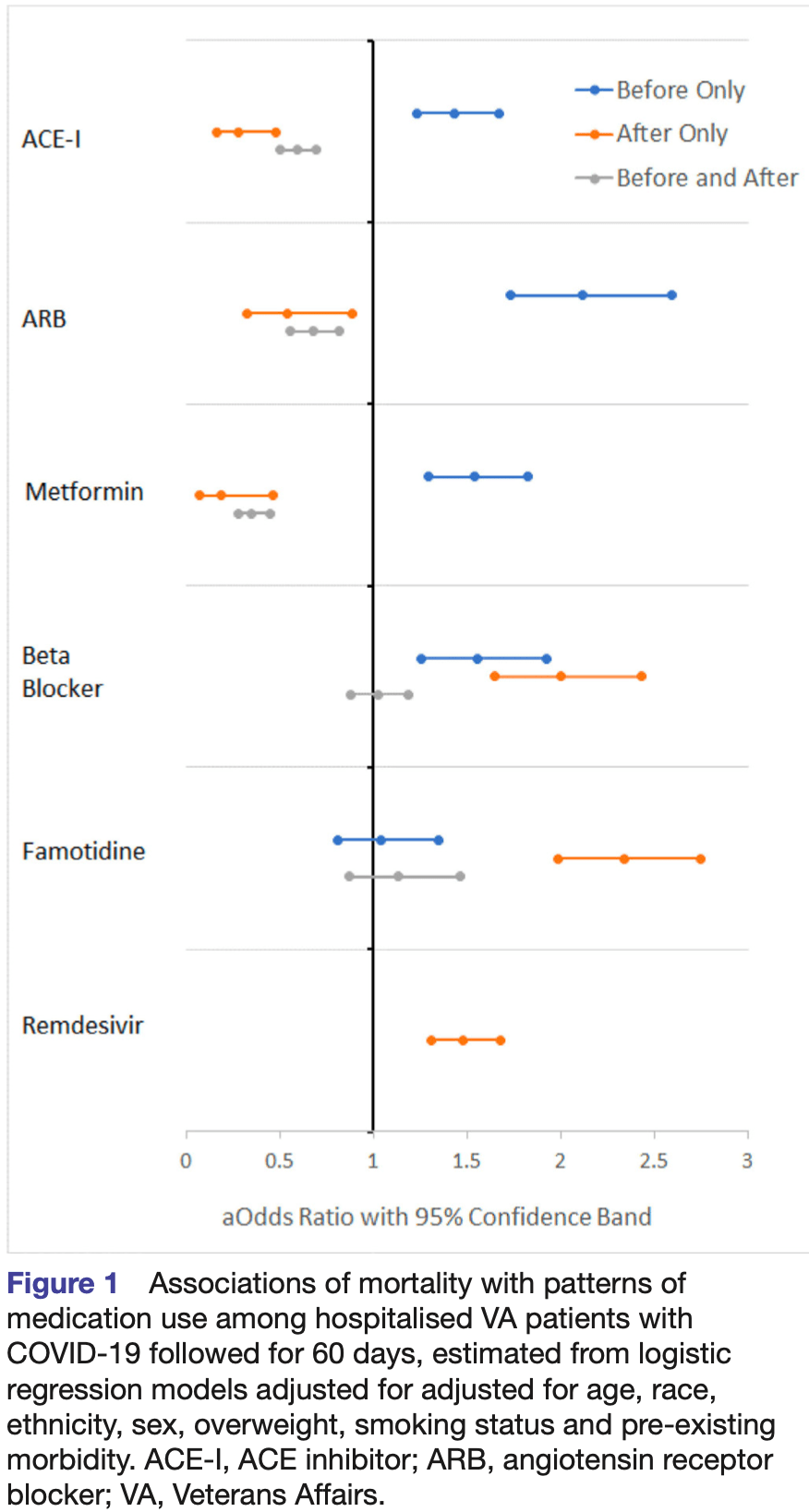
Association of the patterns of use of medications with mortality of COVID-19 infection: a hospital-based observational study
et al., BMJ Open, doi:10.1136/bmjopen-2021-050051, Dec 2021
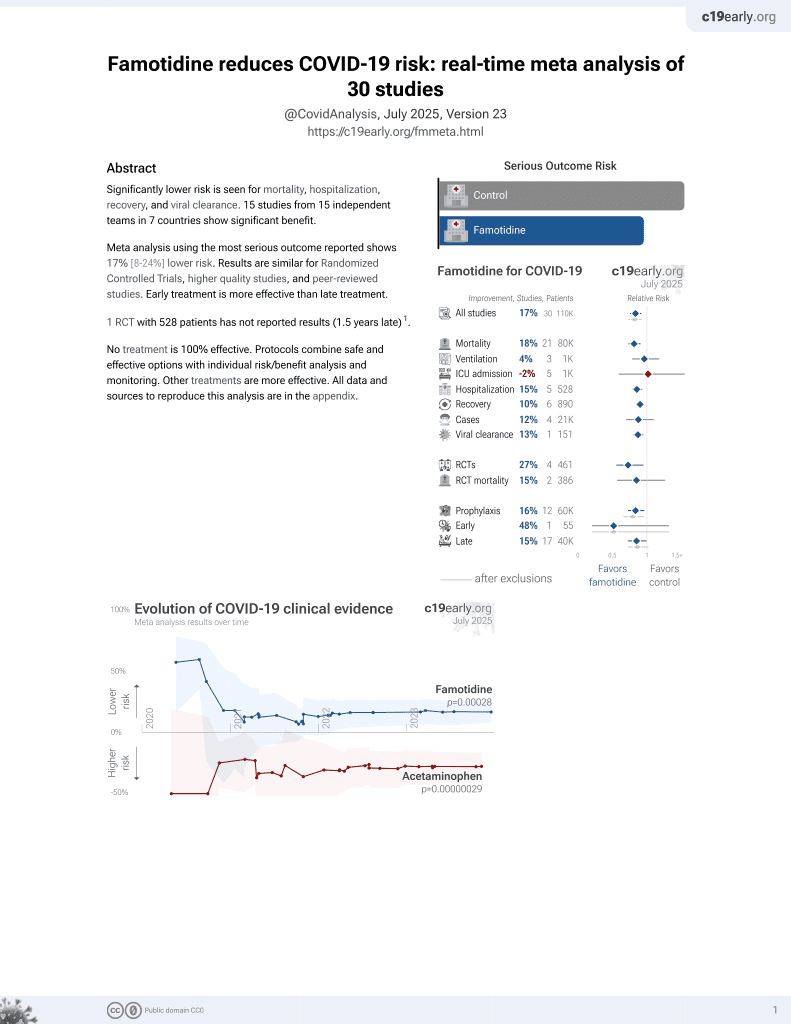
Famotidine for COVID-19
29th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 9,532 hospitalized COVID+ veterans in the USA, showing no significant difference in mortality with famotidine use. The study provides results for use before, after, and before+after. Before+after should more accurately represent prophylaxis up to COVID-19 infection (and continued use). Before included use up to 2 years before, and after included use up to 60 days later.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers metformin and famotidine.
|
risk of death, 11.0% higher, RR 1.11, p = 0.33, treatment 98 of 423 (23.2%), control 1,436 of 7,521 (19.1%), adjusted per study, odds ratio converted to relative risk, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Wallace et al., 31 Dec 2021, retrospective, database analysis, USA, peer-reviewed, 6 authors.
DOI record:
{
"DOI": "10.1136/bmjopen-2021-050051",
"ISSN": [
"2044-6055",
"2044-6055"
],
"URL": "http://dx.doi.org/10.1136/bmjopen-2021-050051",
"abstract": "<jats:sec><jats:title>Objectives</jats:title><jats:p>SARS-CoV-2 enters cells using the ACE2 receptor. Medications that affect ACE2 expression or function such as angiotensin receptor blockers (ARBs) and ACE inhibitors (ACE-I) and metformin have the potential to counter the dysregulation of ACE2 by the virus and protect against viral injury. Here, we describe COVID-19 survival associated with ACE-I, ARB and metformin use.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>This is a hospital-based observational study of patients with COVID-19 infection using logistic regression with correction for pre-existing conditions and propensity score weighted Cox proportional hazards models to estimate associations between medication use and mortality.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>Medical record data from the US Veterans Affairs (VA) were used to identify patients with a reverse transcription PCR diagnosis of COVID-19 infection, to classify patterns of ACE inhibitors (ACE-I), ARB, beta blockers, metformin, famotidine and remdesivir use, and, to capture mortality.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>9532 hospitalised patients with COVID-19 infection followed for 60 days were analysed.</jats:p></jats:sec><jats:sec><jats:title>Outcome measure</jats:title><jats:p>Death from any cause within 60 days of COVID-19 diagnosis was examined.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Discontinuation of ACE-I was associated with increased risk of death (OR: 1.4; 95% CI 1.2–1.7). Initiating (OR: 0.3; 95% CI 0.2–0.5) or continuous (OR: 0.6; 95% CI 0.5–0.7) ACE-I was associated with reduced risk of death. ARB and metformin associations were similar in direction and magnitude and also statistically significant. Results were unchanged when accounting for pre-existing morbidity and propensity score adjustment.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Recent randomised clinical trials support the safety of continuing ACE-I and ARB treatment in patients with COVID-19 where indicated. Our study extends these findings to suggest a possible COVID-19 survival benefit for continuing or initiating ACE-I, ARB and metformin medications. Randomised trials are appropriate to confirm or refute the therapeutic potential for ACE-I, ARBs and metformin.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmjopen-2021-050051"
],
"author": [
{
"affiliation": [],
"family": "Wallace",
"given": "Arthur W",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-4571-4544",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cirillo",
"given": "Piera M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ryan",
"given": "James C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krigbaum",
"given": "Nickilou Y",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badathala",
"given": "Anusha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohn",
"given": "Barbara A",
"sequence": "additional"
}
],
"container-title": [
"BMJ Open"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
31
]
],
"date-time": "2021-12-31T15:30:28Z",
"timestamp": 1640964628000
},
"deposited": {
"date-parts": [
[
2021,
12,
31
]
],
"date-time": "2021-12-31T15:30:42Z",
"timestamp": 1640964642000
},
"funder": [
{
"award": [
"#2207"
],
"name": "Mercatus Center, George Mason University Fast Grants"
},
{
"award": [
"R00RG3118"
],
"name": "UC Office of the President, Emergency COVID-19 Research Seed Funding"
},
{
"award": [
"#2005"
],
"name": "Mercatus Center, George Mason University Fast Grants"
}
],
"indexed": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T05:55:33Z",
"timestamp": 1641016533940
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2044-6055"
},
{
"type": "electronic",
"value": "2044-6055"
}
],
"issue": "12",
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2021,
12,
31
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 29,
"start": {
"date-parts": [
[
2021,
12,
30
]
],
"date-time": "2021-12-30T00:00:00Z",
"timestamp": 1640822400000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjopen-2021-050051",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e050051",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2021,
12
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
31
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1016/j.jare.2020.03.005",
"article-title": "COVID-19 infection: origin, transmission, and characteristics of human coronaviruses",
"author": "Shereen",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "J Adv Res",
"key": "2021123107251020000_11.12.e050051.1",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1002/path.1570",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.2"
},
{
"article-title": "Short-term dexamethasone in Sars-CoV-2 patients",
"author": "Selvaraj",
"first-page": "39",
"journal-title": "R I Med J",
"key": "2021123107251020000_11.12.e050051.3",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.1056/NE-JMoa2007016",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.4"
},
{
"DOI": "10.1136/gutjnl-2020-321852",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.5"
},
{
"DOI": "10.26434/chemrxiv.12382265",
"doi-asserted-by": "crossref",
"key": "2021123107251020000_11.12.e050051.6",
"unstructured": "Sen Gupta PS , Biswal S , Singha D . Binding insight of clinically oriented drug famotidine with the identified potential target of SARS-CoV-2. J Biomol Struct Dyn 2020:1–7."
},
{
"DOI": "10.1053/j.gastro.2020.05.053",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.7"
},
{
"DOI": "10.1101/2020.04.16.20065920",
"doi-asserted-by": "crossref",
"key": "2021123107251020000_11.12.e050051.8",
"unstructured": "Magagnoli J , Narendran S , Pereira F . Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv 2020;2020.04.16.20065920."
},
{
"DOI": "10.1016/j.tmaid.2020.101647",
"doi-asserted-by": "crossref",
"key": "2021123107251020000_11.12.e050051.9",
"unstructured": "Cao YC , Deng QX , Dai SX . Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med Infect Dis 2020;101647."
},
{
"DOI": "10.1056/nejmsr2005760",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.10"
},
{
"DOI": "10.4269/ajtmh.20-0375",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.11"
},
{
"DOI": "10.3414/me17-01-0051",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.12"
},
{
"DOI": "10.1161/01.RES.87.5.e1",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.13"
},
{
"DOI": "10.1007/s11684-020-0754-0",
"article-title": "Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection",
"author": "Zou",
"doi-asserted-by": "crossref",
"first-page": "185",
"journal-title": "Front Med",
"key": "2021123107251020000_11.12.e050051.14",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1155/2012/307315",
"article-title": "Angiotensin-converting enzyme 2: the first decade",
"author": "Clarke",
"doi-asserted-by": "crossref",
"journal-title": "Int J Hypertens",
"key": "2021123107251020000_11.12.e050051.15",
"volume": "2012",
"year": "2012"
},
{
"DOI": "10.1152/ajplung.00498.2016",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.16"
},
{
"DOI": "10.1002/jmv.25726",
"article-title": "Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "595",
"journal-title": "J Med Virol",
"key": "2021123107251020000_11.12.e050051.17",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1038/ncomms4594",
"article-title": "Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections",
"author": "Zou",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "2021123107251020000_11.12.e050051.18",
"volume": "5",
"year": "2014"
},
{
"article-title": "Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus",
"author": "Gu",
"journal-title": "Sci Rep",
"key": "2021123107251020000_11.12.e050051.19",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1074/jbc.M200581200",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.20"
},
{
"DOI": "10.1186/s13054-017-1823-x",
"article-title": "A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome",
"author": "Khan",
"doi-asserted-by": "crossref",
"journal-title": "Crit Care",
"key": "2021123107251020000_11.12.e050051.21",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.1038/nature00786",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.22"
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"article-title": "Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2",
"author": "Monteil",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "Cell",
"key": "2021123107251020000_11.12.e050051.23",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1056/NEJMe2012924",
"doi-asserted-by": "crossref",
"key": "2021123107251020000_11.12.e050051.24",
"unstructured": "Jarcho JA , Ingelfinger JR , Hamel MB . Inhibitors of the renin-angiotensin-aldosterone system and Covid-19. N Engl J Med 2020."
},
{
"DOI": "10.1016/S2213-2600(20)30558-0",
"article-title": "Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "275",
"journal-title": "Lancet Respir Med",
"key": "2021123107251020000_11.12.e050051.25",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.25864",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.26"
},
{
"DOI": "10.7326/M20-1515",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.27"
},
{
"DOI": "10.1002/hep.22399",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.28"
},
{
"DOI": "10.1038/s41577-020-0331-4",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.29"
},
{
"DOI": "10.1016/j.chom.2020.04.009",
"doi-asserted-by": "publisher",
"key": "2021123107251020000_11.12.e050051.30"
},
{
"DOI": "10.1016/j.mce.2017.09.025",
"article-title": "Metformin improves obesity-associated inflammation by altering macrophages polarization",
"author": "Jing",
"doi-asserted-by": "crossref",
"first-page": "256",
"journal-title": "Mol Cell Endocrinol",
"key": "2021123107251020000_11.12.e050051.31",
"volume": "461",
"year": "2018"
},
{
"DOI": "10.1016/j.isci.2020.101425",
"article-title": "ACE2, metformin, and COVID-19",
"author": "Malhotra",
"doi-asserted-by": "crossref",
"journal-title": "iScience",
"key": "2021123107251020000_11.12.e050051.32",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.26633/RPSP.2020.40",
"article-title": "Clinical trials on drug repositioning for COVID-19 treatment",
"author": "Rosa",
"doi-asserted-by": "crossref",
"journal-title": "Rev Panam Salud Publica",
"key": "2021123107251020000_11.12.e050051.33",
"volume": "44",
"year": "2020"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"score": 1,
"short-container-title": [
"BMJ Open"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Association of the patterns of use of medications with mortality of COVID-19 infection: a hospital-based observational study"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "11"
}