Dietary supplements to reduce symptom severity and duration in people with SARS-CoV-2: a double-blind randomised controlled trial
et al., BMJ Open, doi:10.1136/bmjopen-2023-073761, NCT04780061, Sep 2023
Early terminated low-risk population (no hospitalization) very late treatment (mean 8 days) RCT with 44 patients treated with vitamin C, D, K, and zinc, and 46 control patients, showing no significant differences.
Authors acknowledge that the very late treatment is a major limitation, noting that in an ideal setting, "patients would begin taking therapeutic interventions immediately after noticing symptoms". Authors note that patients already had a low symptom burden at baseline and that "it is likely that the majority of the participants had almost fully recovered before starting treatment."
Authors note that most participants were young, had few comorbidities and had excellent self-rated health at baseline, leaving less room for improvement.
There was low compliance with completing surveys. Data from only 64% of patients was in the main analysis.
Authors claim "high internal validity", but the loss of data was statistically significantly different between arms, without analysis or mention. Since the study involves widely available treatments, one possibility is that patients in the control arm who feel sick may be more likely to independently take the treatments (via supplementation or food/sun exposure), believing that they are in the control arm or that additional dosing is safe, and they may then feel it's inappropriate to continue submitting the surveys.
Discussion is biased, stating that "evidence for the use of these products in people with COVID-19 is limited", however there were 219 controlled studies at the time, including 8, 27, and 16 RCTs for vitamin C, D, and zinc. Authors claim high similarity between arms however there was 60% vs. 41% male patients, and 88% vs. 68% of patients that received a third dose.
Authors claim that treatment "showed no beneficial effects for overall health or symptom burden". However 48% lower ER visits is beneficial, and most outcomes show a benefit. The only statistically significant effect was the loss of data, however significant clinical effects are not expected based on the small sample, very late treatment, event rates, and outcomes.
This study is excluded in meta-analysis:
combined treatments may contribute more to the effect seen.
|
ER visit, 47.6% lower, RR 0.52, p = 0.68, treatment 2 of 42 (4.8%), control 4 of 44 (9.1%), NNT 23.
|
|
relative mean cumulative symptom score, 13.8% better, RR 0.86, p = 0.41, treatment mean 166.3 (±92.3) n=34, control mean 192.9 (±153.6) n=24.
|
|
EQ-VAS average score <80, 29.4% lower, RR 0.71, p = 0.54, treatment 7 of 34 (20.6%), control 7 of 24 (29.2%), NNT 12, average daily EQ-VAS score <80.
|
|
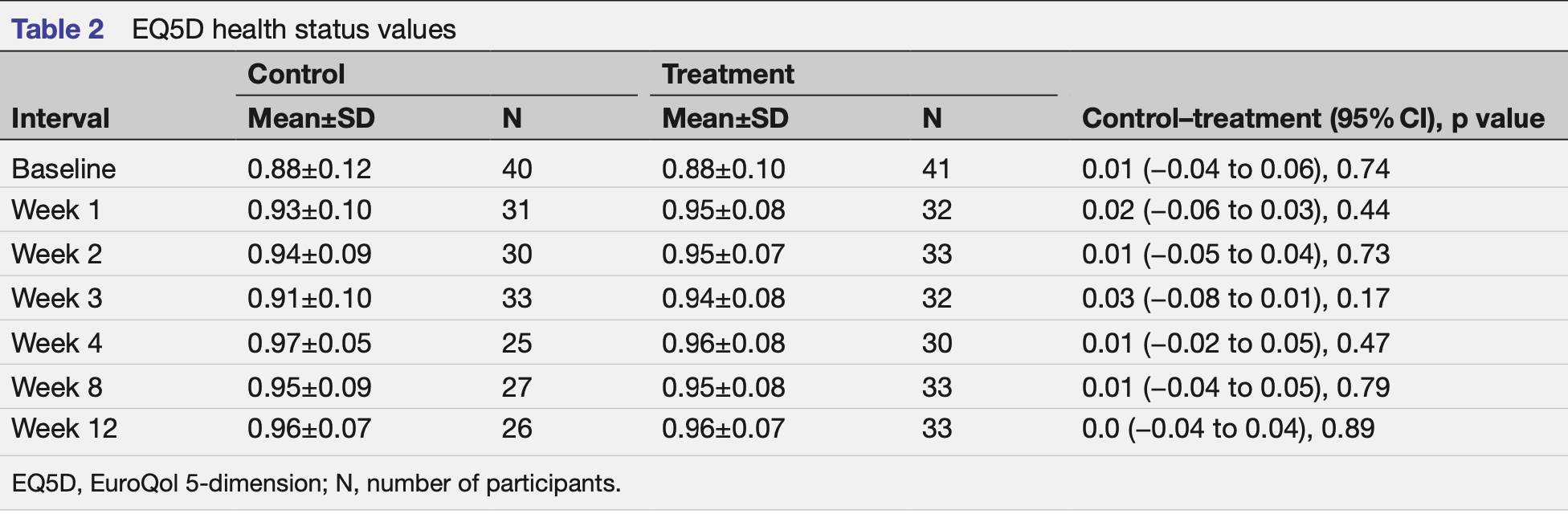
relative EQ5D improvement, 28.6% better, RR 0.71, p = 0.44, treatment 32, control 31, relative improvement in EQ5D, week 1.
|
|
relative EQ5D improvement, 14.3% better, RR 0.86, p = 0.73, treatment 33, control 30, relative improvement in EQ5D, week 2.
|
|
relative EQ5D improvement, 50.0% better, RR 0.50, p = 0.17, treatment 32, control 33, relative improvement in EQ5D, week 3.
|
|
relative EQ5D improvement, 12.5% worse, RR 1.12, p = 0.47, treatment 30, control 25, relative improvement in EQ5D, week 4.
|
|
recovery time, 4.0% higher, relative time 1.04, p = 0.81, treatment 34, control 24.
|
|
risk of long COVID, 12.1% lower, RR 0.88, p = 1.00, treatment 3 of 33 (9.1%), control 3 of 29 (10.3%), NNT 80, 12 weeks.
|
|
risk of long COVID, 35.7% lower, RR 0.64, p = 0.69, treatment 3 of 35 (8.6%), control 4 of 30 (13.3%), NNT 21, 8 weeks.
|
|
risk of long COVID, 0.6% lower, RR 0.99, p = 1.00, treatment 6 of 35 (17.1%), control 5 of 29 (17.2%), NNT 1015, 4 weeks.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Seely et al., 22 Sep 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Canada, peer-reviewed, mean age 39.9, 10 authors, study period September 2021 - April 2022, this trial uses multiple treatments in the treatment arm (combined with vitamin C, D, K2, and zinc) - results of individual treatments may vary, trial NCT04780061 (history).
Contact: dseely@thechi.c, mlegacy@thechi.ca.
Dietary supplements to reduce symptom severity and duration in people with SARS-CoV-2: a double-blind randomised controlled trial
BMJ Open, doi:10.1136/bmjopen-2023-073761
Background COVID-19 has caused morbidity, hospitalisation and mortality worldwide. Despite effective vaccines, there is still a need for effective treatments, especially for people in the community. Dietary supplements have long been used to treat respiratory infections, and preliminary evidence indicates some may be effective in people with COVID-19. We sought to evaluate whether a combination of vitamin C, vitamin D 3 , vitamin K 2 and zinc could improve overall health and decrease symptom burden in outpatients diagnosed with COVID-19. Methods Participants were randomised to receive either vitamin C (6 g), vitamin D 3 (1000 units), vitamin K 2 (240 μg) and zinc acetate (75 mg) or placebo daily for 21 days and were followed for 12 weeks. An additional loading dose of 50 000 units vitamin D 3 (or placebo) was given on day one. The primary outcome was participant-reported overall health using the EuroQol Visual Assessment Scale summed over 21 days. Secondary outcomes included health status, symptom severity, symptom duration, delayed return to usual health, frequency of hospitalisation and mortality. Results 90 patients (46 control, 44 treatment) were randomised. The study was stopped prematurely due to insufficient capacity for recruitment. The mean difference (control-treatment) in cumulative overall health was -37.4 (95% CI -157.2 to 82.3), p=0.53 on a scale of 0-2100. No clinically or statistically significant differences were seen in any secondary outcomes. Interpretation In this double-blind, placebo-controlled, randomised trial of outpatients diagnosed with COVID-19, the dietary supplements vitamin C, vitamin D 3 , vitamin K 2 and zinc acetate showed no clinically or statistically significant effects on the documented measures of health compared with a placebo when given for 21 days. Termination due to feasibility limited our ability to demonstrate the efficacy of these supplements for COVID-19. Further research is needed to determine clinical utility. Trial registration number NCT04780061.
Competing interests None declared. Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not applicable. Ethics approval This study involves human participants and this study was approved by the Research Ethics Boards of the Ottawa Health Sciences Network (20210072-01H) and Canadian College of Naturopathic Medicine (CCNMREB036. Seely.Wilson). No study activities took place before approval by both organisations. Each participant signed an informed consent form approved by both organisations prior to participation in the study. Participants gave informed consent to participate in the study before taking part. Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request. Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages),..
References
Desai, Dirajlal-Fargo, Durieux, Vitamin K & D deficiencies are independently associated with COVID-19 disease severity, Open Forum Infect Dis, doi:10.1093/ofid/ofab408
Ding, Chen, Wu, Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-ΚB and JAK-STAT signaling pathway, Microbes Infect, doi:10.1016/j.micinf.2017.08.009
Dofferhoff, Piscaer, Schurgers, Reduced vitamin K status as a potentially modifiable prognostic risk factor in cOVID-19
Douglas, Hemila, Souza, Vitamin C for preventing and treating the common cold, Cochrane Database Syst Rev, doi:10.1002/14651858.CD000980.pub2
Feng, Parkin, Devlin, Assessing the performance of the EQ-VAS in the NHS Proms programme, Qual Life Res, doi:10.1007/s11136-013-0537-z
Frederiksen, Zhang, Foged, The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies, Front Immunol, doi:10.3389/fimmu.2020.01817
Geist, Bateman, Hayden, In vitro activity of zinc salts against human rhinoviruses, Antimicrob Agents Chemother, doi:10.1128/AAC.31.4.622
Heinz, Henson, Austin, Quercetin supplementation and upper respiratory tract infection: a randomized community clinical trial, Pharmacol Res, doi:10.1016/j.phrs.2010.05.001
Hemilä, Vitamin C and infections, Nutrients, doi:10.3390/nu9040339
Hu, Wu, Logue, Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in adults and children: a systematic review and meta-analysis, PLoS One, doi:10.1371/journal.pone.0181780
Jung, Seo, Lee, Vitamin D₃ supplementation reduces the symptoms of upper respiratory tract infection during winter training in vitamin D-insufficient taekwondo athletes: a randomized controlled trial, Int J Environ Res Public Health, doi:10.3390/ijerph15092003
Korant, Butterworth, Inhibition by zinc of Rhinovirus protein cleavage: interaction of zinc with capsid polypeptides, J Virol, doi:10.1128/JVI.18.1.298-306.1976
Larkin, Preventing COVID-19, saving lives in lower-income countries, JAMA, doi:10.1001/jama.2022.13667
Legacy, Seely, Conte, Dietary supplements to reduce symptom severity and duration in people with SARS-Cov-2: study on September 23, 2023 by guest, BMJ Open, doi:10.1136/bmjopen-2021-057024
Lorenzo-Redondo, Ozer, Hultquist, Covid-19: is Omicron less lethal than delta?, BMJ, doi:10.1136/bmj.o1806
Martineau, Jolliffe, Hooper, Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Menachemi, Dixon, Wools-Kaloustian, How many SARS-Cov-2-infected people require hospitalization? Using random sample testing to better inform preparedness efforts, J Public Health Manag Pract, doi:10.1097/PHH.0000000000001331
Milne, Hames, Scotton, Does infection with or vaccination against SARS-Cov-2 lead to lasting immunity?, Lancet Respir Med, doi:10.1016/S2213-2600(21)00407-0
Novick, Godfrey, Godfrey, How does zinc modify the common cold? clinical observations and implications regarding mechanisms of action, Med Hypotheses, doi:10.1016/s0306-9877(96)90259-5
Polack, Thomas, Kitchin, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Rabin, De Charro, EQ-5D: a measure of health status from the Euroqol group, Ann Med, doi:10.3109/07853890109002087
Schulz, Altman, Moher, 2010 statement: updated guidelines for reporting parallel group randomised trials, BMC Med, doi:10.1186/1741-7015-8-18
Science, Johnstone, Roth, Zinc for the treatment of the common cold: a systematic review and meta-analysis of randomized controlled trials, CMAJ, doi:10.1503/cmaj.111990
Sharif, Alzahrani, Ahmed, Efficacy, Immunogenicity and safety of COVID-19 vaccines: a systematic review and metaanalysis, Front Immunol, doi:10.3389/fimmu.2021.714170
Shimizu, Ito, Yui, Intake of 25-Hydroxyvitamin D3 reduces duration and severity of upper respiratory tract infection: a randomized, double-blind, placebo-controlled, parallel group comparison study, J Nutr Health Aging, doi:10.1007/s12603-017-0952-x
Stolk, Ludwig, Rand, Overview, update, and lessons learned from the International EQ-5D-5L valuation work: version 2 of the EQ-5D-5L valuation protocol, Value Health, doi:10.1016/j.jval.2018.05.010
Swann, Anarchist technologies': anarchism, cybernetics and mutual aid in community responses to the COVID-19 crisis, Organization (Lond), doi:10.1177/13505084221090632
Tenforde, Kim, Lindsell, Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network -United States, March-June 2020, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6930e1
Thomas, Patel, Bittel, Effect of high-dose zinc and Ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-Cov-2 infection: the COVID A to Z randomized clinical trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.0369
Wiersinga, Rhodes, Cheng, Pathophysiology, transmission, diagnosis, and treatment of Coronavirus disease 2019 (COVID-19): A review, JAMA, doi:10.1001/jama.2020.12839
Yisak, Ewunetei, Kefale, Effects of vitamin D on COVID-19 infection and prognosis: a systematic review, Risk Manag Healthc Policy, doi:10.2147/RMHP.S291584
DOI record:
{
"DOI": "10.1136/bmjopen-2023-073761",
"ISSN": [
"2044-6055",
"2044-6055"
],
"URL": "http://dx.doi.org/10.1136/bmjopen-2023-073761",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>COVID-19 has caused morbidity, hospitalisation and mortality worldwide. Despite effective vaccines, there is still a need for effective treatments, especially for people in the community. Dietary supplements have long been used to treat respiratory infections, and preliminary evidence indicates some may be effective in people with COVID-19. We sought to evaluate whether a combination of vitamin C, vitamin D<jats:sub>3</jats:sub>, vitamin K<jats:sub>2</jats:sub>and zinc could improve overall health and decrease symptom burden in outpatients diagnosed with COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Participants were randomised to receive either vitamin C (6 g), vitamin D<jats:sub>3</jats:sub>(1000 units), vitamin K<jats:sub>2</jats:sub>(240 μg) and zinc acetate (75 mg) or placebo daily for 21 days and were followed for 12 weeks. An additional loading dose of 50 000 units vitamin D<jats:sub>3</jats:sub>(or placebo) was given on day one. The primary outcome was participant-reported overall health using the EuroQol Visual Assessment Scale summed over 21 days. Secondary outcomes included health status, symptom severity, symptom duration, delayed return to usual health, frequency of hospitalisation and mortality.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>90 patients (46 control, 44 treatment) were randomised. The study was stopped prematurely due to insufficient capacity for recruitment. The mean difference (control–treatment) in cumulative overall health was −37.4 (95% CI −157.2 to 82.3), p=0.53 on a scale of 0–2100. No clinically or statistically significant differences were seen in any secondary outcomes.</jats:p></jats:sec><jats:sec><jats:title>Interpretation</jats:title><jats:p>In this double-blind, placebo-controlled, randomised trial of outpatients diagnosed with COVID-19, the dietary supplements vitamin C, vitamin D<jats:sub>3</jats:sub>, vitamin K<jats:sub>2</jats:sub>and zinc acetate showed no clinically or statistically significant effects on the documented measures of health compared with a placebo when given for 21 days. Termination due to feasibility limited our ability to demonstrate the efficacy of these supplements for COVID-19. Further research is needed to determine clinical utility.</jats:p></jats:sec><jats:sec><jats:title>Trial registration number</jats:title><jats:p><jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04780061\">NCT04780061</jats:ext-link>.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmjopen-2023-073761"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-4893-5254",
"affiliation": [],
"authenticated-orcid": false,
"family": "Seely",
"given": "Dugald",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-4417-1656",
"affiliation": [],
"authenticated-orcid": false,
"family": "Legacy",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Conte",
"given": "Ellen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Keates",
"given": "Caitlyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Psihogios",
"given": "Athanasios",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8478-8170",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ramsay",
"given": "Tim",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3389-2485",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fergusson",
"given": "Dean A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kanji",
"given": "Salmaan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simmons",
"given": "John-Graydon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilson",
"given": "Kumanan",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04780061",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "BMJ Open",
"container-title-short": "BMJ Open",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2023,
9,
22
]
],
"date-time": "2023-09-22T15:30:29Z",
"timestamp": 1695396629000
},
"deposited": {
"date-parts": [
[
2023,
9,
22
]
],
"date-time": "2023-09-22T15:30:45Z",
"timestamp": 1695396645000
},
"indexed": {
"date-parts": [
[
2023,
9,
23
]
],
"date-time": "2023-09-23T14:47:10Z",
"timestamp": 1695480430155
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2023,
9
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2023,
9,
22
]
]
},
"published-print": {
"date-parts": [
[
2023,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 21,
"start": {
"date-parts": [
[
2023,
9,
22
]
],
"date-time": "2023-09-22T00:00:00Z",
"timestamp": 1695340800000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjopen-2023-073761",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e073761",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2023,
9
]
]
},
"published-online": {
"date-parts": [
[
2023,
9,
22
]
]
},
"published-print": {
"date-parts": [
[
2023,
9
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1177/13505084221090632",
"article-title": "'Anarchist technologies': anarchism, cybernetics and mutual aid in community responses to the COVID-19 crisis",
"author": "Swann",
"doi-asserted-by": "crossref",
"first-page": "193",
"journal-title": "Organization (Lond)",
"key": "2023092208301025000_13.9.e073761.1",
"volume": "30",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.2"
},
{
"DOI": "10.1016/S2213-2600(21)00407-0",
"article-title": "Does infection with or vaccination against SARS-Cov-2 lead to lasting immunity?",
"author": "Milne",
"doi-asserted-by": "crossref",
"first-page": "1450",
"journal-title": "Lancet Respir Med",
"key": "2023092208301025000_13.9.e073761.3",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.714170",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.4"
},
{
"DOI": "10.3389/fimmu.2020.01817",
"doi-asserted-by": "crossref",
"key": "2023092208301025000_13.9.e073761.5",
"unstructured": "Frederiksen LSF , Zhang Y , Foged C , et al . The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front Immunol 2020;11:1817. doi:10.3389/fimmu.2020.01817"
},
{
"article-title": "Preventing COVID-19, saving lives in lower-income countries",
"author": "Larkin",
"first-page": "611",
"journal-title": "JAMA",
"key": "2023092208301025000_13.9.e073761.6",
"volume": "328",
"year": "2022"
},
{
"key": "2023092208301025000_13.9.e073761.7",
"unstructured": "Therapeutics and COVID-19: living guideline. World Health Organization; 2022."
},
{
"DOI": "10.1371/journal.pone.0181780",
"doi-asserted-by": "crossref",
"key": "2023092208301025000_13.9.e073761.8",
"unstructured": "Hu X-Y , Wu R-H , Logue M , et al . Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in adults and children: a systematic review and meta-analysis. PLoS One 2017;12:e0181780. doi:10.1371/journal.pone.0181780"
},
{
"DOI": "10.1016/j.micinf.2017.08.009",
"article-title": "Andrographolide inhibits influenza A virus-induced inflammation in a murine model through NF-ΚB and JAK-STAT signaling pathway",
"author": "Ding",
"doi-asserted-by": "crossref",
"first-page": "605",
"journal-title": "Microbes Infect",
"key": "2023092208301025000_13.9.e073761.9",
"volume": "19",
"year": "2017"
},
{
"DOI": "10.1016/j.phrs.2010.05.001",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.10"
},
{
"DOI": "10.1136/bmj.i6583",
"doi-asserted-by": "crossref",
"key": "2023092208301025000_13.9.e073761.11",
"unstructured": "Martineau AR , Jolliffe DA , Hooper RL , et al . Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583. doi:10.1136/bmj.i6583"
},
{
"DOI": "10.1007/s12603-017-0952-x",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.12"
},
{
"DOI": "10.3390/ijerph15092003",
"doi-asserted-by": "crossref",
"key": "2023092208301025000_13.9.e073761.13",
"unstructured": "Jung HC , Seo MW , Lee S , et al . Vitamin D₃ supplementation reduces the symptoms of upper respiratory tract infection during winter training in vitamin D-insufficient taekwondo athletes: a randomized controlled trial. Int J Environ Res Public Health 2018;15:2003. doi:10.3390/ijerph15092003"
},
{
"DOI": "10.1503/cmaj.111990",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.14"
},
{
"DOI": "10.20944/preprints202004.0457.v2",
"doi-asserted-by": "crossref",
"key": "2023092208301025000_13.9.e073761.15",
"unstructured": "Dofferhoff ASM , Piscaer I , Schurgers LJ . Reduced vitamin K status as a potentially modifiable prognostic risk factor in cOVID-19. doi:10.20944/preprints202004.0457.v2"
},
{
"DOI": "10.1093/ofid/ofab408",
"doi-asserted-by": "crossref",
"key": "2023092208301025000_13.9.e073761.16",
"unstructured": "Desai AP , Dirajlal-Fargo S , Durieux JC , et al . Vitamin K & D deficiencies are independently associated with COVID-19 disease severity. Open Forum Infect Dis 2021;8. doi:10.1093/ofid/ofab408"
},
{
"DOI": "10.2147/RMHP.S291584",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.17"
},
{
"DOI": "10.1097/PHH.0000000000001331",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.18"
},
{
"DOI": "10.1186/1741-7015-8-18",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.19"
},
{
"DOI": "10.1136/bmjopen-2021-057024",
"doi-asserted-by": "crossref",
"key": "2023092208301025000_13.9.e073761.20",
"unstructured": "Legacy M , Seely D , Conte E , et al . Dietary supplements to reduce symptom severity and duration in people with SARS-Cov-2: study protocol for a randomised, double-blind, placebo controlled clinical trial. BMJ Open 2022;12:e057024. doi:10.1136/bmjopen-2021-057024"
},
{
"DOI": "10.1007/s11136-013-0537-z",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.21"
},
{
"DOI": "10.3109/07853890109002087",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.22"
},
{
"DOI": "10.1016/j.jval.2018.05.010",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.23"
},
{
"key": "2023092208301025000_13.9.e073761.24",
"unstructured": "US Food and Drug Admiinistration . [Guidance for Industry 2020 [Available from]. Assessing COVID-19-RelatedSymptoms in Outpatient Adult and Adolescent Subjects in clinical trials of drugs and biological products for covid-19 prevention or treatment, Available: https://www.fda.gov/media/142143/download"
},
{
"DOI": "10.15585/mmwr.mm6930e1",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.25"
},
{
"DOI": "10.1001/jama.2020.12839.e4",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.26"
},
{
"DOI": "10.1002/14651858.CD000980.pub2",
"doi-asserted-by": "crossref",
"key": "2023092208301025000_13.9.e073761.27",
"unstructured": "Douglas RM , Hemila H , D’Souza R , et al . Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2004:CD000980. doi:10.1002/14651858.CD000980.pub2"
},
{
"DOI": "10.3390/nu9040339",
"doi-asserted-by": "crossref",
"key": "2023092208301025000_13.9.e073761.28",
"unstructured": "Hemilä H . Vitamin C and infections. Nutrients 2017;9:339. doi:10.3390/nu9040339"
},
{
"DOI": "10.1016/S0306-9877(96)90259-5",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.29"
},
{
"DOI": "10.1128/jvi.18.1.298-306.1976",
"article-title": "Inhibition by zinc of Rhinovirus protein cleavage: interaction of zinc with capsid polypeptides",
"author": "Korant",
"doi-asserted-by": "crossref",
"first-page": "298",
"journal-title": "J Virol",
"key": "2023092208301025000_13.9.e073761.30",
"volume": "18",
"year": "1976"
},
{
"DOI": "10.1128/AAC.31.4.622",
"doi-asserted-by": "publisher",
"key": "2023092208301025000_13.9.e073761.31"
},
{
"DOI": "10.1001/jamanetworkopen.2021.0369",
"doi-asserted-by": "crossref",
"key": "2023092208301025000_13.9.e073761.32",
"unstructured": "Thomas S , Patel D , Bittel B , et al . Effect of high-dose zinc and Ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-Cov-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open 2021;4:e210369. doi:10.1001/jamanetworkopen.2021.0369"
},
{
"DOI": "10.1136/bmj.o1806",
"article-title": "Covid-19: is Omicron less lethal than delta?",
"author": "Lorenzo-Redondo",
"doi-asserted-by": "crossref",
"first-page": "1806",
"journal-title": "BMJ",
"key": "2023092208301025000_13.9.e073761.33",
"volume": "378",
"year": "2022"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2023-073761"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Dietary supplements to reduce symptom severity and duration in people with SARS-CoV-2: a double-blind randomised controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "13"
}
seely