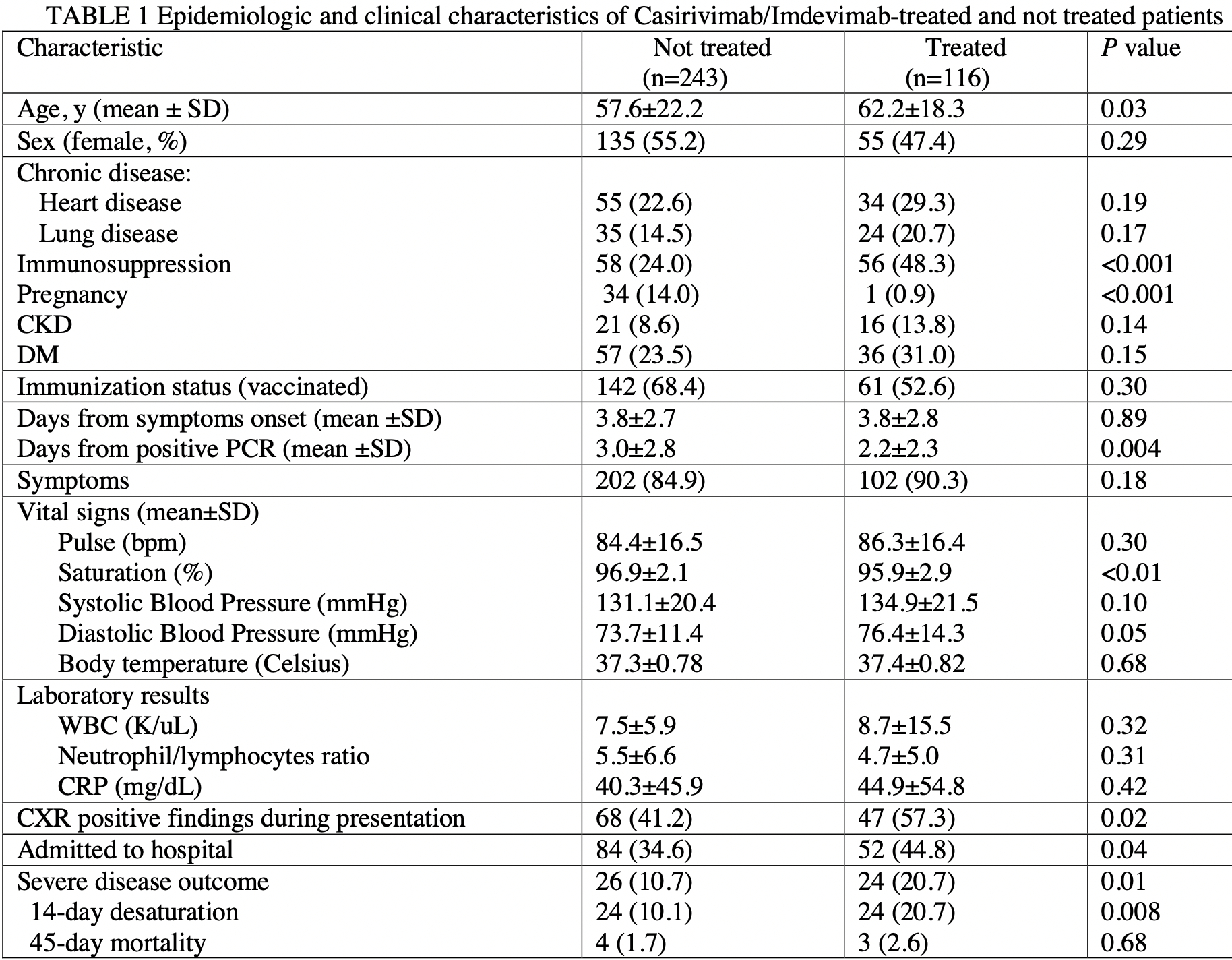
Doubtful Clinical Benefit of Casirivimab-Imdevimab Treatment for Disease Severity Outcome of High-Risk Patients with SARS-CoV-2 Delta Variant Infection
et al., medRxiv, doi:10.1101/2022.01.29.22270090, Jan 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 359 COVID+ patients in Israel, 116 treated with casirivimab/imdevimab, showing no significant difference with treatment in multivariable analysis.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
|
risk of severe case, 45.6% higher, RR 1.46, p = 0.26, treatment 24 of 116 (20.7%), control 26 of 243 (10.7%), adjusted per study, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Shopen et al., 31 Jan 2022, retrospective, Israel, preprint, 11 authors, study period June 2021 - September 2021.
Doubtful Clinical Benefit of Casirivimab-Imdevimab Treatment for Disease Severity Outcome of High-Risk Patients with SARS-CoV-2 Delta Variant Infection
doi:10.1101/2022.01.29.22270090
Casirivimab/Imdevimab therapy reportedly retains neutralization potency against circulating SARS-CoV-2 variants, including Delta (B.1.617.2), but there are sparse data on its clinical benefit against the Delta variant among vaccinated and unvaccinated patients. We explored its therapeutic effect on COVID-19 severity outcome in terms of room air saturation <93% within 14 days of initial presentation and 45-day all-cause mortality among high-risk patients with SARS-CoV-2 Delta variant infection and compared its effect between vaccinated and unvaccinated patients. We conducted a retrospective cohort study at a tertiary care medical center between 6/2021 and 9/2021 and included patients who presented with a positive PCR for SARS-CoV-2 and fulfilled the criteria for Casirivimab/Imdevimab treatment. Of the 359 suitable patients (52% female, median age 63 years), 116 were treated with Casirivimab/Imdevimab and 243 were not. Two-hundred and one (56%) patients had received at least 2 SARS-CoV-2 vaccinations. Casirivimab/Imdevimab treatment was not an independent protective factor of COVID-19 severity outcome (multivariable analysis). Chronic kidney disease (aOR=3.51 [95%CI: 1.34-9.20], P=0.01), lower saturation levels (aOR=0.7 [95%CI: 0.58-0.85], P<0.01), abnormal chest x-ray findings (aOR=2.92, [95%CI: 1.24-6.87, P=0.01), and higher C-reactive protein levels (aOR=1.01 [95%CI: 1.00-1.01], P=0.008) were independent risk factors of COVID-19 severity. Positive immunization status was an independent protective factor (aOR=0.33 [95%CI: 0.14-0.77], P=0.01). A sub analysis of patients treated with Casirivimab/Imdevimab revealed no significant difference in COVID-19 severity between vaccinated and unvaccinated patients. These findings demonstrate no added benefit of Casrivimab/Imdevinab treatment for high-risk patients with the SARS-CoV-2 Delta variant infection, regardless of their vaccination status.
References
Balbi, Caroli, Corsi, Milanese, Surace et al., Chest X-ray for predicting mortality and the need for ventilatory support in COVID-19 patients presenting to the emergency department, Eur Radiol, doi:10.1007/s00330-020-07270-1
Baum, Fulton, Wloga, Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies, Science
Bierle, Ganesh, Tulledge-Scheitel, Monoclonal Antibody Treatment of Breakthrough COVID-19 in Fully Vaccinated Individuals with High-Risk Comorbidities
Bierle, Ganesh, Wilker, Hanson, Moehnke et al., Influence of Social and Cultural Factors on the Decision to Consent for Monoclonal Antibody Treatment among High-Risk Patients with Mild-Moderate COVID-19, J Prim Care Community Health, doi:10.1177/21501327211019282
Cameroni, Saliba, Bowen, Rosen, Culap et al., Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift bioRxiv, doi:10.1101/2021.12.12.472269
Casirivimab + Imdevimab Injection, Elliott, William, Chan, None, Internal Medicine Alert and Atlanta
Copin, Baum, Wloga, The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies, Cell
Cozzi, Albanesi, Cavigli, Moroni, Bindi et al., Chest X-ray in new Coronavirus Disease 2019 (COVID-19) infection: findings and correlation with clinical outcome, Radiol Med, doi:10.1007/s11547-020-01232-9
Docherty, Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterization Protocol: prospective observational cohort study, BMJ
Embi, Levy, Naleway, Patel, Gaglani et al., Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults -Nine States, January, MMWR Morbidity and mortality weekly report
Falcone, Tiseo, Valoriani, Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in Preventing Progression to Severe COVID-19 and Role of Variants of Concern, Infect Dis Ther, doi:10.1007/s40121-021-00525-4
Fda, Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19
Ganesh, Philpot, Bierle, Real-World Clinical Outcomes of Bamlanivimab and Casirivimab-Imdevimab Among High-Risk Patients With Mild to Moderate Coronavirus Disease 2019, J Infect Dis, doi:10.1093/infdis/jiab377
Gao, Dong, Risk Factors for Severe and Critically Ill COVID 19 Patients: A Review, Allergy (Copenhagen)
Gottlieb, Nirula, Chen, Boscia, Heller et al., Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.0202
Haas, Angulo, Mclaughlin, Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data, Lancet
He, He, Hong, Zhang, Wei, The challenges of COVID-19 Delta variant: Prevention and vaccine development
Horby, Mafham, Peto, Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Mair, Berger, Berghoff, Starzer, Ortmayr et al., Humoral Immune Response in Hematooncological Patients and Health Care Workers Who Received SARS-CoV-2 Vaccinations, JAMA Oncology
Marrams, Kobayashi, Suzuki, patients: a systematic literature review and meta-analysis
Planas, Saunders, Maes, Guivel-Benhassine, Planchais et al., Considerable escape of SARS-CoV-2 variant Omicron to antibody neutralization, bioRxiv, doi:10.1101/2021.12.14.472630
Tabata, Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis, Lancet Infect. Dis
Von Elm, Altman, Egger, Pocock, Gøtzsche et al., The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, Lancet
Wang, Nair, Liu, Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody cocktail clinical outcomes study in Covid-19 outpatients, doi:10.1101/2021.05.19.21257469v2
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Williamson, Walker, Bhaskaran, Factors associ-ated with COVID-19-related death using OpenSAFELY, Nature
Zhang, Cao, Tan, Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients, Allergy
DOI record:
{
"DOI": "10.1101/2022.01.29.22270090",
"URL": "http://dx.doi.org/10.1101/2022.01.29.22270090",
"abstract": "<jats:p>Casirivimab/Imdevimab therapy reportedly retains neutralization potency against circulating SARS-CoV-2 variants, including Delta (B.1.617.2), but there are sparse data on its clinical benefit against the Delta variant among vaccinated and unvaccinated patients. We explored its therapeutic effect on COVID-19 severity outcome in terms of room air saturation <93% within 14 days of initial presentation and 45 day all cause mortality among high-risk patients with SARS-CoV-2 Delta variant infection and compared its effect between vaccinated and unvaccinated patients. We conducted a retrospective cohort study at a tertiary care medical center between 6/2021 and 9/2021 and included patients who presented with a positive PCR for SARS-CoV-2 and fulfilled the criteria for Casirivimab/Imdevimab treatment. Of the 359 suitable patients (52% female, median age 63 years), 116 were treated with Casirivimab/Imdevimab and 243 were not. Two hundred and one (56%) patients had received at least 2 SARS-CoV-2 vaccinations. Casirivimab/Imdevimab treatment was not an independent protective factor of COVID 19 severity outcome (multivariable analysis). Chronic kidney disease (aOR=3.51 [95%CI: 1.34 to 9.20], P=0.01), lower saturation levels (aOR=0.7 [95%CI: 0.58 to 0.85], P<0.01), abnormal chest x ray findings (aOR=2.92, [95%CI: 1.24 to 6.87, P=0.01), and higher C-reactive protein levels (aOR=1.01 [95%CI: 1.00 to 1.01], P=0.008) were independent risk factors of COVID-19 severity. Positive immunization status was an independent protective factor (aOR=0.33 [95%CI: 0.14 to 0.77], P=0.01). A sub analysis of patients treated with Casirivimab/Imdevimab revealed no significant difference in COVID-19 severity between vaccinated and unvaccinated patients. These findings demonstrate no added benefit of Casrivimab/Imdevinab treatment for high-risk patients with the SARS-CoV-2 Delta variant infection, regardless of their vaccination status.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
1,
31
]
]
},
"author": [
{
"affiliation": [],
"family": "Shopen",
"given": "Noah",
"sequence": "first"
},
{
"affiliation": [],
"family": "Dekel",
"given": "Michal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mizrahi",
"given": "Michal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zandberg",
"given": "Efrat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bishouty",
"given": "Nancy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Talmud",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vaknin",
"given": "Ben",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haberman",
"given": "Shira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Katz Shalhav",
"given": "Malka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zeltser",
"given": "David",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7694-6407",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cohen",
"given": "Neta",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
31
]
],
"date-time": "2022-01-31T21:10:26Z",
"timestamp": 1643663426000
},
"deposited": {
"date-parts": [
[
2022,
1,
31
]
],
"date-time": "2022-01-31T21:10:26Z",
"timestamp": 1643663426000
},
"group-title": "Emergency Medicine",
"indexed": {
"date-parts": [
[
2022,
1,
31
]
],
"date-time": "2022-01-31T21:41:28Z",
"timestamp": 1643665288563
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
1,
31
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.01.29.22270090",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
1,
31
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
1,
31
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Doubtful Clinical Benefit of Casirivimab-Imdevimab Treatment for Disease Severity Outcome of High-Risk Patients with SARS-CoV-2 Delta Variant Infection"
],
"type": "posted-content"
}
