Association of the patterns of use of medications with mortality of COVID-19 infection: a hospital-based observational study
et al., BMJ Open, doi:10.1136/bmjopen-2021-050051, Dec 2021
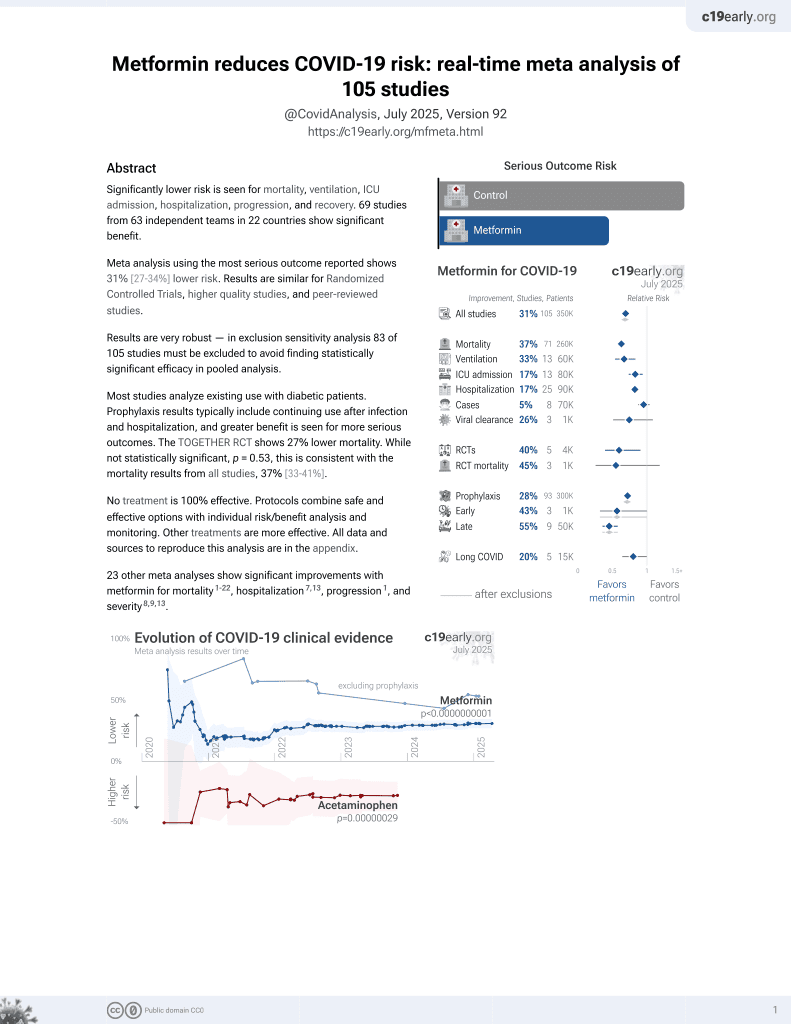
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
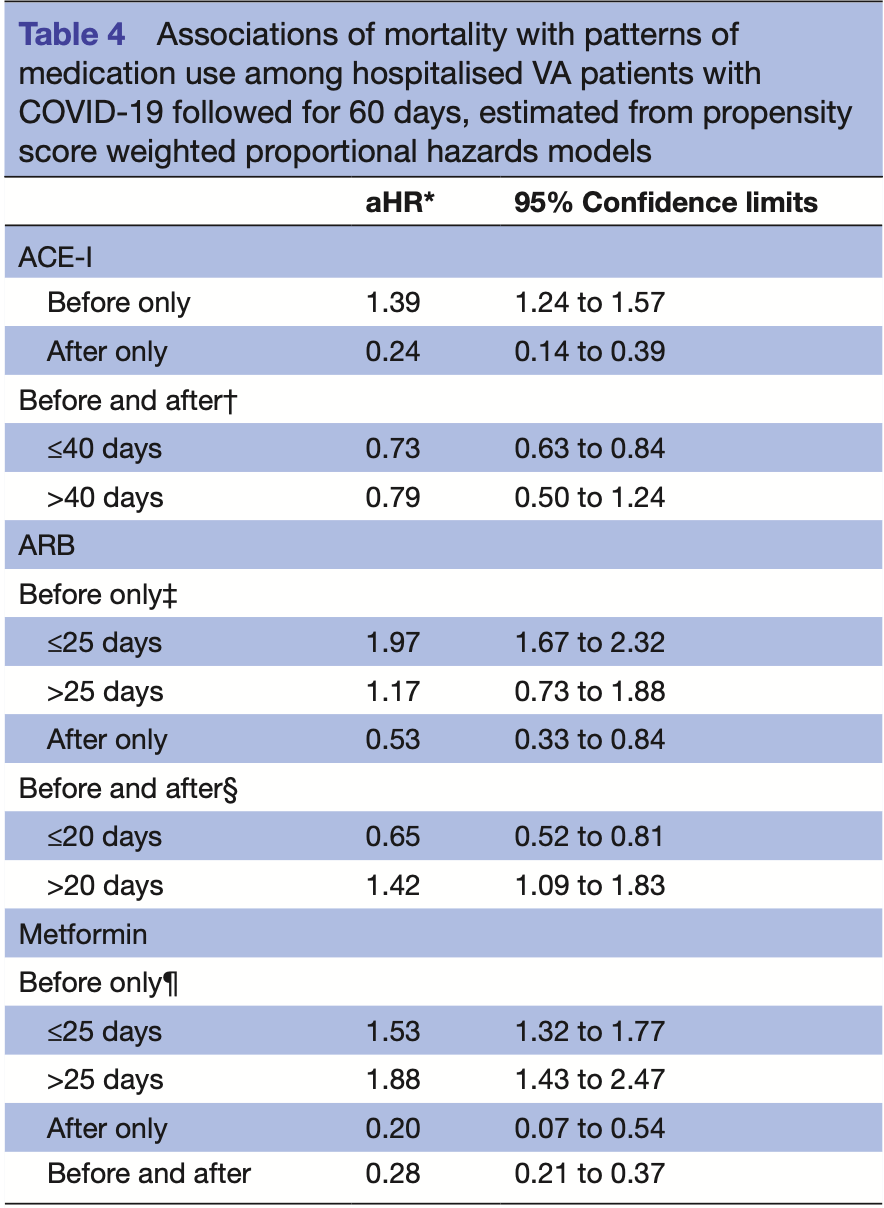
Retrospective 9,532 hospitalized COVID+ veterans in the USA, showing lower mortality with metformin use. The study provides results for use before, after, and before+after. Before+after should more accurately represent prophylaxis up to COVID-19 infection (and continued use). Before included use up to 2 years before, and after included use up to 60 days later.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
Study covers metformin and famotidine.
|
risk of death, 72.0% lower, HR 0.28, p < 0.001, treatment 103 of 1,203 (8.6%), control 1,536 of 6,970 (22.0%), NNT 7.4, adjusted per study, before+after, propensity score weighting, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Wallace et al., 31 Dec 2021, retrospective, database analysis, USA, peer-reviewed, 6 authors.
Association of the patterns of use of medications with mortality of COVID-19 infection: a hospital-based observational study
BMJ Open, doi:10.1136/bmjopen-2021-050051
Objectives SARS-CoV-2 enters cells using the ACE2 receptor. Medications that affect ACE2 expression or function such as angiotensin receptor blockers (ARBs) and ACE inhibitors (ACE-I) and metformin have the potential to counter the dysregulation of ACE2 by the virus and protect against viral injury. Here, we describe COVID-19 survival associated with ACE-I, ARB and metformin use. Design This is a hospital-based observational study of patients with COVID-19 infection using logistic regression with correction for pre-existing conditions and propensity score weighted Cox proportional hazards models to estimate associations between medication use and mortality. Setting Medical record data from the US Veterans Affairs (VA) were used to identify patients with a reverse transcription PCR diagnosis of COVID-19 infection, to classify patterns of ACE inhibitors (ACE-I), ARB, beta blockers, metformin, famotidine and remdesivir use, and, to capture mortality. Participants 9532 hospitalised patients with COVID-19 infection followed for 60 days were analysed. Outcome measure Death from any cause within 60 days of COVID-19 diagnosis was examined. Results Discontinuation of ACE-I was associated with increased risk of death (OR: 1.4; 95% CI 1.2-1.7). Initiating (OR: 0.3; 95% CI 0.2-0.5) or continuous (OR: 0.6; 95% CI 0.5-0.7) ACE-I was associated with reduced risk of death. ARB and metformin associations were similar in direction and magnitude and also statistically significant. Results were unchanged when accounting for pre-existing morbidity and propensity score adjustment. Conclusions Recent randomised clinical trials support the safety of continuing ACE-I and ARB treatment in patients with COVID-19 where indicated. Our study extends these findings to suggest a possible COVID-19 survival benefit for continuing or initiating ACE-I, ARB and metformin medications. Randomised trials are appropriate to confirm or refute the therapeutic potential for ACE-I, ARBs and metformin.
Competing interests All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. AW, PC, NYK and BAC declare support from grants from Mercatus Center, George Mason University, and from UC Office of the President, during the conduct of the study. All authors declare no other competing interests, no relationships with any organisations that might have a financial interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not applicable. Ethics approval This study involves human participants and was approved by the University of California San Francisco's Institutional Review Board (IRB), the San Francisco VA Research and Development (R&D) committee and the Public Health Institute's IRB (US VA IRB project number: 10-03609). This study uses existing data available from the US Department of Veterans Affairs Corporate Data Warehouse and does not require informed consent but does require IRB approval. Provenance and peer review Not commissioned; externally peer reviewed. Data availability statement Data may be obtained from a third party and are not publicly available. Data requests for access to the de-identified (anonymised) data must be submitted to AWW (the..
References
Brusselaers, Lagergren, The Charlson comorbidity index in registry-based research, Methods Inf Med, doi:10.3414/ME17-01-0051
Cao, Deng, Dai, Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence, Travel Med Infect Dis
Clarke, Turner, Angiotensin-converting enzyme 2: the first decade, Int J Hypertens, doi:10.1155/2012/307315
Cohen, Hanff, William, Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial, Lancet Respir Med, doi:10.1016/S2213-2600(20)30558-0
Crackower, Sarao, Oudit, Angiotensin-converting enzyme 2 is an essential regulator of heart function, Nature, doi:10.1038/nature00786
Donoghue, Hsieh, Baronas, A novel angiotensinconverting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9, Circ Res, doi:10.1161/01.RES.87.5.e1
Freedberg, Conigliaro, Wang, Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study, Gastroenterology, doi:10.1053/j.gastro.2020.05.053
Grein, Ohmagari, Shin, Compassionate use of remdesivir for patients with severe Covid-19, N Engl J Med, doi:10.1056/NEJMoa2007016
Gu, Xie, Li, Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus, Sci Rep, doi:10.1038/srep19840
Gupta, Biswal, Singha, Binding insight of clinically oriented drug famotidine with the identified potential target of SARS-CoV-2, J Biomol Struct Dyn
Hamming, Timens, Bulthuis, Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis, J Pathol, doi:10.1002/path.1570
Janowitz, Gablenz, Pattinson, Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series, Gut, doi:10.1136/gutjnl-2020-321852
Jarcho, Ingelfinger, Hamel, Inhibitors of the reninangiotensin-aldosterone system and Covid-19, N Engl J Med
Jing, Wu, Li, Metformin improves obesity-associated inflammation by altering macrophages polarization, Mol Cell Endocrinol, doi:10.1016/j.mce.2017.09.025
Khan, Benthin, Zeno, A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome, Crit Care, doi:10.1186/s13054-017-1823-x
Liu, Xiao, Wei, Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2, J Med Virol, doi:10.1002/jmv.25726
Lopes, Macedo, De, Silva, Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.25864
Luo, Qiu, Liu, Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis, Am J Trop Med Hyg, doi:10.4269/ajtmh.20-0375
Mackey, King, Gurley, Risks and impact of angiotensinconverting enzyme inhibitors or angiotensin-receptor blockers on SARS-CoV-2 infection in adults: a living systematic review, Ann Intern Med, doi:10.7326/M20-1515
Magagnoli, Narendran, Pereira, Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19, medRxiv
Maher, Leon, Ryan, Beyond insulin resistance: innate immunity in nonalcoholic steatohepatitis, Hepatology, doi:10.1002/hep.22399
Malhotra, Hepokoski, Mccowen, ACE2, metformin, and COVID-19, iScience, doi:10.1016/j.isci.2020.101425
Merad, Martin, Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages, Nat Rev Immunol, doi:10.1038/s41577-020-0331-4
Monteil, Kwon, Prado, Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell, doi:10.1016/j.cell.2020.04.004
Netea, Rovina, Complex immune dysregulation in COVID-19 patients with severe respiratory failure, Cell Host Microbe, doi:10.1016/j.chom.2020.04.009
Rosa, Santos, Clinical trials on drug repositioning for COVID-19 treatment, Rev Panam Salud Publica, doi:10.26633/RPSP.2020.40
Selvaraj, Dapaah-Afriyie, Finn, Short-term dexamethasone in Sars-CoV-2 patients, R I Med J
Shereen, Khan, Kazmi, COVID-19 infection: origin, transmission, and characteristics of human coronaviruses, J Adv Res, doi:10.1016/j.jare.2020.03.005
Sodhi, Wohlford-Lenane, Yamaguchi, Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg 9 bradykinin/ BKB1R axis and facilitates LPS-induced neutrophil infiltration, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00498.2016
Vaduganathan, Vardeny, Michel, Renin-angiotensinaldosterone system inhibitors in patients with Covid-19, N Engl J Med, doi:10.1056/NEJMsr2005760
Vickers, Hales, Kaushik, Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase, J Biol Chem, doi:10.1074/jbc.M200581200
Zou, Chen, Zou, Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection, Front Med, doi:10.1007/s11684-020-0754-0
Zou, Yan, Shu, Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections, Nat Commun, doi:10.1038/ncomms4594
DOI record:
{
"DOI": "10.1136/bmjopen-2021-050051",
"ISSN": [
"2044-6055",
"2044-6055"
],
"URL": "http://dx.doi.org/10.1136/bmjopen-2021-050051",
"abstract": "<jats:sec><jats:title>Objectives</jats:title><jats:p>SARS-CoV-2 enters cells using the ACE2 receptor. Medications that affect ACE2 expression or function such as angiotensin receptor blockers (ARBs) and ACE inhibitors (ACE-I) and metformin have the potential to counter the dysregulation of ACE2 by the virus and protect against viral injury. Here, we describe COVID-19 survival associated with ACE-I, ARB and metformin use.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>This is a hospital-based observational study of patients with COVID-19 infection using logistic regression with correction for pre-existing conditions and propensity score weighted Cox proportional hazards models to estimate associations between medication use and mortality.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>Medical record data from the US Veterans Affairs (VA) were used to identify patients with a reverse transcription PCR diagnosis of COVID-19 infection, to classify patterns of ACE inhibitors (ACE-I), ARB, beta blockers, metformin, famotidine and remdesivir use, and, to capture mortality.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>9532 hospitalised patients with COVID-19 infection followed for 60 days were analysed.</jats:p></jats:sec><jats:sec><jats:title>Outcome measure</jats:title><jats:p>Death from any cause within 60 days of COVID-19 diagnosis was examined.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Discontinuation of ACE-I was associated with increased risk of death (OR: 1.4; 95% CI 1.2–1.7). Initiating (OR: 0.3; 95% CI 0.2–0.5) or continuous (OR: 0.6; 95% CI 0.5–0.7) ACE-I was associated with reduced risk of death. ARB and metformin associations were similar in direction and magnitude and also statistically significant. Results were unchanged when accounting for pre-existing morbidity and propensity score adjustment.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Recent randomised clinical trials support the safety of continuing ACE-I and ARB treatment in patients with COVID-19 where indicated. Our study extends these findings to suggest a possible COVID-19 survival benefit for continuing or initiating ACE-I, ARB and metformin medications. Randomised trials are appropriate to confirm or refute the therapeutic potential for ACE-I, ARBs and metformin.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmjopen-2021-050051"
],
"author": [
{
"affiliation": [],
"family": "Wallace",
"given": "Arthur W",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-4571-4544",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cirillo",
"given": "Piera M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ryan",
"given": "James C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krigbaum",
"given": "Nickilou Y",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badathala",
"given": "Anusha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohn",
"given": "Barbara A",
"sequence": "additional"
}
],
"container-title": [
"BMJ Open"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
31
]
],
"date-time": "2021-12-31T15:30:28Z",
"timestamp": 1640964628000
},
"deposited": {
"date-parts": [
[
2021,
12,
31
]
],
"date-time": "2021-12-31T15:30:42Z",
"timestamp": 1640964642000
},
"funder": [
{
"award": [
"#2207"
],
"name": "Mercatus Center, George Mason University Fast Grants"
},
{
"award": [
"R00RG3118"
],
"name": "UC Office of the President, Emergency COVID-19 Research Seed Funding"
},
{
"award": [
"#2005"
],
"name": "Mercatus Center, George Mason University Fast Grants"
}
],
"indexed": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T05:55:33Z",
"timestamp": 1641016533940
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2044-6055"
},
{
"type": "electronic",
"value": "2044-6055"
}
],
"issue": "12",
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2021,
12,
31
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 29,
"start": {
"date-parts": [
[
2021,
12,
30
]
],
"date-time": "2021-12-30T00:00:00Z",
"timestamp": 1640822400000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjopen-2021-050051",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e050051",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2021,
12
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
31
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1016/j.jare.2020.03.005",
"article-title": "COVID-19 infection: origin, transmission, and characteristics of human coronaviruses",
"author": "Shereen",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "J Adv Res",
"key": "2021123107251020000_11.12.e050051.1",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1002/path.1570",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.2"
},
{
"article-title": "Short-term dexamethasone in Sars-CoV-2 patients",
"author": "Selvaraj",
"first-page": "39",
"journal-title": "R I Med J",
"key": "2021123107251020000_11.12.e050051.3",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.1056/NE-JMoa2007016",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.4"
},
{
"DOI": "10.1136/gutjnl-2020-321852",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.5"
},
{
"DOI": "10.26434/chemrxiv.12382265",
"doi-asserted-by": "crossref",
"key": "2021123107251020000_11.12.e050051.6",
"unstructured": "Sen Gupta PS , Biswal S , Singha D . Binding insight of clinically oriented drug famotidine with the identified potential target of SARS-CoV-2. J Biomol Struct Dyn 2020:1–7."
},
{
"DOI": "10.1053/j.gastro.2020.05.053",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.7"
},
{
"DOI": "10.1101/2020.04.16.20065920",
"doi-asserted-by": "crossref",
"key": "2021123107251020000_11.12.e050051.8",
"unstructured": "Magagnoli J , Narendran S , Pereira F . Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv 2020;2020.04.16.20065920."
},
{
"DOI": "10.1016/j.tmaid.2020.101647",
"doi-asserted-by": "crossref",
"key": "2021123107251020000_11.12.e050051.9",
"unstructured": "Cao YC , Deng QX , Dai SX . Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med Infect Dis 2020;101647."
},
{
"DOI": "10.1056/nejmsr2005760",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.10"
},
{
"DOI": "10.4269/ajtmh.20-0375",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.11"
},
{
"DOI": "10.3414/me17-01-0051",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.12"
},
{
"DOI": "10.1161/01.RES.87.5.e1",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.13"
},
{
"DOI": "10.1007/s11684-020-0754-0",
"article-title": "Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection",
"author": "Zou",
"doi-asserted-by": "crossref",
"first-page": "185",
"journal-title": "Front Med",
"key": "2021123107251020000_11.12.e050051.14",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1155/2012/307315",
"article-title": "Angiotensin-converting enzyme 2: the first decade",
"author": "Clarke",
"doi-asserted-by": "crossref",
"journal-title": "Int J Hypertens",
"key": "2021123107251020000_11.12.e050051.15",
"volume": "2012",
"year": "2012"
},
{
"DOI": "10.1152/ajplung.00498.2016",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.16"
},
{
"DOI": "10.1002/jmv.25726",
"article-title": "Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "595",
"journal-title": "J Med Virol",
"key": "2021123107251020000_11.12.e050051.17",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1038/ncomms4594",
"article-title": "Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections",
"author": "Zou",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "2021123107251020000_11.12.e050051.18",
"volume": "5",
"year": "2014"
},
{
"article-title": "Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus",
"author": "Gu",
"journal-title": "Sci Rep",
"key": "2021123107251020000_11.12.e050051.19",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1074/jbc.M200581200",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.20"
},
{
"DOI": "10.1186/s13054-017-1823-x",
"article-title": "A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome",
"author": "Khan",
"doi-asserted-by": "crossref",
"journal-title": "Crit Care",
"key": "2021123107251020000_11.12.e050051.21",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.1038/nature00786",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.22"
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"article-title": "Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2",
"author": "Monteil",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "Cell",
"key": "2021123107251020000_11.12.e050051.23",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1056/NEJMe2012924",
"doi-asserted-by": "crossref",
"key": "2021123107251020000_11.12.e050051.24",
"unstructured": "Jarcho JA , Ingelfinger JR , Hamel MB . Inhibitors of the renin-angiotensin-aldosterone system and Covid-19. N Engl J Med 2020."
},
{
"DOI": "10.1016/S2213-2600(20)30558-0",
"article-title": "Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "275",
"journal-title": "Lancet Respir Med",
"key": "2021123107251020000_11.12.e050051.25",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.25864",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.26"
},
{
"DOI": "10.7326/M20-1515",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.27"
},
{
"DOI": "10.1002/hep.22399",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.28"
},
{
"DOI": "10.1038/s41577-020-0331-4",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.29"
},
{
"DOI": "10.1016/j.chom.2020.04.009",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.30"
},
{
"DOI": "10.1016/j.mce.2017.09.025",
"article-title": "Metformin improves obesity-associated inflammation by altering macrophages polarization",
"author": "Jing",
"doi-asserted-by": "crossref",
"first-page": "256",
"journal-title": "Mol Cell Endocrinol",
"key": "2021123107251020000_11.12.e050051.31",
"volume": "461",
"year": "2018"
},
{
"DOI": "10.1016/j.isci.2020.101425",
"article-title": "ACE2, metformin, and COVID-19",
"author": "Malhotra",
"doi-asserted-by": "crossref",
"journal-title": "iScience",
"key": "2021123107251020000_11.12.e050051.32",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.26633/RPSP.2020.40",
"article-title": "Clinical trials on drug repositioning for COVID-19 treatment",
"author": "Rosa",
"doi-asserted-by": "crossref",
"journal-title": "Rev Panam Salud Publica",
"key": "2021123107251020000_11.12.e050051.33",
"volume": "44",
"year": "2020"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"score": 1,
"short-container-title": [
"BMJ Open"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Association of the patterns of use of medications with mortality of COVID-19 infection: a hospital-based observational study"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "11"
}