Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study
et al., Critical Care, doi:10.1186/s13054-020-03371-x, Nov 2020
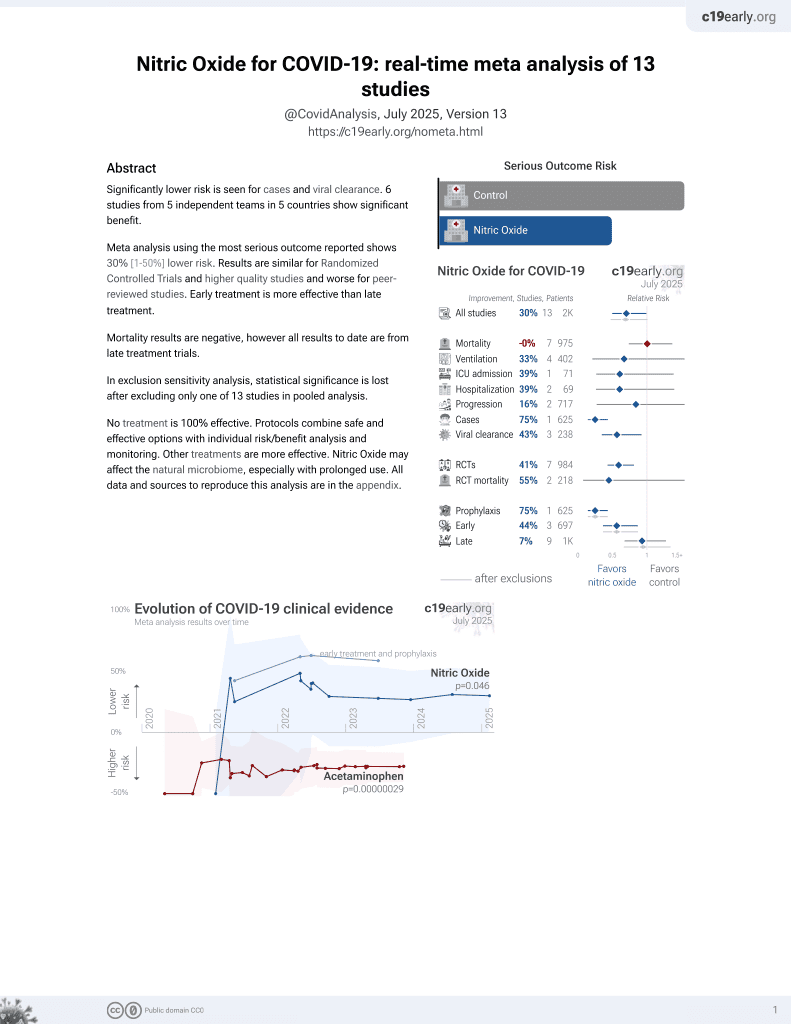
43rd treatment shown to reduce risk in
June 2022, now with p = 0.012 from 12 studies, recognized in 10 countries.
Lower risk for cases and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
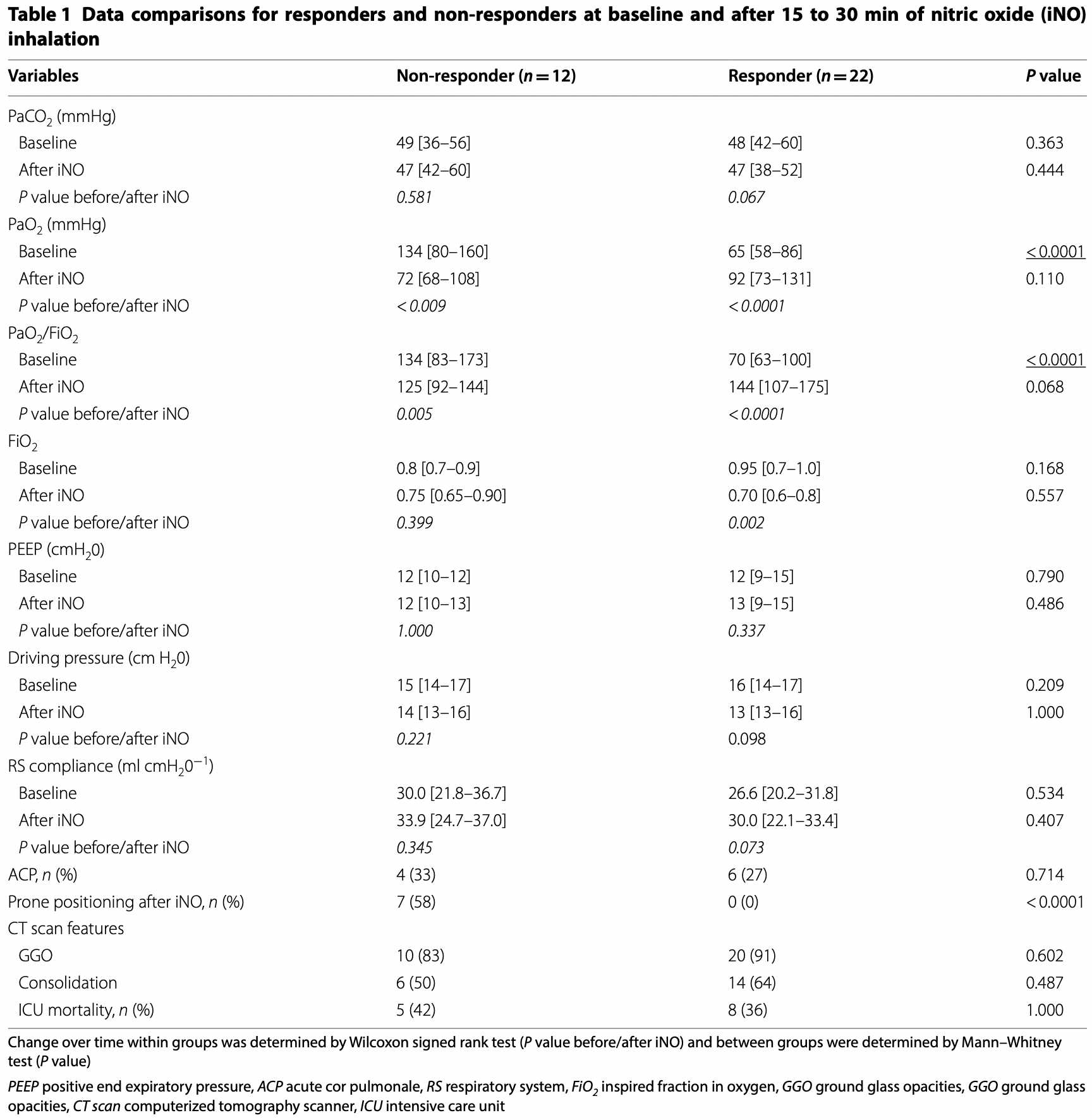
Retrospective 34 ICU patients in France treated with inhaled nitric oxide, showing 65% had an increase in PaO2/FiO2 of >20% 30 min following administration.
Abou-Arab et al., 12 Nov 2020, prospective, France, peer-reviewed, 6 authors.
Contact: osama.abouarab@gmail.com (corresponding author).
Abstract: (2020) 24:645
Abou‑Arab et al. Crit Care
https://doi.org/10.1186/s13054-020-03371-x
RESEARCH LETTER
Open Access
Inhaled nitric oxide for critically ill Covid‑19
patients: a prospective study
Osama Abou‑Arab1* , Pierre Huette1, Fanny Debouvries1, Hervé Dupont1, Vincent Jounieaux2
and Yazine Mahjoub1
Dear editor,
The role of inhaled nitric oxide (iNO) in the management of severe hypoxia due to coronavirus disease 2019
(Covid-19) is a subject of debate. Despite the lack of clinical data, the surviving sepsis campaign recommended the
use of iNO as a rescue therapy in such patients with persistent hypoxemia and, at the same time, reminded that
this treatment must be tapered off in the absence of rapid
improvement [1].
The aim of the present study is to record the effect of
iNO administration in COVID-19 patients with severe
pneumonia.
We conducted a single-center prospective study at
Amiens Hospital University (France), (ancillary study of
a prospective COVID-19 critically patient database registered on ClinicalTrials.gov: NCT04354558 and declared
to the CNIL number: PI2020_843_0026).
The population study was conducted on adults admitted in our intensive care unit for a COVID-19 severe
pneumonia defined according to the WHO case definition [2]. All patients underwent a chest CT scan before
iNO administration.
We administered 10 ppm of iNO (Kinox, Air Liquid
Healthcare, Canada) through the inspiratory limb of the
ventilator tubing when PaO2/FiO2 ratio was under 150
according to our local protocol management. Response
to iNO was defined as an increase in PaO2/FiO2 over
20% during over 30 min following its administration. In
*Correspondence: osama.abouarab@gmail.com
1
Department of Anaesthesiology and Critical Care Medicine, Amiens
Picardie University Hospital, 1 rue du Professeur Christian Cabrol,
80054 Amiens, France
Full list of author information is available at the end of the article
the absence of response to iNO administration, patients
received one session of prone positioning. The following respiratory parameters were collected at baseline and
after 15 to 30 min of iNO administration: positive end
expiratory pressure (PEEP), respiratory lung compliance
(RS compliance), driving pressure, fraction in inspired
oxygen (FiO2), PaO2, PaCO2 and the echocardiographic
presence of an acute cor pulmonale (ACP).
Data were presented as median [interquartile range]
or as number (percentage). Responders group and
non-responders group were compared using Wilcoxon–Mann–Whitney, chi-2 or Fischer exact test, as
appropriate. Statistical tests were performed using SPSS
software version 24. A P value under 0.05 was considered
as significant.
From 1st of March to 31st of May 2020, 34 of 80
patients with COVID-19 severe pneumonia received
iNO. Twenty-two of 34 patients (65%) were responders and twelve were non-responders (35%). After iNO
administration, PEEP, RS compliance and driving pressure remained un1
changed both in responders and in non-responders.
At baseline, P
aO2/FiO2 was significantly lower in the
responders group in comparison with the non-responders group (respectively, 70 [63–100] vs 134 [83–173];
P < 0.0001) and was similar between groups after iNO
administration (P = 0.068). PaCO2 levels were comparable between groups at baseline and after iNO administration. Prone positioning was not performed in the
responders group.
We found a response rate of 65% to iNO administration. Our results differ from two recent reports on iNO
use in..
DOI record:
{
"DOI": "10.1186/s13054-020-03371-x",
"ISSN": [
"1364-8535"
],
"URL": "http://dx.doi.org/10.1186/s13054-020-03371-x",
"alternative-id": [
"3371"
],
"article-number": "645",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "3 September 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "3 November 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "12 November 2020"
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The study was approved by Amiens Hospital University and declared at CNIL (registration number: PI2020_843_0026). Oral and written information was delivered to the patients. No written consent was required."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3766-716X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Abou-Arab",
"given": "Osama",
"sequence": "first"
},
{
"affiliation": [],
"family": "Huette",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Debouvries",
"given": "Fanny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dupont",
"given": "Hervé",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jounieaux",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahjoub",
"given": "Yazine",
"sequence": "additional"
}
],
"container-title": "Critical Care",
"container-title-short": "Crit Care",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2020,
11,
12
]
],
"date-time": "2020-11-12T22:02:41Z",
"timestamp": 1605218561000
},
"deposited": {
"date-parts": [
[
2020,
11,
12
]
],
"date-time": "2020-11-12T22:02:42Z",
"timestamp": 1605218562000
},
"indexed": {
"date-parts": [
[
2022,
7,
9
]
],
"date-time": "2022-07-09T23:29:30Z",
"timestamp": 1657409370368
},
"is-referenced-by-count": 25,
"issue": "1",
"issued": {
"date-parts": [
[
2020,
11,
12
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2020,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
12
]
],
"date-time": "2020-11-12T00:00:00Z",
"timestamp": 1605139200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
12
]
],
"date-time": "2020-11-12T00:00:00Z",
"timestamp": 1605139200000
}
}
],
"link": [
{
"URL": "http://link.springer.com/content/pdf/10.1186/s13054-020-03371-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://link.springer.com/article/10.1186/s13054-020-03371-x/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://link.springer.com/content/pdf/10.1186/s13054-020-03371-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2020,
11,
12
]
]
},
"published-online": {
"date-parts": [
[
2020,
11,
12
]
]
},
"published-print": {
"date-parts": [
[
2020,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"author": "W Alhazzani",
"first-page": "1",
"journal-title": "Crit Care Med",
"key": "3371_CR1",
"unstructured": "Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2019;2020:1.",
"volume": "2020",
"year": "2019"
},
{
"DOI": "10.1001/jama.2020.2648",
"author": "Z Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "JAMA.",
"key": "3371_CR2",
"unstructured": "Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03222-9",
"author": "G Tavazzi",
"doi-asserted-by": "publisher",
"first-page": "508",
"journal-title": "Crit Care",
"key": "3371_CR3",
"unstructured": "Tavazzi G, Marco P, Mongodi S, Dammassa V, Romito G, Mojoli F. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit Care. 2020;24:508.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.jcrc.2020.08.007",
"author": "M Ferrari",
"doi-asserted-by": "publisher",
"first-page": "159",
"journal-title": "J Crit Care",
"key": "3371_CR4",
"unstructured": "Ferrari M, Santini A, Protti A, Andreis DT, Iapichino G, Castellani G, et al. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J Crit Care. 2020;60:159–60.",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-02972-w",
"author": "Y Mahjoub",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Crit Care",
"key": "3371_CR5",
"unstructured": "Mahjoub Y, Rodenstein DO, Jounieaux V. Severe Covid-19 disease: rather AVDS than ARDS? Crit Care. 2020;24:1–2. https://doi.org/10.1186/s13054-020-02972-w.",
"volume": "24",
"year": "2020"
}
],
"reference-count": 5,
"references-count": 5,
"relation": {},
"resource": {
"primary": {
"URL": "https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03371-x"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Critical Care and Intensive Care Medicine"
],
"subtitle": [],
"title": "Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}