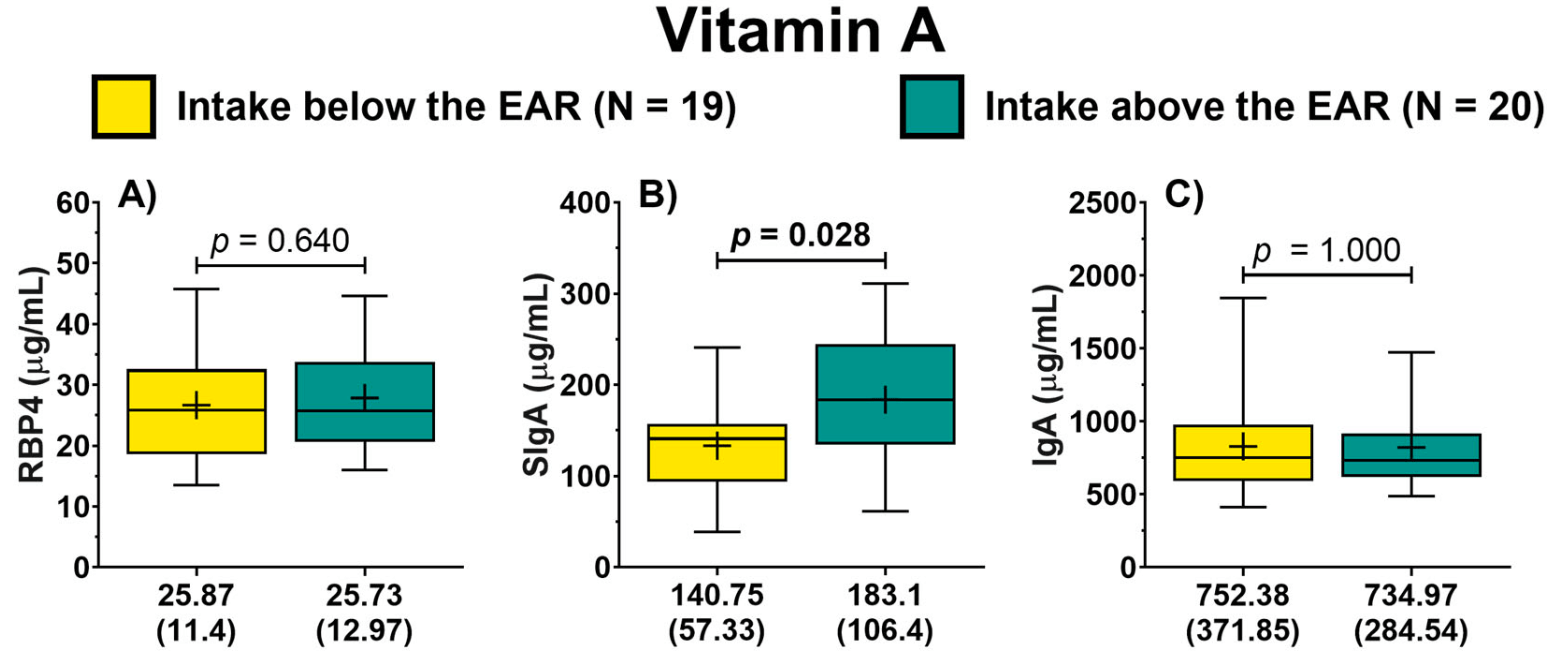
Vitamin A Positively Correlates with Secretory Immunoglobulin A: A Cross-Sectional Study in Omicron COVID-19 Outpatients
et al., Journal of Clinical Medicine, doi:10.3390/jcm13061538, Mar 2024
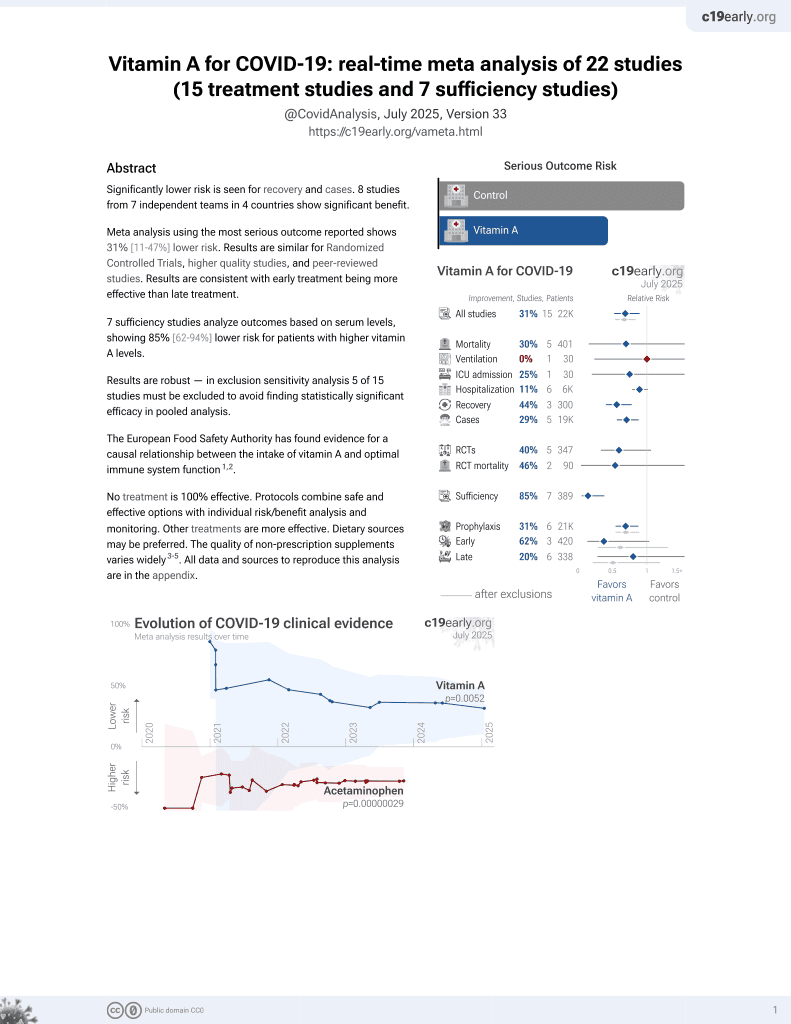
Vitamin A for COVID-19
49th treatment shown to reduce risk in
May 2023, now with p = 0.004 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Analysis of 39 COVID-19 outpatients showing a positive correlation between vitamin A nutritional status and secretory immunoglobulin A (SIgA) levels. Patients with higher dietary vitamin A intake showed higher SIgA/IgG1 and SIgA/IgG3 ratios, while those with higher retinol-binding protein 4 (RBP4) levels showed higher SIgA/IgM, SIgA/IgG1, and SIgA/IgG2 ratios. The authors suggest maintaining optimal vitamin A levels may be important for preventing viral infections.
Turrubiates-Hernández et al., 7 Mar 2024, peer-reviewed, 11 authors.
Vitamin A Positively Correlates with Secretory Immunoglobulin A: A Cross-Sectional Study in Omicron COVID-19 Outpatients
Journal of Clinical Medicine, doi:10.3390/jcm13061538
Background: Respiratory tract infections remain among the leading causes of mortality worldwide. The COVID-19 pandemic has highlighted the importance of mucosal immunity in defending against infectious agents. Vitamin A is known to influence the production of secretory immunoglobulin A (SIgA) predominantly in the gut, where it is a critical component of the first line of defense on mucosal surfaces. Methods: This cross-sectional study, conducted 14 days post-positive COVID-19 diagnosis, aimed to determine the relationship between the nutritional status of vitamin A and SIgA levels in COVID-19 outpatients. Serum and saliva samples were collected. Vitamin A nutritional status was determined based on the assessment of dietary intake and the analysis of retinol-binding protein 4 (RBP4). SIgA levels were analyzed from salivary samples. In addition, serum antibodies were analyzed. Results: Dietary vitamin A intake and RBP4 levels positively correlated with SIgA. Patients with higher vitamin A intake showed higher SIgA/IgG1 and SIgA/IgG3 ratios, while those with higher RBP4 levels showed higher SIgA/IgM, SIgA/IgG1, and SIgA/IgG2 ratios. Conclusions: These findings underscore a significant correlation between vitamin A nutritional status and SIgA levels in COVID-19 outpatients, which may suggest the potential importance of maintaining optimal vitamin A levels for the prevention of viral infections.
Conflicts of Interest: The authors have no relevant financial or non-financial interests to disclose.
References
Abdelkader, Wahba, El-Tonsy, Zewail, Shams Eldin, Recurrent Respiratory Infections and Vitamin A Levels: A Link? It Is Cross-Sectional, Medicine, doi:10.1097/MD.0000000000030108
Almoosawi, Palla, Association between Vitamin Intake and Respiratory Complaints in Adults from the UK National Diet and Nutrition Survey Years 1-8, BMJ Nutr. Prev. Health, doi:10.1136/bmjnph-2020-000150
Amimo, Michael, Chepngeno, Raev, Saif et al., Immune Impairment Associated with Vitamin A Deficiency: Insights from Clinical Studies and Animal Model Research, Nutrients, doi:10.3390/nu14235038
Bemark, Angeletti, Know Your Enemy or Find Your Friend?-Induction of IgA at Mucosal Surfaces, Immunol. Rev, doi:10.1111/imr.13014
Bonanni, Ceddia, Dawson, A Call to Action: Current Challenges and Considerations for COVID-19 Vaccination in Immunocompromised Populations, J. Infect. Dis, doi:10.1093/infdis/jiad150
Bos, Van Egmond, Mebius, The Role of Retinoic Acid in the Production of Immunoglobulin A, Mucosal Immunol, doi:10.1038/s41385-022-00509-8
Calderaro, Buttrini, Farina, Montecchini, De Conto et al., Respiratory Tract Infections and Laboratory Diagnostic Methods: A Review with A Focus on Syndromic Panel-Based Assays, Microorganisms, doi:10.3390/microorganisms10091856
Carazo, Macáková, Matoušová, Krčmová, Protti et al., Vitamin A Update: Forms, Sources, Kinetics, Detection, Function, Deficiency, Therapeutic Use and Toxicity, Nutrients, doi:10.3390/nu13051703
Cui, Stephensen, Moldoveanu, High-Level Dietary Vitamin A Enhances T-Helper Type 2 Cytokine Production and Secretory Immunoglobulin A Response to Influenza A Virus Infection in BALB/c Mice, J. Nutr, doi:10.1093/jn/130.5.1132
Czerkinsky, Svennerholm, Quiding, Jonsson, Holmgren, Antibody-Producing Cells in Peripheral Blood and Salivary Glands after Oral Cholera Vaccination of Humans, Infect. Immun, doi:10.1128/iai.59.3.996-1001.1991
De Pee, Dary, Biochemical Indicators of Vitamin A Deficiency: Serum Retinol and Serum Retinol Binding Protein, J. Nutr, doi:10.1093/jn/132.9.2895S
González-Estevez, Turrubiates-Hernández, Herrera-Jiménez, Sánchez-Zuno, Herrera-Godina et al., Association of Food Intake Quality with Vitamin D in SARS-CoV-2 Positive Patients from Mexico: A Cross-Sectional Study, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph18147266
Grewal, Pilgrim, Grewal, Kasman, Werner et al., Salivary Glands Act as Mucosal Inductive Sites via the Formation of Ectopic Germinal Centers after Site-restricted MCMV Infection, FASEB J, doi:10.1096/fj.10-174656
Huiming, Chaomin, Meng, Vitamin A for Treating Measles in Children
Isho, Abe, Zuo, Jamal, Rathod et al., Persistence of Serum and Saliva Antibody Responses to SARS-CoV-2 Spike Antigens in COVID-19 Patients, Sci. Immunol, doi:10.1126/sciimmunol.abe5511
Jackson, Lally, Nakamura, Montgomery, Migration of IgA-Bearing Lymphocytes into Salivary Glands, Cell. Immunol, doi:10.1016/0008-8749(81)90042-3
Jin, Ren, Li, Gao, Zhang et al., Global Burden of Upper Respiratory Infections in 204 Countries and Territories, from 1990 to 2019, eClinicalMedicine, doi:10.1016/j.eclinm.2021.100986
Jones, Oshansky, Bajracharya, Tang, Sun et al., Retinol Binding Protein and Vitamin D Associations with Serum Antibody Isotypes, Serum Influenza Virus-Specific Neutralizing Activities and Airway Cytokine Profiles, Clin. Exp. Immunol, doi:10.1111/cei.12718
Kirtana, Kumar, Kumar, Singh, Shankar et al., Mild COVID-19 Infection-Predicting Symptomatic Phase and Outcome: A Study from AIIMS, New Delhi, J. Fam. Med. Prim. Care, doi:10.4103/jfmpc.jfmpc_1610_20
Kunkel, Butcher, Plasma-Cell Homing, Nat. Rev. Immunol, doi:10.1038/nri1203
Li, Jin, Chen, The Effects of Secretory IgA in the Mucosal Immune System, BioMed Res. Int, doi:10.1155/2020/2032057
Lisulo, Kapulu, Banda, Sinkala, Kayamba et al., Adjuvant Potential of Low Dose All-Trans Retinoic Acid during Oral Typhoid Vaccination in Zambian Men, Clin. Exp. Immunol, doi:10.1111/cei.12238
Liu, Li, Liu, Liang, Wang et al., Longitudinal Characteristics of Lymphocyte Responses and Cytokine Profiles in the Peripheral Blood of SARS-CoV-2 Infected Patients, eBioMedicine, doi:10.1016/j.ebiom.2020.102763
Ma, Ross, The Anti-Tetanus Immune Response of Neonatal Mice Is Augmented by Retinoic Acid Combined with Polyriboinosinic:Polyribocytidylic Acid, Proc. Natl. Acad. Sci, doi:10.1073/pnas.0506438102
Macedo-Ojeda, Muñoz-Valle, Yokogawa-Teraoka, Machado-Sulbarán, Loza-Rojas et al., COVID-19 Screening by Anti-SARS-CoV-2 Antibody Seropositivity: Clinical and Epidemiological Characteristics, Comorbidities, and Food Intake Quality, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph18178995
Machin, Campbell, Tan, Tan, Sample Size Tables for Clinical Studies
Marrón-Ponce, Sánchez-Pimienta, Rodríguez-Ramírez, Batis, Cediel, Ultra-processed Foods Consumption Reduces Dietary Diversity and Micronutrient Intake in the Mexican Population, J. Hum. Nutr. Diet, doi:10.1111/jhn.13003
Martini, Godos, Bonaccio, Vitaglione, Grosso, Ultra-Processed Foods and Nutritional Dietary Profile: A Meta-Analysis of Nationally Representative Samples, Nutrients, doi:10.3390/nu13103390
Midha, Kumar, Kumar, Madan, Mega Doses of Retinol: A Possible Immunomodulation in COVID-19 Illness in Resource-limited Settings, Rev. Med. Virol, doi:10.1002/rmv.2204
Mohd, Ahmad Norudin, Muhammad, Transcriptional Regulation of Retinol Binding Protein 4 by Interleukin-6 via Peroxisome Proliferator-Activated Receptor α and CCAAT/Enhancer Binding Proteins, Mol. Cell. Endocrinol, doi:10.1016/j.mce.2020.110702
Muñoz-Valle, Venancio-Landeros, Sánchez-Sánchez, Reyes-Díaz, Galindo-Ornelas et al., An Upgrade on the Surveillance System of SARS-CoV-2: Deployment of New Methods for Genetic Inspection, Int. J. Mol. Sci, doi:10.3390/ijms23063143
Patel, Penkert, Sealy, Surman, Jones et al., Retinol Binding Protein, Sunlight Hours, and the Influenza Virus-Specific Immune Response, Biomedicines, doi:10.3390/biomedicines10092322
Pedroza-Tobías, Hernández-Barrera, López-Olmedo, García-Guerra, Rodríguez-Ramírez et al., Usual Vitamin Intakes by Mexican Populations, J. Nutr, doi:10.3945/jn.115.219162
Polato Glu, Oncu-Oner, Dalman, Ozdogan, COVID-19 in Early 2023: Structure, Replication Mechanism, Variants of SARS-CoV-2, Diagnostic Tests, and Vaccine & Drug Development Studies, MedComm, doi:10.1002/mco2.228
Popkin, Ng, The Nutrition Transition to a Stage of High Obesity and Noncommunicable Disease Prevalence Dominated by Ultra-processed Foods Is Not Inevitable, Obes. Rev, doi:10.1111/obr.13366
Ramírez-Silva, Rodríguez-Ramírez, Barragán-Vázquez, Castellanos-Gutiérrez, Reyes-García et al., Prevalence of Inadequate Intake of Vitamins and Minerals in the Mexican Population Correcting by Nutrient Retention Factors, Ensanut 2016, Salud Pública México, doi:10.21149/11096
Ross, Chen, Ma, Vitamin A and Retinoic Acid in the Regulation of B-Cell Development and Antibody Production, Vitamins & Hormones
Rudraraju, Jones, Surman, Sealy, Thomas et al., Respiratory Tract Epithelial Cells Express Retinaldehyde Dehydrogenase ALDH1A and Enhance IgA Production by Stimulated B Cells in the Presence of Vitamin A, PLoS ONE, doi:10.1371/journal.pone.0086554
Russell, Mestecky, Mucosal Immunity: The Missing Link in Comprehending SARS-CoV-2 Infection and Transmission, Front. Immunol, doi:10.3389/fimmu.2022.957107
Safiri, Mahmoodpoor, Kolahi, Nejadghaderi, Sullman et al., Global Burden of Lower Respiratory Infections during the Last Three Decades, Front. Public Health, doi:10.3389/fpubh.2022.1028525
Said, Amer, Sheta, Makled, Arafa et al., Nano-Encapsulated Antioxidant: Retinoic Acid as a Natural Mucosal Adjuvant for Intranasal Immunization against Chronic Experimental Toxoplasmosis, Trop. Med. Infect. Dis
Sano, Bhavsar, Singh, Floda, Srivastava et al., SARS-CoV-2 Vaccination Induces Mucosal Antibody Responses in Previously Infected Individuals, Nat. Commun, doi:10.1038/s41467-022-32389-8
Sheikh-Mohamed, Isho, Chao, Zuo, Cohen et al., Systemic and Mucosal IgA Responses Are Variably Induced in Response to SARS-CoV-2 mRNA Vaccination and Are Associated with Protection against Subsequent Infection, Mucosal Immunol, doi:10.1038/s41385-022-00511-0
Sirisinha, Darip, Moongkarndi, Ongsakul, Lamb, Impaired Local Immune Response in Vitamin A-Deficient Rats, Clin. Exp. Immunol
Su, Patel, Hu, Chen, Induction of Mucosal Immunity through Systemic Immunization: Phantom or Reality?, Hum. Vaccines Immunother, doi:10.1080/21645515.2015.1114195
Surman, Jones, Rudraraju, Sealy, Hurwitz, Intranasal Administration of Retinyl Palmitate with a Respiratory Virus Vaccine Corrects Impaired Mucosal IgA Response in the Vitamin A-Deficient Host, Clin. Vaccine Immunol, doi:10.1128/CVI.00757-13
Surman, Jones, Sealy, Rudraraju, Hurwitz, Oral Retinyl Palmitate or Retinoic Acid Corrects Mucosal IgA Responses toward an Intranasal Influenza Virus Vaccine in Vitamin A Deficient Mice, Vaccine, doi:10.1016/j.vaccine.2014.03.025
Surman, Penkert, Jones, Sealy, Hurwitz, Vitamin Supplementation at the Time of Immunization with a Cold-Adapted Influenza Virus Vaccine Corrects Poor Mucosal Antibody Responses in Mice Deficient for Vitamins A and D, Clin. Vaccine Immunol, doi:10.1128/CVI.00739-15
Tanumihardjo, Russell, Stephensen, Gannon, Craft et al., Biomarkers of Nutrition for Development (BOND)-Vitamin A Review, J. Nutr, doi:10.3945/jn.115.229708
Tepasse, Vollenberg, Fobker, Kabar, Schmidt et al., Vitamin A Plasma Levels in COVID-19 Patients: A Prospective Multicenter Study and Hypothesis, Nutrients, doi:10.3390/nu13072173
Velikova, Snegarova, Kukov, Batselova, Mihova et al., Gastrointestinal Mucosal Immunity and COVID-19, World J. Gastroenterol, doi:10.3748/wjg.v27.i30.5047
Vizmanos-Lamotte, López-Uriarte, Hunot-Alexander, Bernal-Orozco, Rodríguez-Rocha et al., Álbum Fotográfico de Alimentos Mexicanos, Editorial Pandora
Vollenberg, Tepasse, Fobker, Hüsing-Kabar, Significantly Reduced Retinol Binding Protein 4 (RBP4) Levels in Critically Ill COVID-19 Patients, Nutrients, doi:10.3390/nu14102007
Wang, Lorenzi, Muecksch, Finkin, Viant et al., Enhanced SARS-CoV-2 Neutralization by Dimeric IgA, Sci. Transl. Med, doi:10.1126/scitranslmed.abf1555
Willett, Howe, Kushi, Adjustment for Total Energy Intake in Epidemiologic Studies, Am. J. Clin. Nutr, doi:10.1093/ajcn/65.4.1220S
Zhao, Liu, Zhang, Zhao, Yu et al., Global Burden of Vitamin A Deficiency in 204 Countries and Territories from 1990-2019, Nutrients, doi:10.3390/nu14050950