IFN-γ-mediated control of SARS-CoV-2 infection through nitric oxide
et al., Frontiers in Immunology, doi:10.3389/fimmu.2023.1284148, Dec 2023
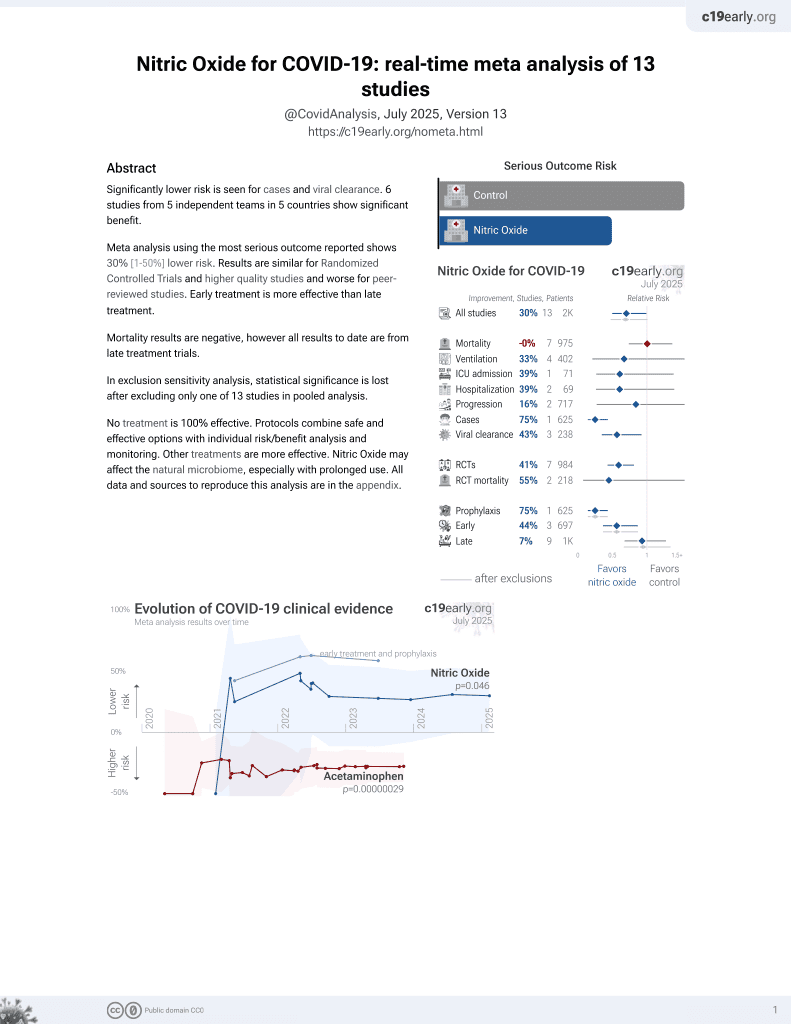
43rd treatment shown to reduce risk in
June 2022, now with p = 0.012 from 12 studies, recognized in 10 countries.
Lower risk for cases and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
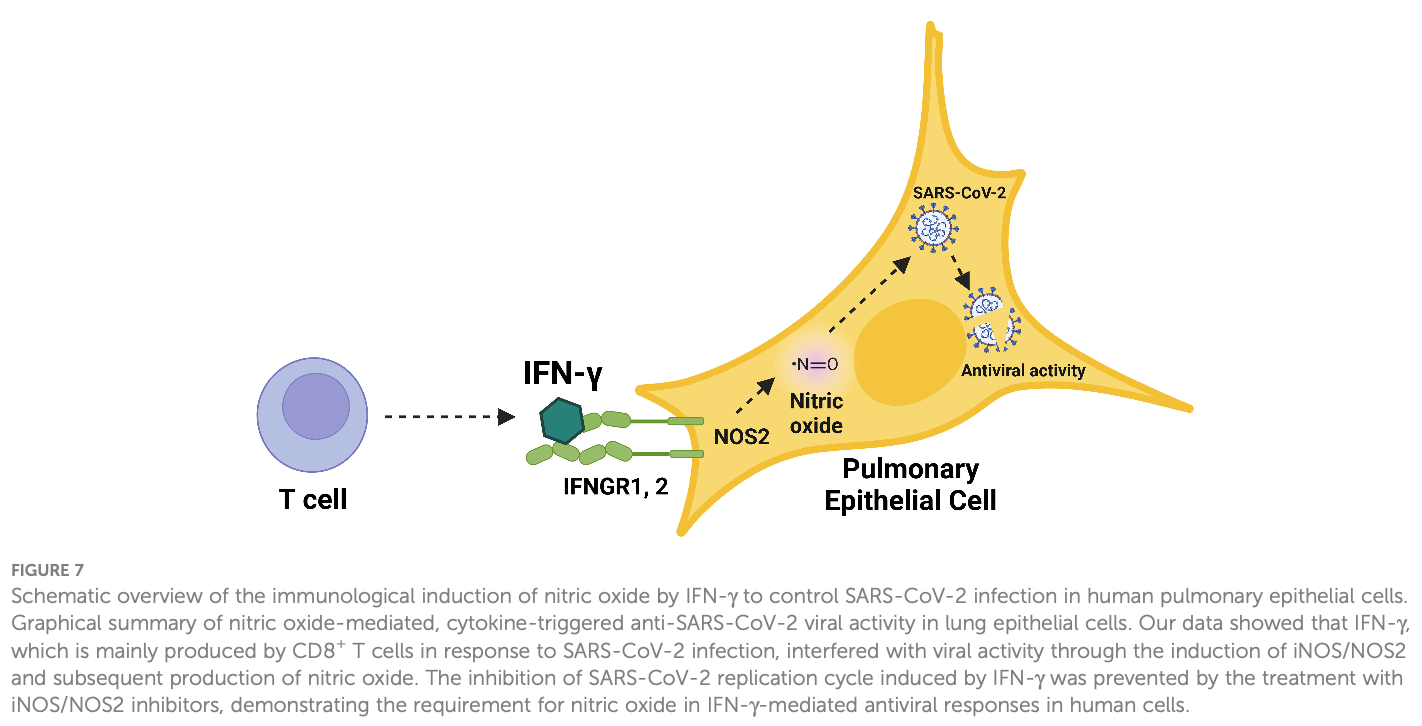
In vitro study showing that treatment with interferon-gamma (IFN-γ) inhibits SARS-CoV-2 infection in simian Vero E6 and human lung epithelial cell lines. The IFN-γ-triggered antiviral effect against SARS-CoV-2 is mediated through the endogenous production of nitric oxide (NO). IFN-γ alone or together with IL-1β induces NO production and reduces SARS-CoV-2 replication. Pharmacologic inhibition of NO production prevents the IFN-γ-induced antiviral activity. As IFN-γ is produced by CD8+ T cells in early response to SARS-CoV-2, these findings link the T cell-mediated adaptive immune response to a NO-dependent innate antiviral pathway in the host defense against SARS-CoV-2 infection. Authors believe that NO-mediated antiviral effects may be intracellular.
3 preclinical studies support the efficacy of nitric oxide for COVID-19:
1.
Martins et al., Broad-Spectrum Virucidal Activity of Nitric Oxide Nasal Spray (NONS) Against SARS-CoV-2 Variants and Major Respiratory Viruses, Viruses, doi:10.3390/v18010091.
Silva et al., 15 Dec 2023, USA, peer-reviewed, 9 authors.
Contact: rmodlin@mednet.ucla.edu.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
IFN-γ-mediated control of SARS-CoV-2 infection through nitric oxide
Frontiers in Immunology, doi:10.3389/fimmu.2023.1284148
Introduction: The COVID-19 pandemic has highlighted the need to identify mechanisms of antiviral host defense against SARS-CoV-2. One such mediator is interferon-g (IFN-g), which, when administered to infected patients, is reported to result in viral clearance and resolution of pulmonary symptoms. IFN-g treatment of a human lung epithelial cell line triggered an antiviral activity against SARS-CoV-2, yet the mechanism for this antiviral response was not identified. Methods: Given that IFN-g has been shown to trigger antiviral activity via the generation of nitric oxide (NO), we investigated whether IFN-g induction of antiviral activity against SARS-CoV-2 infection is dependent upon the generation of NO in human pulmonary epithelial cells. We treated the simian epithelial cell line Vero E6 and human pulmonary epithelial cell lines, including A549-ACE2, and Calu-3, with IFN-g and observed the resulting induction of NO and its effects on SARS-CoV-2 replication. Pharmacological inhibition of inducible nitric oxide synthase (iNOS) was employed to assess the dependency on NO production. Additionally, the study examined the effect of interleukin-1b (IL-1b) on the IFN-g-induced NO production and its antiviral efficacy. Results: Treatment of Vero E6 cells with IFN-g resulted in a dose-responsive induction of NO and an inhibitory effect on SARS-CoV-2 replication. This antiviral activity was blocked by pharmacologic inhibition of iNOS. IFN-g also triggered a NO-mediated antiviral activity in SARS-CoV-2 infected human lung epithelial cell lines A549-ACE2 and Calu-3. IL-1b enhanced IFN-g induction of NO, but it had little effect on antiviral activity. Discussion: Given that IFN-g has been shown to be produced by CD8+ T cells in the early response to SARS-CoV-2, our findings in human lung epithelial cell lines,
Ethics statement Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1284148/ full#supplementary-material
References
Abdelmassih, Hozaien, Shershaby, Kamel, Ismail et al., The potential role of inhaled nitric oxide for postexposure chemoprophylaxis of COVID-19, J Genet Eng Biotechnol, doi:10.1186/s43141-021-00249-5
Abou-Arab, Huette, Debouvries, Dupont, Jounieaux et al., Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study, Crit Care, doi:10.1186/s13054-020-03371-x
Adusumilli, Zhang, Friedman, Friedman, Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19, Nitric Oxide, doi:10.1016/j.niox.2020.07.003
Ahmed, Liu, Nawshad, Mechanisms of palatal epithelial seam disintegration by transforming growth factor (TGF) beta3, Dev Biol, doi:10.1016/j.ydbio.2007.06.018
Akaberi, Krambrich, Ling, Luni, Hedenstierna et al., Mitigation of the replication of SARS-CoV-2 by nitric oxide. vitro, Redox Biol, doi:10.1016/j.redox.2020.101734
Akerström, Mousavi-Jazi, Klingström, Leijon, Lundkvist et al., Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus, J Virol, doi:10.1128/JVI.79.3.1966-1969.2005
Alavi Darazam, Hatami, Rabiei, Pourhoseingholi, Shabani et al., An investigation into the beneficial effects of high-dose interferon beta 1-a, compared to low-dose interferon beta 1-a in severe COVID-19: The COVIFERON II randomized controlled trial, Int Immunopharmacol, doi:10.1016/j.intimp.2021.107916
Balin, Pellegrini, Klechevsky, Won, Weiss et al., Human antimicrobial cytotoxic T lymphocytes, defined by NK receptors and antimicrobial proteins, kill intracellular bacteria, Sci Immunol, doi:10.1126/sciimmunol.aat7668
Blanco-Melo, Nilsson-Payant, Liu, Uhl, Hoagland et al., Imbalanced host response to SARS-coV-2 drives development of COVID-19, Cell, doi:10.1016/j.cell.2020.04.026
Bogdan, Nitric oxide and the immune response, Nat Immunol, doi:10.1038/ni1001-907
Broggi, Ghosh, Sposito, Spreafico, Balzarini et al., Type III interferons disrupt the lung epithelial barrier upon viral recognition, Science, doi:10.1126/science.abc3545
Busnadiego, Fernbach, Pohl, Karakus, Huber et al., Antiviral activity of II, and III interferons counterbalances ACE2 inducibility and restricts SARS-coV-2, mBio, doi:10.1128/mBio.01928-20
Chang, Parsi, Somasundaran, Vanderleeden, Liu et al., A newly engineered A549 cell line expressing ACE2 and TMPRSS2 is highly permissive to SARS-coV-2, including the delta and omicron variants, Viruses, doi:10.3390/v14071369
Chu, Marks-Konczalik, Wu, Banks, Moss, Analysis of the cytokinestimulated human inducible nitric oxide synthase (iNOS) gene: characterization of differences between human and mouse iNOS promoters, Biochem Biophys Res Commun, doi:10.1006/bbrc.1998.9062
Cooper, Abdullatif, Burnett, Kempsell, Conforti et al., Long term culture of the A549 cancer cell line promotes multilamellar body formation and differentiation towards an alveolar type II pneumocyte phenotype, PloS One, doi:10.1371/journal.pone.0164438
Cremoni, Allouche, Graca, Zorzi, Fernandez et al., Low baseline IFN-g response could predict hospitalization in COVID-19 patients, Front Immunol, doi:10.3389/fimmu.2022.953502
Diao, Wang, Tan, Chen, Liu et al., Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19), Front Immunol, doi:10.3389/fimmu.2020.00827
Diaz, Ziemin, Beau, Pitha, Smith et al., Homozygous deletion of the alpha-and beta 1-interferon genes in human leukemia and derived cell lines, Proc Natl Acad Sci U.S.A, doi:10.1073/pnas.85.14.5259
Diefenbach, Schindler, Donhauser, Lorenz, Laskay et al., Type 1 interferon (IFNalpha/beta) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite, Immunity, doi:10.1016/s1074-7613(00)80460-4
Eitner, Müller, König, Wilharm, Raab et al., Inhibition of inducible nitric oxide synthase prevents IL-1b-induced mitochondrial dysfunction in human chondrocytes, Int J Mol Sci, doi:10.3390/ijms22052477
Elliott, Wang, Interferon gamma runs interference on persistent COVID-19, Med, doi:10.1016/j.medj.2021.09.004
Fabri, Stenger, Shin, Liu, Realegeno, Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages, Sci Transl Med, doi:10.1126/scitranslmed.3003045
Felgenhauer, Schoen, Gad, Hartmann, Schaubmar et al., Inhibition of SARS-CoV-2 by type I and type III interferons, J Biol Chem, doi:10.1074/jbc.AC120.013788
Fulda, Gorman, Hori, Samali, Cellular stress responses: cell survival and cell death, Int J Cell Biol, doi:10.1155/2010/214074
Furfine, Harmon, Paith, Garvey, Selective inhibition of constitutive nitric oxide synthase by L-NG-nitroarginine, Biochemistry, doi:10.1021/bi00084a017
Galani, Rovina, Lampropoulou, Triantafyllia, Manioudaki et al., Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison, Nat Immunol, doi:10.1038/s41590-020-00840-x
Geers, Shamier, Bogers, Den Hartog, Gommers et al., SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees, Sci Immunol, doi:10.1126/sciimmunol.abj1750
Gelmez, Oktelik, Tahrali, Yilmaz, Kucuksezer et al., Immune modulation as a consequence of SARS-CoV-2 infection, Front Immunol, doi:10.3389/fimmu.2022.954391
Guthikonda, Baker, Mattson, Interferon-beta-1-b (IFN-B) decreases induced nitric oxide (NO) production by a human astrocytoma cell line, J Neuroimmunol, doi:10.1016/s0165-5728(97)00172-0
Hadjadj, Yatim, Barnabei, Corneau, Boussier et al., Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients, Science, doi:10.1126/science.abc6027
Harcourt, Caidi, Anderson, Haynes, Evaluation of the Calu-3 cell line as a model of in vitro respiratory syncytial virus infection, J Virol Methods, doi:10.1016/j.jviromet.2011.03.027
Harcourt, Tamin, Lu, Kamili, Sakthivel et al., Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States, Emerg Infect Dis, doi:10.3201/eid2606.200516
Harding, Carretero, Lapointe, Effects of interleukin-1 beta and nitric oxide on cardiac myocytes, Hypertension, doi:10.1161/01.hyp.25.3.421
Hu, Xu, Yin, Li, Hou et al., Lower circulating interferon-gamma is a risk factor for lung fibrosis in COVID-19 patients, Front Immunol, doi:10.3389/fimmu.2020.585647
Isaacs, Lindenmann, Virus interference. I, Interferon Proc R Soc Lond B Biol Sci, doi:10.1098/rspb.1957.0048
Jagannathan, Andrews, Bonilla, Hedlin, Jacobson et al., Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial, Nat Commun, doi:10.1038/s41467-021-22177-1
Johnson, Xie, Bailey, Kalveram, Lokugamage et al., Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis, Nature, doi:10.1038/s41586-021-03237-4
Karki, Sharma, Tuladhar, Williams, Zalduondo et al., Synergism of TNF-a and IFN-g Triggers inflammatory cell death, tissue damage, and mortality in SARS-coV-2 infection and cytokine shock syndromes, Cell, doi:10.1016/j.cell.2020.11.025
Karupiah, Xie, Buller, Nathan, Duarte et al., Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase, Science, doi:10.1126/science.7690156
Kitade, Sakitani, Inoue, Masu, Kawada et al., Interleukin 1 beta markedly stimulates nitric oxide formation in the absence of other cytokines or lipopolysaccharide in primary cultured rat hepatocytes but not in Kupffer cells, Hepatology, doi:10.1053/jhep.1996.v23.pm0008666334
Klein, Winter, Wachsmuth-Melm, Neufeldt, Cerikan, SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography, Nat Commun, doi:10.1038/s41467-020-19619-7
Klingström, Akerström, Hardestam, Stoltz, Simon et al., Nitric oxide and peroxynitrite have different antiviral effects against hantavirus replication and free mature virions, Eur J Immunol, doi:10.1002/eji.200535587
Kojima, Nakatsubo, Kikuchi, Kawahara, Kirino et al., Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins, Anal Chem, doi:10.1021/ac9801723
Kotenko, Gallagher, Baurin, Lewis-Antes, Shen et al., IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex, Nat Immunol, doi:10.1038/ni875
Landes, Rajaram, Nguyen, Schlesinger, Role for NOD2 in Mycobacterium tuberculosis-induced iNOS expression and NO production in human macrophages, J Leukoc Biol, doi:10.1189/jlb.3A1114-557R
Lei, Su, Dong, Bellavia, Fenza et al., Protocol of a randomized controlled trial testing inhaled Nitric Oxide in mechanically ventilated patients with severe acute respiratory syndrome in COVID-19 (SARS-CoV-2), medRxiv, doi:10.1101/2020.03.09.20033530
Lin, Chung, Wu, Fung, Hsu et al., Inhibition of nitric oxide production reverses diabetes-induced Kupffer cell activation and Klebsiella pneumonia liver translocation, PloS One, doi:10.1371/journal.pone.0177269
Londino, Gulick, Lear, Suber, Weathington et al., Post-translational modification of the interferon-gamma receptor alters its stability and signaling, Biochem J, doi:10.1042/BCJ20170548
Lowery, Sariol, Perlman, Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19, Cell Host Microbe, doi:10.1016/j.chom.2021.05.004
Lucas, Wong, Klein, Castro, Silva et al., Longitudinal analyses reveal immunological misfiring in severe COVID-19, Nature, doi:10.1038/s41586-020-2588-y
Lukacikova, Oveckova, Betakova, Laposova, Polcicova et al., Antiviral effect of interferon lambda against lymphocytic choriomeningitis virus, J Interferon Cytokine Res, doi:10.1089/jir.2014.0083
Lukaszewicz, Venet, Faure, Vignot, Monneret, Immunostimulation with interferon-g in protracted SARS-CoV-2 pneumonia, J Med Virol, doi:10.1002/jmv.27172
Macmicking, Interferon-inducible effector mechanisms in cell-autonomous immunity, Nat Rev Immunol, doi:10.1038/nri3210
Macmicking, Xie, Nathan, Nitric oxide and macrophage function, Annu Rev Immunol, doi:10.1146/annurev.immunol.15.1.323
Mateus, Dan, Zhang, Moderbacher, Lammers et al., Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells, Science, doi:10.1126/science.abj9853
Mccallum, Czudnochowski, Rosen, Zepeda, Bowen et al., Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement, Science, doi:10.1126/science.abn8652
Miller, Megson, Recent developments in nitric oxide donor drugs, Br J Pharmacol, doi:10.1038/sj.bjp.0707224
Moderbacher, Ramirez, Dan, Grifoni, Hastie et al., Antigen-specific adaptive immunity to SARS-coV-2 in acute COVID-19 and associations with age and disease severity, Cell, doi:10.1016/j.cell.2020.09.038
Murphy, Nitric oxide and cell death, Biochim Biophys Acta, doi:10.1016/s0005-2728(99)00029-8
Nagaoka, Kawasuji, Murai, Kaneda, Ueno et al., Circulating type I interferon levels in the early phase of COVID-19 are associated with the development of respiratory failure, Front Immunol, doi:10.3389/fimmu.2022.844304
Nelde, Bilich, Heitmann, Maringer, Salih et al., SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition, Nat Immunol, doi:10.1038/s41590-020-00808-x
Nguyen, Rowntree, Petersen, Chua, Hensen et al., CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope display high naive precursor frequency and TCR promiscuity, Immunity, doi:10.1016/j.immuni.2021.04.009
Oberhardt, Luxenburger, Kemming, Schulien, Ciminski et al., Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine, Nature, doi:10.1038/s41586-021-03841-4
Orzalli, Smith, Jurado, Iwasaki, Garlick et al., An antiviral branch of the IL-1 signaling pathway restricts immune-evasive virus replication, Mol Cell, doi:10.1016/j.molcel.2018.07.009
Patil, More, Rane, Mukherjee, Suresh et al., Proinflammatory cytokine Interleukin-1b (IL-1b) controls Leishmania infection, Cytokine, doi:10.1016/j.cyto.2018.06.033
Pervolaraki, Talemi, Albrecht, Bormann, Bamford et al., Differential induction of interferon stimulated genes between type I and type III interferons is independent of interferon receptor abundance, PloS Pathog, doi:10.1371/journal.ppat.1007420
Poljakovic, Karpman, Svanborg, Persson, Human renal epithelial cells express iNOS in response to cytokines but not bacteria, Kidney Int, doi:10.1046/j.1523-1755.2002.00138.x
Ramakrishnan, Determination of 50% endpoint titer using a simple formula, World J Virol, doi:10.5501/wjv.v5.i2.85
Reis, Silva, Silva, Thabane, Campos et al., Early treatment with pegylated interferon lambda for covid-19, New Engl J Med, doi:10.1056/NEJMoa2209760
Ren, Fan, Hou, Su, Cai, COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas, Cell, doi:10.1016/j.cell.2021.01.053
Robichon, Maiwald, Schilling, Schneider, Willemsen et al., Identification of interleukin1b as an amplifier of interferon alpha-induced antiviral responses, PloS Pathog, doi:10.1371/journal.ppat.1008461
Sadanandam, Bopp, Dixit, Knapp, Emperumal et al., A blood transcriptome-based analysis of disease progression, immune regulation, and symptoms in coronavirus-infected patients, Cell Death Discovery, doi:10.1038/s41420-020-00376-x
Schapira, Wiessner, Morrisey, Almagro, Nelin, L-arginine uptake and metabolism by lung macrophages and neutrophils following intratracheal instillation of silica in vivo, Am J Respir Cell Mol Biol, doi:10.1165/ajrcmb.19.2.2814
Schindelin, Arganda-Carreras, Frise, Kaynig, Longair et al., Fiji: an open-source platform for biological-image analysis, Nat Methods, doi:10.1038/nmeth.2019
Schulien, Kemming, Oberhardt, Wild, Seidel et al., Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells, Nat Med, doi:10.1038/s41591-020-01143-2
Sharara, Perkins, Misukonis, Chan, Dominitz et al., Interferon (IFN)-alpha activation of human blood mononuclear cells in vitro and in vivo for nitric oxide synthase (NOS) type 2 mRNA and protein expression: possible relationship of induced NOS2 to the anti-hepatitis C effects of IFN-alpha in vivo, J Exp Med, doi:10.1084/jem.186.9.1495
Silva Bj De, Barbosa Mg De, Andrade, Ferreira, Nery et al., Autophagy is an innate mechanism associated with leprosy polarization, PloS Pathog, doi:10.1371/journal.ppat.1006103
Silva Bj De, Bittencourt, Leal-Calvo, Mendes, Prata et al., Autophagy-associated IL-15 production is involved in the pathogenesis of leprosy type 1 reaction, Cells, doi:10.3390/cells10092215
Simpson, Pang, Weir, Kong, Fritsch et al., Interferon-g primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway, Immunity, doi:10.1016/j.immuni.2022.01.003
Spp Pharmaclon, Prospective randomized open-label comparative study of the use of intranasal form of interferon gamma human recombinant in patients for the prevention of acute respiratory viral infections, including COVID-19
Stenger, Thuring, Rollinghoff, Manning, Bogdan, L-N6-(1iminoethyl)-lysine potently inhibits inducible nitric oxide synthase and is superior to NG-monomethyl-arginine in vitro and in vivo, Eur J Pharmacol, doi:10.1016/0014-2999(95)00618-4
Stolp, Stern, Ambiel, Hofmann, Morath et al., SARS-CoV-2 variants of concern display enhanced intrinsic pathogenic properties and expanded organ tropism in mouse models, Cell Rep, doi:10.1016/j.celrep.2022.110387
Stoltz, Ahlm, Lundkvist, Klingström, Lambda interferon (IFN-lambda) in serum is decreased in hantavirus-infected patients, and in vitro-established infection is insensitive to treatment with all IFNs and inhibits IFN-gamma-induced nitric oxide production, J Virol, doi:10.1128/JVI.00415-07
Sun, Zhuang, Zheng, Li, Wong et al., Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment, Cell, doi:10.1016/j.cell.2020.06.010
Thoma-Uszynski, Stenger, Takeuchi, Ochoa, Engele et al., Induction of direct antimicrobial activity through mammalian toll-like receptors, Science, doi:10.1126/science.291.5508.1544
Trillo-Tinoco, Sierra, Cao, De Mingo-Pulido, Gilvary, AMPK alpha-1 intrinsically regulates the function and differentiation of tumor myeloid-derived suppressor cells, Cancer Res, doi:10.1158/0008-5472.CAN-19-0880
Van Laarhoven, Kurver, Overheul, Kooistra, Abdo et al., Interferon gamma immunotherapy in five critically ill COVID-19 patients with impaired cellular immunity: A case series, Med (N Y), doi:10.1016/j.medj.2021.09.003
Weiss, Ma, Merleev, Maverakis, Gilliet et al., IL-1b Induces the rapid secretion of the antimicrobial protein IL-26 from th17 cells, J Immunol, doi:10.4049/jimmunol.1900318
Wu, Chiu, Peng, Lin, Lu, Epithelial inducible nitric oxide synthase causes bacterial translocation by impairment of enterocytic tight junctions via intracellular signals of Rho-associated kinase and protein kinase C zeta, Crit Care Med, doi:10.1097/CCM.0b013e31821cb40e
Xia, Cao, Xie, Zhang, Chen et al., Evasion of type I interferon by SARS-coV-2, Cell Rep, doi:10.1016/j.celrep.2020.108234
Xie, Muruato, Lokugamage, Narayanan, Zhang et al., An infectious cDNA clone of SARS-coV-2, Cell Host Microbe, doi:10.1016/j.chom.2020.04.004
Zhang, Wang, Xing, Xu, Zhang et al., Single-cell landscape of immunological responses in patients with COVID-19, Nat Immunol, doi:10.1038/s41590-020-0762-x
Zheng, Gao, Wang, Song, Liu et al., Functional exhaustion of antiviral lymphocytes in COVID-19 patients, Cell Mol Immunol, doi:10.1038/s41423-020-0402-2
Zhu, Chidekel, Shaffer, Cultured human airway epithelial cells (calu-3): a model of human respiratory function, structure, and inflammatory responses, Crit Care Res Pract, doi:10.1155/2010/394578
Zhu, Wang, Liu, Liang, Wang et al., Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells, Nat Commun, doi:10.1038/s41467-020-17796-z
Zhu, Zhang, Yang, Song, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.3389/fimmu.2023.1284148",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2023.1284148",
"abstract": "<jats:sec><jats:title>Introduction</jats:title><jats:p>The COVID-19 pandemic has highlighted the need to identify mechanisms of antiviral host defense against SARS-CoV-2. One such mediator is interferon-g (IFN-γ), which, when administered to infected patients, is reported to result in viral clearance and resolution of pulmonary symptoms. IFN-γ treatment of a human lung epithelial cell line triggered an antiviral activity against SARS-CoV-2, yet the mechanism for this antiviral response was not identified.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Given that IFN-γ has been shown to trigger antiviral activity via the generation of nitric oxide (NO), we investigated whether IFN-γ induction of antiviral activity against SARS-CoV-2 infection is dependent upon the generation of NO in human pulmonary epithelial cells. We treated the simian epithelial cell line Vero E6 and human pulmonary epithelial cell lines, including A549-ACE2, and Calu-3, with IFN-γ and observed the resulting induction of NO and its effects on SARS-CoV-2 replication. Pharmacological inhibition of inducible nitric oxide synthase (iNOS) was employed to assess the dependency on NO production. Additionally, the study examined the effect of interleukin-1b (IL-1β) on the IFN-g-induced NO production and its antiviral efficacy.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Treatment of Vero E6 cells with IFN-γ resulted in a dose-responsive induction of NO and an inhibitory effect on SARS-CoV-2 replication. This antiviral activity was blocked by pharmacologic inhibition of iNOS. IFN-γ also triggered a NO-mediated antiviral activity in SARS-CoV-2 infected human lung epithelial cell lines A549-ACE2 and Calu-3. IL-1β enhanced IFN-γ induction of NO, but it had little effect on antiviral activity.</jats:p></jats:sec><jats:sec><jats:title>Discussion</jats:title><jats:p>Given that IFN-g has been shown to be produced by CD8+ T cells in the early response to SARS-CoV-2, our findings in human lung epithelial cell lines, of an IFN-γ-triggered, NO-dependent, links the adaptive immune response to an innate antiviral pathway in host defense against SARS-CoV-2. These results underscore the importance of IFN-γ and NO in the antiviral response and provide insights into potential therapeutic strategies for COVID-19.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fimmu.2023.1284148"
],
"author": [
{
"affiliation": [],
"family": "Silva",
"given": "Bruno J. de Andrade",
"sequence": "first"
},
{
"affiliation": [],
"family": "Krogstad",
"given": "Paul A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teles",
"given": "Rosane M. B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andrade",
"given": "Priscila R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rajfer",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrini",
"given": "Monica G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Otto O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bloom",
"given": "Barry R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Modlin",
"given": "Robert L.",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
12,
15
]
],
"date-time": "2023-12-15T10:32:02Z",
"timestamp": 1702636322000
},
"deposited": {
"date-parts": [
[
2023,
12,
15
]
],
"date-time": "2023-12-15T10:32:09Z",
"timestamp": 1702636329000
},
"indexed": {
"date-parts": [
[
2023,
12,
16
]
],
"date-time": "2023-12-16T00:46:37Z",
"timestamp": 1702687597319
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
12,
15
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
15
]
],
"date-time": "2023-12-15T00:00:00Z",
"timestamp": 1702598400000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2023.1284148/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
12,
15
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
15
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03841-4",
"article-title": "Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine",
"author": "Oberhardt",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B2",
"volume": "597",
"year": "2021"
},
{
"DOI": "10.1126/science.abj9853",
"article-title": "Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells",
"author": "Mateus",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B3",
"volume": "374",
"year": "2021"
},
{
"key": "B4",
"unstructured": "COVID-19 Treatment GuidelinesInformation on COVID-19 treatment, Prevention and research"
},
{
"DOI": "10.1016/j.celrep.2022.110387",
"article-title": "SARS-CoV-2 variants of concern display enhanced intrinsic pathogenic properties and expanded organ tropism in mouse models",
"author": "Stolp",
"doi-asserted-by": "publisher",
"journal-title": "Cell Rep",
"key": "B5",
"volume": "38",
"year": "2022"
},
{
"DOI": "10.1126/science.abn8652",
"article-title": "Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement",
"author": "McCallum",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B6",
"volume": "375",
"year": "2022"
},
{
"DOI": "10.1126/sciimmunol.abj1750",
"article-title": "SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees",
"author": "Geers",
"doi-asserted-by": "publisher",
"journal-title": "Sci Immunol",
"key": "B7",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1098/rspb.1957.0048",
"article-title": "Virus interference",
"author": "Isaacs",
"doi-asserted-by": "publisher",
"journal-title": "I. Interferon Proc R Soc Lond B Biol Sci",
"key": "B8",
"volume": "147",
"year": "1957"
},
{
"DOI": "10.1126/science.abc6027",
"article-title": "Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients",
"author": "Hadjadj",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B9",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2588-y",
"article-title": "Longitudinal analyses reveal immunological misfiring in severe COVID-19",
"author": "Lucas",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B10",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1016/j.celrep.2020.108234",
"article-title": "Evasion of type I interferon by SARS-coV-2",
"author": "Xia",
"doi-asserted-by": "publisher",
"journal-title": "Cell Rep",
"key": "B11",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1038/s41590-020-00840-x",
"article-title": "Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison",
"author": "Galani",
"doi-asserted-by": "publisher",
"first-page": "32",
"journal-title": "Nat Immunol",
"key": "B12",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1038/s41420-020-00376-x",
"article-title": "A blood transcriptome-based analysis of disease progression, immune regulation, and symptoms in coronavirus-infected patients",
"author": "Sadanandam",
"doi-asserted-by": "publisher",
"first-page": "141",
"journal-title": "Cell Death Discovery",
"key": "B13",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2021.01.053",
"article-title": "COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas",
"author": "Ren",
"doi-asserted-by": "publisher",
"first-page": "1895",
"journal-title": "Cell",
"key": "B14",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/j.medj.2021.09.003",
"article-title": "Interferon gamma immunotherapy in five critically ill COVID-19 patients with impaired cellular immunity: A case series",
"author": "van Laarhoven",
"doi-asserted-by": "publisher",
"first-page": "1163",
"journal-title": "Med (N Y)",
"key": "B15",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27172",
"article-title": "Immunostimulation with interferon-γ in protracted SARS-CoV-2 pneumonia",
"author": "Lukaszewicz",
"doi-asserted-by": "publisher",
"journal-title": "J Med Virol",
"key": "B16",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.medj.2021.09.004",
"article-title": "Interferon gamma runs interference on persistent COVID-19",
"author": "Elliott",
"doi-asserted-by": "publisher",
"journal-title": "Med (N Y)",
"key": "B17",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1128/mBio.01928-20",
"article-title": "Antiviral activity of II, and III interferons counterbalances ACE2 inducibility and restricts SARS-coV-2",
"author": "Busnadiego",
"doi-asserted-by": "publisher",
"journal-title": "mBio",
"key": "B18",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/nri3210",
"article-title": "Interferon-inducible effector mechanisms in cell-autonomous immunity",
"author": "MacMicking",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Immunol",
"key": "B19",
"volume": "12",
"year": "2012"
},
{
"DOI": "10.1128/JVI.79.3.1966-1969.2005",
"article-title": "Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus",
"author": "Akerström",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B20",
"volume": "79",
"year": "2005"
},
{
"DOI": "10.1126/science.7690156",
"article-title": "Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase",
"author": "Karupiah",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B21",
"volume": "261",
"year": "1993"
},
{
"DOI": "10.1146/annurev.immunol.15.1.323",
"article-title": "Nitric oxide and macrophage function",
"author": "MacMicking",
"doi-asserted-by": "publisher",
"journal-title": "Annu Rev Immunol",
"key": "B22",
"volume": "15",
"year": "1997"
},
{
"DOI": "10.1038/s41467-020-19619-7",
"article-title": "SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography",
"author": "Klein",
"doi-asserted-by": "publisher",
"first-page": "5885",
"journal-title": "Nat Commun",
"key": "B23",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3201/eid2606.200516",
"article-title": "Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States",
"author": "Harcourt",
"doi-asserted-by": "publisher",
"journal-title": "Emerg Infect Dis",
"key": "B24",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2020.04.004",
"article-title": "An infectious cDNA clone of SARS-coV-2",
"author": "Xie",
"doi-asserted-by": "publisher",
"first-page": "841",
"journal-title": "Cell Host Microbe",
"key": "B25",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03237-4",
"article-title": "Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis",
"author": "Johnson",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B26",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-17796-z",
"article-title": "Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells",
"author": "Zhu",
"doi-asserted-by": "publisher",
"first-page": "3910",
"journal-title": "Nat Commun",
"key": "B27",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1074/jbc.AC120.013788",
"article-title": "Inhibition of SARS-CoV-2 by type I and type III interferons",
"author": "Felgenhauer",
"doi-asserted-by": "publisher",
"journal-title": "J Biol Chem",
"key": "B28",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1126/science.291.5508.1544",
"article-title": "Induction of direct antimicrobial activity through mammalian toll-like receptors",
"author": "Thoma-Uszynski",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B29",
"volume": "291",
"year": "2001"
},
{
"DOI": "10.1016/s1074-7613(00)80460-4",
"article-title": "Type 1 interferon (IFNalpha/beta) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite",
"author": "Diefenbach",
"doi-asserted-by": "publisher",
"first-page": "77",
"journal-title": "Immunity",
"key": "B30",
"volume": "8",
"year": "1998"
},
{
"DOI": "10.1165/ajrcmb.19.2.2814",
"article-title": "L-arginine uptake and metabolism by lung macrophages and neutrophils following intratracheal instillation of silica in vivo",
"author": "Schapira",
"doi-asserted-by": "publisher",
"journal-title": "Am J Respir Cell Mol Biol",
"key": "B31",
"volume": "19",
"year": "1998"
},
{
"DOI": "10.1016/0014-2999(95)00618-4",
"article-title": "L-N6-(1-iminoethyl)-lysine potently inhibits inducible nitric oxide synthase and is superior to NG-monomethyl-arginine in vitro and in vivo",
"author": "Stenger",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Pharmacol",
"key": "B32",
"volume": "294",
"year": "1995"
},
{
"DOI": "10.1016/j.redox.2020.101734",
"article-title": "Mitigation of the replication of SARS-CoV-2 by nitric oxide",
"author": "Akaberi",
"doi-asserted-by": "publisher",
"journal-title": "vitro. Redox Biol",
"key": "B33",
"volume": "37",
"year": "2020"
},
{
"DOI": "10.5501/wjv.v5.i2.85",
"article-title": "Determination of 50% endpoint titer using a simple formula",
"author": "Ramakrishnan",
"doi-asserted-by": "publisher",
"journal-title": "World J Virol",
"key": "B34",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.1021/ac9801723",
"article-title": "Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins",
"author": "Kojima",
"doi-asserted-by": "publisher",
"journal-title": "Anal Chem",
"key": "B35",
"volume": "70",
"year": "1998"
},
{
"DOI": "10.1189/jlb.3A1114-557R",
"article-title": "Role for NOD2 in Mycobacterium tuberculosis-induced iNOS expression and NO production in human macrophages",
"author": "Landes",
"doi-asserted-by": "publisher",
"journal-title": "J Leukoc Biol",
"key": "B36",
"volume": "97",
"year": "2015"
},
{
"DOI": "10.1158/0008-5472.CAN-19-0880",
"article-title": "AMPK alpha-1 intrinsically regulates the function and differentiation of tumor myeloid-derived suppressor cells",
"author": "Trillo-Tinoco",
"doi-asserted-by": "publisher",
"journal-title": "Cancer Res",
"key": "B37",
"volume": "79",
"year": "2019"
},
{
"DOI": "10.1038/nmeth.2019",
"article-title": "Fiji: an open-source platform for biological-image analysis",
"author": "Schindelin",
"doi-asserted-by": "publisher",
"journal-title": "Nat Methods",
"key": "B38",
"volume": "9",
"year": "2012"
},
{
"DOI": "10.1371/journal.ppat.1006103",
"article-title": "Autophagy is an innate mechanism associated with leprosy polarization",
"author": "Silva BJ de",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B39",
"volume": "13",
"year": "2017"
},
{
"DOI": "10.3390/cells10092215",
"article-title": "Autophagy-associated IL-15 production is involved in the pathogenesis of leprosy type 1 reaction",
"author": "Silva BJ de",
"doi-asserted-by": "publisher",
"journal-title": "Cells",
"key": "B40",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1073/pnas.85.14.5259",
"article-title": "Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines",
"author": "Diaz",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci U.S.A.",
"key": "B41",
"volume": "85",
"year": "1988"
},
{
"DOI": "10.1002/eji.200535587",
"article-title": "Nitric oxide and peroxynitrite have different antiviral effects against hantavirus replication and free mature virions",
"author": "Klingström",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Immunol",
"key": "B42",
"volume": "36",
"year": "2006"
},
{
"DOI": "10.1016/j.molcel.2018.07.009",
"article-title": "An antiviral branch of the IL-1 signaling pathway restricts immune-evasive virus replication",
"author": "Orzalli",
"doi-asserted-by": "publisher",
"first-page": "825",
"journal-title": "Mol Cell",
"key": "B43",
"volume": "71",
"year": "2018"
},
{
"DOI": "10.1016/j.ydbio.2007.06.018",
"article-title": "Mechanisms of palatal epithelial seam disintegration by transforming growth factor (TGF) beta3",
"author": "Ahmed",
"doi-asserted-by": "publisher",
"first-page": "193",
"journal-title": "Dev Biol",
"key": "B44",
"volume": "309",
"year": "2007"
},
{
"DOI": "10.1016/s0005-2728(99)00029-8",
"article-title": "Nitric oxide and cell death",
"author": "Murphy",
"doi-asserted-by": "publisher",
"journal-title": "Biochim Biophys Acta",
"key": "B45",
"volume": "1411",
"year": "1999"
},
{
"DOI": "10.1021/bi00084a017",
"article-title": "Selective inhibition of constitutive nitric oxide synthase by L-NG-nitroarginine",
"author": "Furfine",
"doi-asserted-by": "publisher",
"journal-title": "Biochemistry",
"key": "B46",
"volume": "32",
"year": "1993"
},
{
"DOI": "10.1371/journal.pone.0177269",
"article-title": "Inhibition of nitric oxide production reverses diabetes-induced Kupffer cell activation and Klebsiella pneumonia liver translocation",
"author": "Lin",
"doi-asserted-by": "publisher",
"journal-title": "PloS One",
"key": "B47",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1097/CCM.0b013e31821cb40e",
"article-title": "Epithelial inducible nitric oxide synthase causes bacterial translocation by impairment of enterocytic tight junctions via intracellular signals of Rho-associated kinase and protein kinase C zeta",
"author": "Wu",
"doi-asserted-by": "publisher",
"journal-title": "Crit Care Med",
"key": "B48",
"volume": "39",
"year": "2011"
},
{
"DOI": "10.1371/journal.pone.0164438",
"article-title": "Long term culture of the A549 cancer cell line promotes multilamellar body formation and differentiation towards an alveolar type II pneumocyte phenotype",
"author": "Cooper",
"doi-asserted-by": "publisher",
"journal-title": "PloS One",
"key": "B49",
"volume": "11",
"year": "2016"
},
{
"DOI": "10.3390/v14071369",
"article-title": "A newly engineered A549 cell line expressing ACE2 and TMPRSS2 is highly permissive to SARS-coV-2, including the delta and omicron variants",
"author": "Chang",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B50",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1155/2010/394578",
"article-title": "Cultured human airway epithelial cells (calu-3): a model of human respiratory function, structure, and inflammatory responses",
"author": "Zhu",
"doi-asserted-by": "publisher",
"journal-title": "Crit Care Res Pract",
"key": "B51",
"volume": "2010",
"year": "2010"
},
{
"DOI": "10.1016/j.jviromet.2011.03.027",
"article-title": "Evaluation of the Calu-3 cell line as a model of in vitro respiratory syncytial virus infection",
"author": "Harcourt",
"doi-asserted-by": "publisher",
"journal-title": "J Virol Methods",
"key": "B52",
"volume": "174",
"year": "2011"
},
{
"DOI": "10.1038/ni1001-907",
"article-title": "Nitric oxide and the immune response",
"author": "Bogdan",
"doi-asserted-by": "publisher",
"journal-title": "Nat Immunol",
"key": "B53",
"volume": "2",
"year": "2001"
},
{
"DOI": "10.1016/j.niox.2020.07.003",
"article-title": "Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19",
"author": "Adusumilli",
"doi-asserted-by": "publisher",
"first-page": "4",
"journal-title": "Nitric Oxide",
"key": "B54",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.1186/s43141-021-00249-5",
"article-title": "The potential role of inhaled nitric oxide for postexposure chemoprophylaxis of COVID-19",
"author": "AbdelMassih",
"doi-asserted-by": "publisher",
"first-page": "165",
"journal-title": "J Genet Eng Biotechnol",
"key": "B55",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1038/sj.bjp.0707224",
"article-title": "Recent developments in nitric oxide donor drugs",
"author": "Miller",
"doi-asserted-by": "publisher",
"journal-title": "Br J Pharmacol",
"key": "B56",
"volume": "151",
"year": "2007"
},
{
"DOI": "10.1101/2020.03.09.20033530",
"article-title": "Protocol of a randomized controlled trial testing inhaled Nitric Oxide in mechanically ventilated patients with severe acute respiratory syndrome in COVID-19 (SARS-CoV-2)",
"author": "Lei",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "B57",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03371-x",
"article-title": "Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study",
"author": "Abou-Arab",
"doi-asserted-by": "publisher",
"first-page": "645",
"journal-title": "Crit Care",
"key": "B58",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.11.025",
"article-title": "Synergism of TNF-α and IFN-γ Triggers inflammatory cell death, tissue damage, and mortality in SARS-coV-2 infection and cytokine shock syndromes",
"author": "Karki",
"doi-asserted-by": "publisher",
"first-page": "149",
"journal-title": "Cell",
"key": "B59",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1155/2010/214074",
"article-title": "Cellular stress responses: cell survival and cell death",
"author": "Fulda",
"doi-asserted-by": "publisher",
"journal-title": "Int J Cell Biol",
"key": "B60",
"volume": "2010",
"year": "2010"
},
{
"DOI": "10.1126/scitranslmed.3003045",
"article-title": "Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages",
"author": "Fabri",
"doi-asserted-by": "publisher",
"first-page": "104ra102",
"journal-title": "Sci Transl Med",
"key": "B61",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.1016/j.immuni.2022.01.003",
"article-title": "Interferon-γ primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway",
"author": "Simpson",
"doi-asserted-by": "publisher",
"journal-title": "Immunity",
"key": "B62",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.4049/jimmunol.1900318",
"article-title": "IL-1β Induces the rapid secretion of the antimicrobial protein IL-26 from th17 cells",
"author": "Weiss",
"doi-asserted-by": "publisher",
"journal-title": "J Immunol",
"key": "B63",
"volume": "203",
"year": "2019"
},
{
"DOI": "10.1016/j.cyto.2018.06.033",
"article-title": "Pro-inflammatory cytokine Interleukin-1β (IL-1β) controls Leishmania infection",
"author": "Patil",
"doi-asserted-by": "publisher",
"first-page": "27",
"journal-title": "Cytokine",
"key": "B64",
"volume": "112",
"year": "2018"
},
{
"DOI": "10.1371/journal.ppat.1008461",
"article-title": "Identification of interleukin1β as an amplifier of interferon alpha-induced antiviral responses",
"author": "Robichon",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B65",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1046/j.1523-1755.2002.00138.x",
"article-title": "Human renal epithelial cells express iNOS in response to cytokines but not bacteria",
"author": "Poljakovic",
"doi-asserted-by": "publisher",
"journal-title": "Kidney Int",
"key": "B66",
"volume": "61",
"year": "2002"
},
{
"DOI": "10.3390/ijms22052477",
"article-title": "Inhibition of inducible nitric oxide synthase prevents IL-1β-induced mitochondrial dysfunction in human chondrocytes",
"author": "Eitner",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "B67",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1053/jhep.1996.v23.pm0008666334",
"article-title": "Interleukin 1 beta markedly stimulates nitric oxide formation in the absence of other cytokines or lipopolysaccharide in primary cultured rat hepatocytes but not in Kupffer cells",
"author": "Kitade",
"doi-asserted-by": "publisher",
"first-page": "797",
"journal-title": "Hepatology",
"key": "B68",
"volume": "23",
"year": "1996"
},
{
"DOI": "10.1161/01.hyp.25.3.421",
"article-title": "Effects of interleukin-1 beta and nitric oxide on cardiac myocytes",
"author": "Harding",
"doi-asserted-by": "publisher",
"journal-title": "Hypertension",
"key": "B69",
"volume": "25",
"year": "1995"
},
{
"DOI": "10.1006/bbrc.1998.9062",
"article-title": "Analysis of the cytokine-stimulated human inducible nitric oxide synthase (iNOS) gene: characterization of differences between human and mouse iNOS promoters",
"author": "Chu",
"doi-asserted-by": "publisher",
"journal-title": "Biochem Biophys Res Commun",
"key": "B70",
"volume": "248",
"year": "1998"
},
{
"DOI": "10.1016/j.cell.2020.04.026",
"article-title": "Imbalanced host response to SARS-coV-2 drives development of COVID-19",
"author": "Blanco-Melo",
"doi-asserted-by": "publisher",
"first-page": "1036",
"journal-title": "Cell",
"key": "B71",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1084/jem.186.9.1495",
"article-title": "Interferon (IFN)-alpha activation of human blood mononuclear cells in vitro and in vivo for nitric oxide synthase (NOS) type 2 mRNA and protein expression: possible relationship of induced NOS2 to the anti-hepatitis C effects of IFN-alpha in vivo",
"author": "Sharara",
"doi-asserted-by": "publisher",
"journal-title": "J Exp Med",
"key": "B72",
"volume": "186",
"year": "1997"
},
{
"DOI": "10.1016/s0165-5728(97)00172-0",
"article-title": "Interferon-beta-1-b (IFN-B) decreases induced nitric oxide (NO) production by a human astrocytoma cell line",
"author": "Guthikonda",
"doi-asserted-by": "publisher",
"journal-title": "J Neuroimmunol",
"key": "B73",
"volume": "82",
"year": "1998"
},
{
"DOI": "10.1126/science.abc3545",
"article-title": "Type III interferons disrupt the lung epithelial barrier upon viral recognition",
"author": "Broggi",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B74",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1038/ni875",
"article-title": "IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex",
"author": "Kotenko",
"doi-asserted-by": "publisher",
"first-page": "69",
"journal-title": "Nat Immunol",
"key": "B75",
"volume": "4",
"year": "2003"
},
{
"DOI": "10.1371/journal.ppat.1007420",
"article-title": "Differential induction of interferon stimulated genes between type I and type III interferons is independent of interferon receptor abundance",
"author": "Pervolaraki",
"doi-asserted-by": "publisher",
"journal-title": "PloS Pathog",
"key": "B76",
"volume": "14",
"year": "2018"
},
{
"DOI": "10.1128/JVI.00415-07",
"article-title": "Lambda interferon (IFN-lambda) in serum is decreased in hantavirus-infected patients, and in vitro-established infection is insensitive to treatment with all IFNs and inhibits IFN-gamma-induced nitric oxide production",
"author": "Stoltz",
"doi-asserted-by": "publisher",
"journal-title": "J Virol",
"key": "B77",
"volume": "81",
"year": "2007"
},
{
"DOI": "10.1042/BCJ20170548",
"article-title": "Post-translational modification of the interferon-gamma receptor alters its stability and signaling",
"author": "Londino",
"doi-asserted-by": "publisher",
"journal-title": "Biochem J",
"key": "B78",
"volume": "474",
"year": "2017"
},
{
"DOI": "10.1089/jir.2014.0083",
"article-title": "Antiviral effect of interferon lambda against lymphocytic choriomeningitis virus",
"author": "Lukacikova",
"doi-asserted-by": "publisher",
"journal-title": "J Interferon Cytokine Res",
"key": "B79",
"volume": "35",
"year": "2015"
},
{
"DOI": "10.1038/s41423-020-0402-2",
"article-title": "Functional exhaustion of antiviral lymphocytes in COVID-19 patients",
"author": "Zheng",
"doi-asserted-by": "publisher",
"journal-title": "Cell Mol Immunol",
"key": "B80",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2022.954391",
"article-title": "Immune modulation as a consequence of SARS-CoV-2 infection",
"author": "Gelmez",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B81",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2020.00827",
"article-title": "Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19)",
"author": "Diao",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B82",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2022.953502",
"article-title": "Low baseline IFN-γ response could predict hospitalization in COVID-19 patients",
"author": "Cremoni",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B83",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.06.010",
"article-title": "Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment",
"author": "Sun",
"doi-asserted-by": "publisher",
"first-page": "734",
"journal-title": "Cell",
"key": "B84",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.09.038",
"article-title": "Antigen-specific adaptive immunity to SARS-coV-2 in acute COVID-19 and associations with age and disease severity",
"author": "Rydyznski Moderbacher",
"doi-asserted-by": "publisher",
"first-page": "996",
"journal-title": "Cell",
"key": "B85",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1038/s41590-020-00808-x",
"article-title": "SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition",
"author": "Nelde",
"doi-asserted-by": "publisher",
"first-page": "74",
"journal-title": "Nat Immunol",
"key": "B86",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1038/s41591-020-01143-2",
"article-title": "Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells",
"author": "Schulien",
"doi-asserted-by": "publisher",
"first-page": "78",
"journal-title": "Nat Med",
"key": "B87",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/j.immuni.2021.04.009",
"article-title": "CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope display high naive precursor frequency and TCR promiscuity",
"author": "Nguyen",
"doi-asserted-by": "publisher",
"first-page": "1066",
"journal-title": "Immunity",
"key": "B88",
"volume": "54",
"year": "2021"
},
{
"DOI": "10.1038/s41590-020-0762-x",
"article-title": "Single-cell landscape of immunological responses in patients with COVID-19",
"author": "Zhang",
"doi-asserted-by": "publisher",
"journal-title": "Nat Immunol",
"key": "B89",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1126/sciimmunol.aat7668",
"article-title": "Human antimicrobial cytotoxic T lymphocytes, defined by NK receptors and antimicrobial proteins, kill intracellular bacteria",
"author": "Balin",
"doi-asserted-by": "publisher",
"first-page": "eaat7668",
"journal-title": "Sci Immunol",
"key": "B90",
"volume": "3",
"year": "2018"
},
{
"DOI": "10.1016/j.chom.2021.05.004",
"article-title": "Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19",
"author": "Lowery",
"doi-asserted-by": "publisher",
"journal-title": "Cell Host Microbe",
"key": "B91",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2022.844304",
"article-title": "Circulating type I interferon levels in the early phase of COVID-19 are associated with the development of respiratory failure",
"author": "Nagaoka",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B92",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2020.585647",
"article-title": "Lower circulating interferon-gamma is a risk factor for lung fibrosis in COVID-19 patients",
"author": "Hu",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B93",
"volume": "11",
"year": "2020"
},
{
"article-title": "SPP Pharmaclon Ltd. Prospective randomized open-label comparative study of the use of intranasal form of interferon gamma human recombinant in patients for the prevention of acute respiratory viral infections, including COVID-19. [Clinical trial registration]. clinicaltrials.gov",
"key": "B94",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-22177-1",
"article-title": "Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial",
"author": "Jagannathan",
"doi-asserted-by": "publisher",
"first-page": "1967",
"journal-title": "Nat Commun",
"key": "B95",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2209760",
"article-title": "Early treatment with pegylated interferon lambda for covid-19",
"author": "Reis",
"doi-asserted-by": "publisher",
"journal-title": "New Engl J Med",
"key": "B96",
"volume": "388",
"year": "2023"
},
{
"DOI": "10.1016/j.intimp.2021.107916",
"article-title": "An investigation into the beneficial effects of high-dose interferon beta 1-a, compared to low-dose interferon beta 1-a in severe COVID-19: The COVIFERON II randomized controlled trial",
"author": "Alavi Darazam",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol",
"key": "B97",
"volume": "99",
"year": "2021"
}
],
"reference-count": 97,
"references-count": 97,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2023.1284148/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "IFN-γ-mediated control of SARS-CoV-2 infection through nitric oxide",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "14"
}