteases of SARS-CoV-2",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "16252",
"journal-title": "J. Med. Chem.",
"key": "ref_105",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1016/j.virs.2023.04.008",
"article-title": "Oridonin Inhibits SARS-CoV-2 Replication by Targeting Viral Proteinase and Polymerase",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "470",
"journal-title": "Virol. Sin.",
"key": "ref_106",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.3390/ph15060744",
"doi-asserted-by": "crossref",
"key": "ref_107",
"unstructured": "Meewan, I., Kattoula, J., Kattoula, J.Y., Skinner, D., Fajtová, P., Giardini, M.A., Woodworth, B., McKerrow, J.H., Lage De Siqueira-Neto, J., and O’Donoghue, A.J. (2022). Discovery of Triple Inhibitors of Both SARS-CoV-2 Proteases and Human Cathepsin L.. Pharmaceuticals, 15."
},
{
"DOI": "10.1016/j.ijantimicag.2023.107039",
"article-title": "Chrysin 7-O-β-D-Glucuronide, a Dual Inhibitor of SARS-CoV-2 3CLpro and PLpro, for the Prevention and Treatment of COVID-19",
"author": "Yi",
"doi-asserted-by": "crossref",
"first-page": "107039",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "ref_108",
"volume": "63",
"year": "2024"
},
{
"DOI": "10.1016/j.phymed.2023.155176",
"article-title": "Compounds Derived from Humulus Lupulus Inhibit SARS-CoV-2 Papain-like Protease and Virus Replication",
"author": "Herzog",
"doi-asserted-by": "crossref",
"first-page": "155176",
"journal-title": "Phytomedicine",
"key": "ref_109",
"volume": "123",
"year": "2024"
},
{
"DOI": "10.1007/s00044-022-02903-0",
"article-title": "Invalidation of Dieckol and 1,2,3,4,6-Pentagalloylglucose (PGG) as SARS-CoV-2 Main Protease Inhibitors and the Discovery of PGG as a Papain-like Protease Inhibitor",
"author": "Tan",
"doi-asserted-by": "crossref",
"first-page": "1147",
"journal-title": "Med. Chem. Res.",
"key": "ref_110",
"volume": "31",
"year": "2022"
},
{
"DOI": "10.1016/j.virol.2022.07.006",
"article-title": "A Robust High-Throughput Fluorescence Polarization Assay for Rapid Screening of SARS-CoV-2 Papain-like Protease Inhibitors",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "18",
"journal-title": "Virology",
"key": "ref_111",
"volume": "574",
"year": "2022"
},
{
"DOI": "10.1016/j.ijbiomac.2023.124772",
"article-title": "Development of 2-Chloroquinoline Based Heterocyclic Frameworks as Dual Inhibitors of SARS-CoV-2 MPro and PLPro",
"author": "Kattula",
"doi-asserted-by": "crossref",
"first-page": "124772",
"journal-title": "Int. J. Biol. Macromol.",
"key": "ref_112",
"volume": "242",
"year": "2023"
},
{
"DOI": "10.1039/D3RA01915B",
"article-title": "Mercapto-Pyrimidines Are Reversible Covalent Inhibitors of the Papain-like Protease (PLpro) and Inhibit SARS-CoV-2 (SCoV-2) Replication",
"author": "Bajaj",
"doi-asserted-by": "crossref",
"first-page": "17667",
"journal-title": "RSC Adv.",
"key": "ref_113",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1016/j.ejmech.2023.115380",
"article-title": "Discovery of Novel Papain-like Protease Inhibitors for Potential Treatment of COVID-19",
"author": "Hersi",
"doi-asserted-by": "crossref",
"first-page": "115380",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_114",
"volume": "254",
"year": "2023"
},
{
"DOI": "10.1007/s13238-022-00909-3",
"article-title": "Targeting Papain-like Protease for Broad-Spectrum Coronavirus Inhibition",
"author": "Yuan",
"doi-asserted-by": "crossref",
"first-page": "940",
"journal-title": "Protein Cell",
"key": "ref_115",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.ejmech.2023.115272",
"article-title": "Repurposing 1,2,4-Oxadiazoles as SARS-CoV-2 PLpro Inhibitors and Investigation of Their Possible Viral Entry Blockade Potential",
"author": "Ayoup",
"doi-asserted-by": "crossref",
"first-page": "115272",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_116",
"volume": "252",
"year": "2023"
},
{
"DOI": "10.1002/cmdc.202100156",
"article-title": "Inspecting the Mechanism of Fragment Hits Binding on SARS-CoV-2 Mpro by Using Supervised Molecular Dynamics (SuMD) Simulations",
"author": "Bissaro",
"doi-asserted-by": "crossref",
"first-page": "2075",
"journal-title": "ChemMedChem",
"key": "ref_117",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1038/s41587-024-02143-0",
"article-title": "A Small-Molecule TNIK Inhibitor Targets Fibrosis in Preclinical and Clinical Models",
"author": "Ren",
"doi-asserted-by": "crossref",
"first-page": "63",
"journal-title": "Nat. Biotechnol.",
"key": "ref_118",
"volume": "43",
"year": "2025"
},
{
"DOI": "10.1021/acs.jcim.2c01191",
"article-title": "Chemistry42: An AI-Driven Platform for Molecular Design and Optimization",
"author": "Ivanenkov",
"doi-asserted-by": "crossref",
"first-page": "695",
"journal-title": "J. Chem. Inf. Model.",
"key": "ref_119",
"volume": "63",
"year": "2023"
},
{
"DOI": "10.1038/s41586-025-09429-6",
"article-title": "One-Shot Design of Functional Protein Binders with BindCraft",
"author": "Pacesa",
"doi-asserted-by": "crossref",
"first-page": "483",
"journal-title": "Nature",
"key": "ref_120",
"volume": "646",
"year": "2025"
},
{
"DOI": "10.1038/s41586-023-06415-8",
"article-title": "De Novo Design of Protein Structure and Function with RFdiffusion",
"author": "Watson",
"doi-asserted-by": "crossref",
"first-page": "1089",
"journal-title": "Nature",
"key": "ref_121",
"volume": "620",
"year": "2023"
},
{
"DOI": "10.1038/s41586-022-04654-9",
"article-title": "Design of Protein-Binding Proteins from the Target Structure Alone",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "551",
"journal-title": "Nature",
"key": "ref_122",
"volume": "605",
"year": "2022"
},
{
"DOI": "10.1021/acs.jcim.5c01708",
"article-title": "De N Ovo-Designed Miniprotein Inhibits the Enzymatic Activity of the SARS-CoV-2 Main Protease",
"author": "Lima",
"doi-asserted-by": "crossref",
"first-page": "11314",
"journal-title": "J. Chem. Inf. Model.",
"key": "ref_123",
"volume": "65",
"year": "2025"
},
{
"DOI": "10.1021/acs.jmedchem.3c02416",
"article-title": "Discovery of First-in-Class PROTAC Degraders of SARS-CoV-2 Main Protease",
"author": "Alugubelli",
"doi-asserted-by": "crossref",
"first-page": "6495",
"journal-title": "J. Med. Chem.",
"key": "ref_124",
"volume": "67",
"year": "2024"
},
{
"DOI": "10.3389/fddsv.2022.892057",
"doi-asserted-by": "crossref",
"key": "ref_125",
"unstructured": "Reboud-Ravaux, M., and El Amri, C. (2022). COVID-19 Therapies: Protease Inhibitions and Novel Degrader Strategies. Front. Drug Discov., 2."
}
],
"reference-count": 125,
"references-count": 125,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1420-3049/31/3/474"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Structural Basis and Inhibitor Development of SARS-CoV-2 Papain-like Protease",
"type": "journal-article",
"update-policy": "https://doi.org/10.3390/mdpi_crossmark_policy",
"volume": "31"
}