The effect of TRV027 on coagulation in COVID‐19: A pilot randomized, placebo‐controlled trial
et al., British Journal of Clinical Pharmacology, doi:10.1111/bcp.15618, COVRAS, NCT04419610, Dec 2022
Double-blind RCT 30 hospitalized COVID-19 patients in the United Kingdom showing a non-statistically significant trend towards decreased D-dimer levels with TRV027, an angiotensin-(1-7) analogue, compared to placebo. The trial was terminated early due to declining cases.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
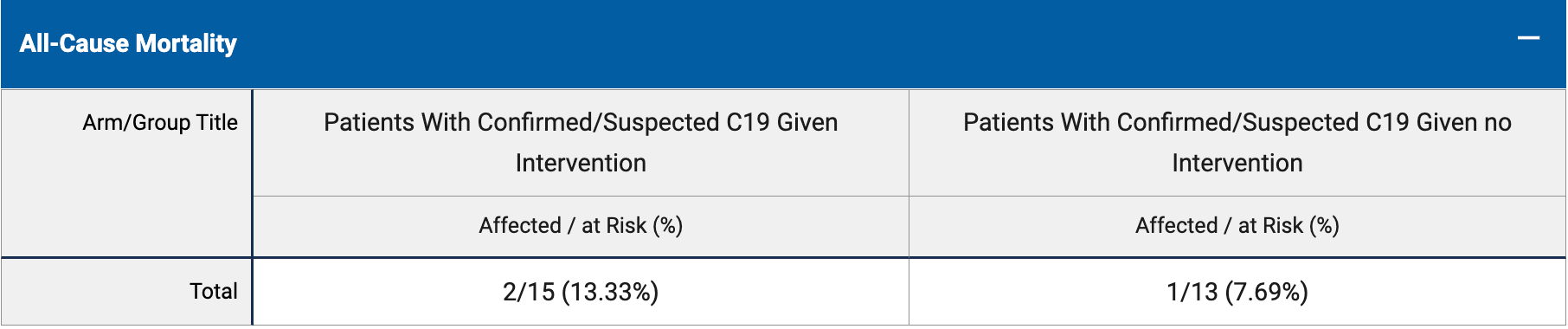
risk of death, 73.3% higher, RR 1.73, p = 1.00, treatment 2 of 15 (13.3%), control 1 of 13 (7.7%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Robbins et al., 14 Dec 2022, Double Blind Randomized Controlled Trial, placebo-controlled, United Kingdom, peer-reviewed, 23 authors, trial NCT04419610 (history) (COVRAS).
Contact: alexander.robbins@nhs.net.
The effect of TRV027 on coagulation in COVID‐19: A pilot randomized, placebo‐controlled trial
British Journal of Clinical Pharmacology, doi:10.1111/bcp.15618
COVID-19 causes significant thrombosis and coagulopathy, with elevated D-dimer a predictor of adverse outcome. The precise mechanism of this coagulopathy remains unclear; one hypothesis is that loss of angiotensin-converting enzyme 2 activity during viral endocytosis leads to pro-inflammatory angiotensin-II accumulation, loss of angiotensin-1-7 and subsequent vascular endothelial activation. We undertook a double-blind randomized, placebo-controlled experimental medicine study to assess the effect of TRV027, a synthetic angiotensin-1-7 analogue on D-dimer in 30 patients admitted to hospital with COVID-19. The study showed a similar rate of adverse events in TRV027 and control groups. There was a numerical decrease in D-dimer in the TRV027 group and increase in D-dimer in the placebo group; however, this did not reach statistical significance (P = .15). A Bayesian analysis demonstrated that there was a 92% probability that this change represented a true drug effect.
COMPETING INTERESTS SUPPORTING INFORMATION Additional supporting information can be found online in the Supporting Information section at the end of this article.
References
Ackermann, Verleden, Kuehnel, Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19, N Engl J Med, doi:info:doi/10.1056/nejmoa2015432
Alexander, Christopoulos, Davenport, The Concise Guide to PHARMACOLOGY 2021/22: G protein-coupled receptors, Br J Pharmacol, doi:info:doi/10.1111/bph.15538
Clinicaltrials, Gov, RAS and coagulopathy in COVID19
Glowacka, Bertram, Herzog, Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63, J Virol, doi:info:doi/10.1128/JVI.01248-09
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform, doi:info:doi/10.1016/j.jbi.2008.08.010
Imai, Kuba, Rao, Angiotensin-converting enzyme 2 protects from severe acute lung failure, Nature, doi:info:doi/10.1038/nature03712
Klein, Gembardt, Supé, Angiotensin-(1-7) protects from experimental acute lung injury, Crit Care Med, doi:info:doi/10.1097/CCM.0b013e31828a6688
Lei, Zhang, Schiavon, SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2, Circ Res, doi:info:doi/10.1101/2020.12.04.409144
Li, Li, Farzan, Harrison, Structure of SARS coronavirus spike receptor-binding domain complexed with receptor, Science, doi:info:doi/10.1126/science.1116480
Li, Receptor recognition mechanisms of coronaviruses: a decade of structural studies, J Virol, doi:info:doi/10.1128/JVI.02615-14
Manglik, Wingler, Rockman, Lefkowitz, β-Arrestin-biased angiotensin II receptor agonists for COVID-19, Circulation, doi:info:doi/10.1161/CIRCULATIONAHA.120.048723
Mccracken, Saginc, He, Lack of evidence of angiotensinconverting enzyme 2 expression and replicative infection by SARS-CoV-2 in human endothelial cells, Circulation, doi:info:doi/10.1161/CIRCULATIONAHA.120.052824
Moher, Hopewell, Schulz, Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials, BMJ, doi:info:doi/10.1136/bmj.c869
Pang, Butler, Collins, Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF), Eur Heart J, doi:info:doi/10.1093/eurheartj/ehx196
Piroth, Cottenet, Mariet, Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study, Lancet Respir Med, doi:info:doi/10.1016/S2213-2600(20)30527-0
Rysz, Al-Saadi, Sjöström, COVID-19 pathophysiology may be driven by an imbalance in the renin-angiotensin-aldosterone system, Nat Commun, doi:info:doi/10.1038/s41467-021-22713-z
Schimmel, Chew, Stocks, Endothelial cells are not productively infected by SARS-CoV-2, Clin Transl Immunol, doi:info:doi/10.1002/cti2.1350
Wan, Shang, Graham, Baric, Li, Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus, J Virol, doi:info:doi/10.1128/JVI.00127-20
Ward, Sarraju, Lee, COVID-19 is associated with higher risk of venous thrombosis, but not arterial thrombosis, compared with influenza: insights from a large US cohort, PLoS ONE, doi:info:doi/10.1371/journal.pone.0261786
Yu, Qin, Chen, Wang, Tian, D-dimer level is associated with the severity of COVID-19, Thromb Res, doi:info:doi/10.1016/j.thromres.2020.07.047
DOI record:
{
"DOI": "10.1111/bcp.15618",
"ISSN": [
"0306-5251",
"1365-2125"
],
"URL": "http://dx.doi.org/10.1111/bcp.15618",
"abstract": "<jats:p>COVID‐19 causes significant thrombosis and coagulopathy, with elevated D‐dimer a predictor of adverse outcome. The precise mechanism of this coagulopathy remains unclear; one hypothesis is that loss of angiotensin‐converting enzyme 2 activity during viral endocytosis leads to pro‐inflammatory angiotensin‐II accumulation, loss of angiotensin‐1‐7 and subsequent vascular endothelial activation. We undertook a double‐blind randomized, placebo‐controlled experimental medicine study to assess the effect of TRV027, a synthetic angiotensin‐1‐7 analogue on D‐dimer in 30 patients admitted to hospital with COVID‐19. The study showed a similar rate of adverse events in TRV027 and control groups. There was a numerical decrease in D‐dimer in the TRV027 group and increase in D‐dimer in the placebo group; however, this did not reach statistical significance (<jats:italic>P</jats:italic> = .15). A Bayesian analysis demonstrated that there was a 92% probability that this change represented a true drug effect.</jats:p>",
"alternative-id": [
"10.1111/bcp.15618"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6929-5802",
"affiliation": [
{
"name": "Imperial College Research Facility Imperial College London London UK"
},
{
"name": "Imperial College Healthcare NHS Trust London UK"
}
],
"authenticated-orcid": false,
"family": "Robbins",
"given": "Alexander J.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Imperial College Healthcare NHS Trust London UK"
},
{
"name": "Department of Surgery and Cancer Imperial College London London UK"
}
],
"family": "Che Bakri",
"given": "Nur Amalina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Healthcare NHS Trust London UK"
}
],
"family": "Toke‐Bjolgerud",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Research Facility Imperial College London London UK"
}
],
"family": "Edwards",
"given": "Aaron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Research Facility Imperial College London London UK"
}
],
"family": "Vikraman",
"given": "Asha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Trevena, Inc. Chesterbrook Pennsylvania USA"
}
],
"family": "Michalsky",
"given": "Cathy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Trevena, Inc. Chesterbrook Pennsylvania USA"
}
],
"family": "Fossler",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Research Facility Imperial College London London UK"
}
],
"family": "Lemm",
"given": "Nana‐Marie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Research Facility Imperial College London London UK"
}
],
"family": "Medhipour",
"given": "Savviz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Research Facility Imperial College London London UK"
}
],
"family": "Budd",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Research Facility Imperial College London London UK"
}
],
"family": "Gravani",
"given": "Athanasia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Research Facility Imperial College London London UK"
},
{
"name": "Imperial College Healthcare NHS Trust London UK"
}
],
"family": "Hurley",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "William Harvey Research Institute, Centre for Cardiovascular Medicine and Devices, Faculty of Medicine and Dentistry Queen Mary University London London UK"
}
],
"family": "Kapil",
"given": "Vikas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cancer Research Clinical Trials Unit University of Birmingham Birmingham UK"
}
],
"family": "Jackson",
"given": "Aimee",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0838-921X",
"affiliation": [
{
"name": "Department of Clinical Pharmacology St George's University of London London UK"
},
{
"name": "Department of Critical Care St George's University Hospitals NHS Foundation Trust London UK"
}
],
"authenticated-orcid": false,
"family": "Lonsdale",
"given": "Dagan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Healthcare NHS Trust London UK"
}
],
"family": "Latham",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Haematology Imperial College London London UK"
}
],
"family": "Laffan",
"given": "Michael",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1307-9275",
"affiliation": [
{
"name": "Imperial College Healthcare NHS Trust London UK"
}
],
"authenticated-orcid": false,
"family": "Chapman",
"given": "Neil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Inflammation and Immunity Imperial College London London UK"
}
],
"family": "Cooper",
"given": "Nichola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Haematology Imperial College London London UK"
}
],
"family": "Szydlo",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Healthcare NHS Trust London UK"
},
{
"name": "National Heart and Lung Institute Imperial College London London UK"
}
],
"family": "Boyle",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Research Facility Imperial College London London UK"
}
],
"family": "Pollock",
"given": "Katrina M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College Research Facility Imperial College London London UK"
},
{
"name": "Department of Brain Sciences Imperial College London London UK"
}
],
"family": "Owen",
"given": "David",
"sequence": "additional"
}
],
"container-title": "British Journal of Clinical Pharmacology",
"container-title-short": "Brit J Clinical Pharma",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
11,
28
]
],
"date-time": "2022-11-28T07:27:42Z",
"timestamp": 1669620462000
},
"deposited": {
"date-parts": [
[
2023,
8,
21
]
],
"date-time": "2023-08-21T20:16:55Z",
"timestamp": 1692649015000
},
"funder": [
{
"DOI": "10.13039/501100000274",
"award": [
"RE/18/4/34215"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000274",
"id-type": "DOI"
}
],
"name": "British Heart Foundation"
},
{
"DOI": "10.13039/501100000761",
"award": [
"COVID 19 Research Fund"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000761",
"id-type": "DOI"
}
],
"name": "Imperial College London"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
12
]
],
"date-time": "2024-09-12T04:31:24Z",
"timestamp": 1726115484210
},
"is-referenced-by-count": 3,
"issue": "4",
"issued": {
"date-parts": [
[
2022,
12,
14
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2023,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
14
]
],
"date-time": "2022-12-14T00:00:00Z",
"timestamp": 1670976000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/bcp.15618",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/bcp.15618",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://bpspubs.onlinelibrary.wiley.com/doi/pdf/10.1111/bcp.15618",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1495-1501",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
12,
14
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
14
]
]
},
"published-print": {
"date-parts": [
[
2023,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1056/nejmoa2015432",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_2_1"
},
{
"DOI": "10.1371/journal.pone.0261786",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_3_1"
},
{
"DOI": "10.1016/S2213‐2600(20)30527‐0",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_4_1"
},
{
"DOI": "10.1016/j.thromres.2020.07.047",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_5_1"
},
{
"DOI": "10.1128/JVI.01248‐09",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_6_1"
},
{
"DOI": "10.1126/science.1116480",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_7_1"
},
{
"DOI": "10.1128/JVI.00127‐20",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_8_1"
},
{
"DOI": "10.1128/JVI.02615‐14",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_9_1"
},
{
"DOI": "10.1101/2020.12.04.409144",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_10_1"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.048723",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_11_1"
},
{
"DOI": "10.1038/s41467‐021‐22713‐z",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_12_1"
},
{
"DOI": "10.1038/nature03712",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_13_1"
},
{
"DOI": "10.1097/CCM.0b013e31828a6688",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_14_1"
},
{
"key": "e_1_2_12_15_1",
"unstructured": "ClinicalTrials.gov RAS and coagulopathy in COVID19 [Internet].https://clinicaltrials.gov/ct2/show/NCT04419610. Accessed June 2 2021."
},
{
"DOI": "10.1136/bmj.c869",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_16_1"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_17_1"
},
{
"DOI": "10.1111/bph.15538",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_18_1"
},
{
"DOI": "10.1093/eurheartj/ehx196",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_19_1"
},
{
"DOI": "10.1002/cti2.1350",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_20_1"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.052824",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_21_1"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.15618"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The effect of TRV027 on coagulation in COVID‐19: A pilot randomized, placebo‐controlled trial",
"type": "journal-article",
"volume": "89"
}