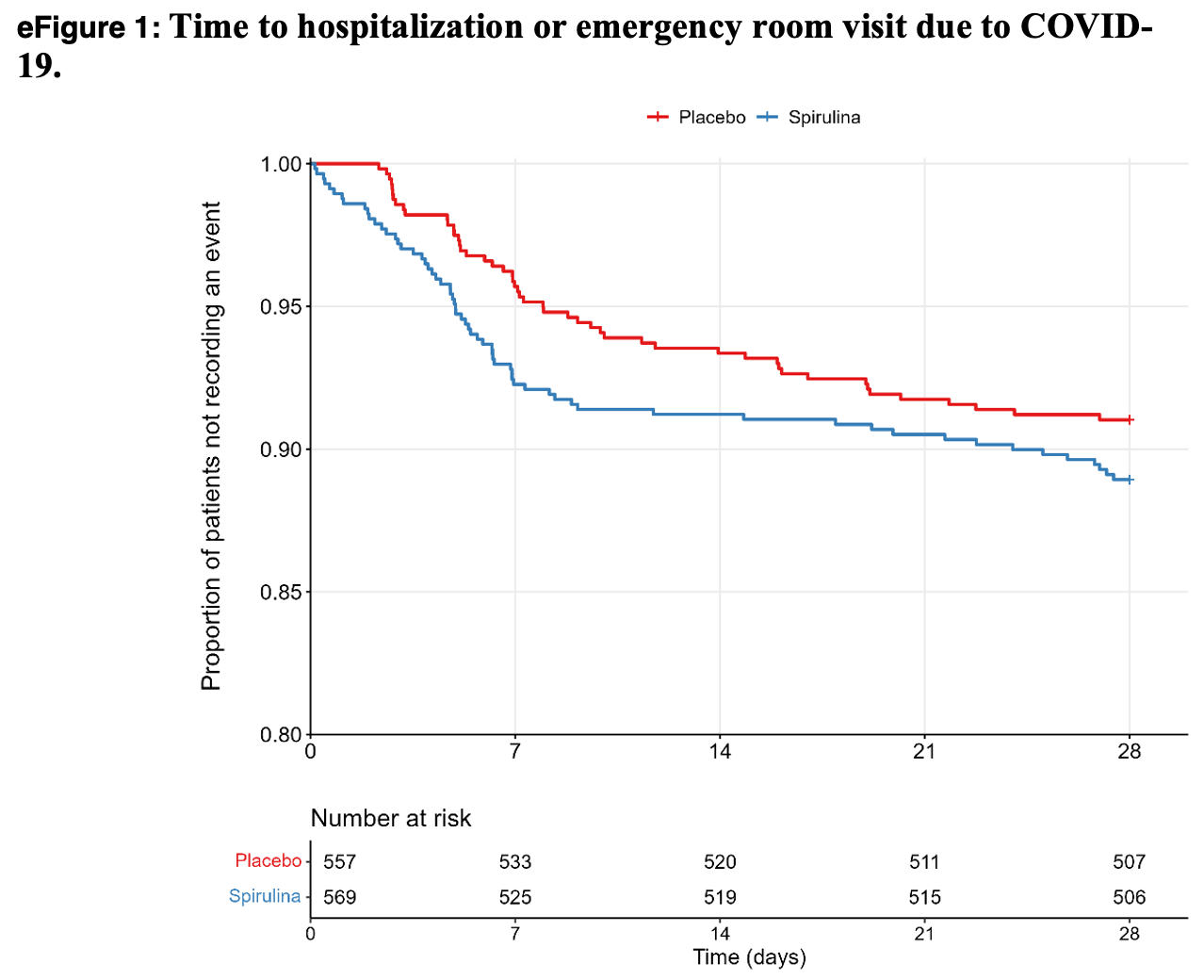
Effect of spirulina on risk of hospitalization among patients with COVID-19: the TOGETHER randomized trial
et al., The American Journal of Clinical Nutrition, doi:10.1016/j.ajcnut.2024.06.016, Aug 2024
RCT 1,126 patients in Brazil showing no significant differences with low dose spirulina. The dose used was 7.6 times lower than the dose used by Aghasadeghi et al. which shows significantly lower mortality.
eFigure 1 shows 12 events in the treatment group before the first event in the placebo group. The probability of this happening is very low, ~ 0.001. One possible cause would be if some process resulted in patients expected to visit the ER soon being more likely to be placed in the treatment group. (Another possibility is treatment side effects causing ER visits, however the were fewer adverse events and fewer severe adverse events in the treatment group).
|
risk of death, 2.1% lower, RR 0.98, p = 1.00, treatment 1 of 569 (0.2%), control 1 of 557 (0.2%), NNT 26411.
|
|
risk of hospitalization, 57.7% higher, RR 1.58, p = 0.66, treatment 3 of 569 (0.5%), control 2 of 557 (0.4%), odds ratio converted to relative risk, COVID-19.
|
|
risk of hospitalization, 67.4% higher, RR 1.67, p = 0.50, treatment 4 of 569 (0.7%), control 3 of 557 (0.5%), odds ratio converted to relative risk, all-cause.
|
|
hospitalization or ER>6 hours, 21.4% higher, RR 1.21, p = 0.29, treatment 63 of 567 (11.1%), control 50 of 557 (9.0%), odds ratio converted to relative risk.
|
|
ER, 18.9% higher, RR 1.19, p = 0.36, treatment 58 of 569 (10.2%), control 47 of 557 (8.4%), odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Reis et al., 15 Aug 2024, Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, median age 49.0, 22 authors, low dose, data anomaly.
Effect of spirulina on risk of hospitalization among patients with COVID-19: the TOGETHER randomized trial
The American Journal of Clinical Nutrition, doi:10.1016/j.ajcnut.2024.06.016
Background: Algae-derived nutraceuticals, such as spirulina, have been reported to have biological activities that may minimize clinical consequences to COVID-19 infections. Objectives: This study aimed to determine whether spirulina is an effective treatment for high-risk patients with early COVID-19 in an outpatient setting. Methods: The TOGETHER trial is a placebo-controlled, randomized, platform trial conducted in Brazil. Eligible participants were symptomatic adults with a positive rapid test for SARS-CoV-2 older than 50 y or with a known risk factor for disease severity. Patients were randomly assigned to receive placebo or spirulina (1 g twice daily for 14 d). The primary end point was hospitalization defined as either retention in a COVID-19 emergency setting for >6 h or transfer to tertiary hospital owing to COVID-19 at 28 d. Secondary outcomes included time-to-hospitalization, mortality, and adverse drug reactions. We used a Bayesian framework to compare spirulina with placebo. Results: We recruited 1126 participants, 569 randomly assigned to spirulina and 557 to placebo. The median age was 49.0 y, and 65.3% were female. The primary outcome occurred in 11.2% in the spirulina group and 8.1% in the placebo group (odds ratio [OR]: 1.24; 95% credible interval: 0.84, 1.86). There were no differences in emergency department visit (OR: 1.21; 95% credible interval: 0.81, 1.83), nor time to symptom relief (hazard ratio: 0.90; 95% credible interval: 0.79, 1.03). Spirulina also not demonstrate important treatment effects in the prespecified subgroups defined by age, sex, BMI, days since symptom onset, or vaccination status. Conclusions: Spirulina has no any clinical benefits as an outpatient therapy for COVID-19 compared with placebo with respect to reducing the retention in an emergency setting or COVID-19-related hospitalization. There are no differences between spirulina and placebo for other secondary outcomes. This trial was registered at clinicaltrials.gov as NCT04727424.
Author Contributions The authors' responsibilities were as follows -GR, EAdSMS, DCMS, OH, JIF, KT, EJM: conceived and designed the study; GR, LCMS, TSF, LLFR, MICS, LBR, DCMS, EAdSMS, APFGA, ADdFN, VHdSC, CB, EDC, RO, PL, OH, JIF, LD, EJM: performed acquisition, analysis, or interpretation of data; GR, DCMS, PL, OH, JIF, LD, EJM: drafted the manuscript; GR, LT, LCMS, TSF, LLFR, MICS, LBR, DCMS, EAdSMS, APFGA, ADdFN, VHdSC, CB, EDC, RO, PL, OH, LD, EJM: critically reviewed the manuscript for important intellectual content; GR, PL, OH, EJM: performed statistical analysis; GR, LCMS, TSF, LLFR, MICS, LBR, JIF, LD, EJM: were responsible for administrative and technical of material support; GR, TSF, LLFR, MICS, LBR, DCMS, KT, EJM: supervised the study; and all authors: read and approved the final manuscript.
Conflict of interest The authors report no conflicts of interest.
Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi. org/10.1016/j.ajcnut.2024.06.016 .
References
Aiello, Li, Boschin, Bollati, Arnoldi et al., Chemical and biological characterization of spirulina protein hydrolysates: focus on ACE and DPP-IV activities modulation, J. Funct. Foods, doi:10.1016/j.jff.2019.103592
Blette, Granholm, Li, Shankar-Hari, Lange et al., Causal Bayesian machine learning to assess treatment effect heterogeneity by dexamethasone dose for patients with COVID-19 and severe hypoxemia, Sci. Rep, doi:10.1038/s41598-023-33425-3
Brown, Obasi, Barrett, Rasch analysis of the WURSS-21 dimensional validation and assessment of invariance, J. Lung Pulm. Respir. Res, doi:10.15406/jlprr.2015.03.00076
Ceban, Ling, Lui, Lee, Gill et al., Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis, Brain Behav Immun, doi:10.1016/j.bbi.2021.12.020
Chei, Oh, Song, Seo, Lee et al., Spirulina maxima extract prevents activation of the NLRP3 inflammasome by inhibiting ERK signaling, Sci. Rep, doi:10.1038/s41598-020-58896-6
Garcia-Ruiz, Villalobos-S Anchez, Alam-Escamilla, Elizondo-Quiroga, In vitro inhibition of SARS-CoV-2 infection by dry algae powders, Sci. Rep, doi:10.1038/s41598-022-22148-6
Goligher, Lawler, Jensen, Talisa, Berry et al., Heterogeneous treatment effects of therapeutic-dose heparin in patients hospitalized for COVID-19, JAMA, doi:10.1001/jama.2023.3651
Herdman, Gudex, Lloyd, Janssen, Kind et al., Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L), Qual. Life Res, doi:10.1007/s11136-011-9903-x
Hirahashi, Matsumoto, Hazeki, Saeki, Ui et al., Activation of the human innate immune system by spirulina: augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis, Int. Immunopharmacol, doi:10.1016/S1567-5769(01)00166-7
Javid, Santos, Norouzi, Taghavi, Hatami et al., The effects of Spirulina platensis supplementation on COVID-19 severity in critically ill patients: a randomized clinical trial, ResearchSquare, doi:10.21203/rs.3.rs-2382997/v1
Niraula, Baral, Lamsal, Bataju, Thapa, Potential role of biochemical markers in the prognosis of COVID-19 patients, SAGE Open Med, doi:10.1177/20503121221108613
Ratha, Renuka, Rawat, Bux, Prospective options of algae-derived nutraceuticals as supplements to combat COVID-19 and human coronavirus diseases, Nutrition, doi:10.1016/j.nut.2020.111089
Reis, Moreira-Silva, Silva, Thabane, Milagres et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob. Health, doi:10.1016/S2214-109X(21)00448-4
Reis, Santos Moreira Silva, Medeiros Silva, Thabane, Cruz Milagres et al., Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial, Lancet Reg. Health Am, doi:10.1016/j.lana.2021.100142
Reis, Santos Moreira Silva, Silva, Thorlund, Thabane et al., A multi-center, adaptive, randomized, platform trial to evaluate the effect of repurposed medicines in outpatients with early coronavirus disease 2019 (COVID-19) and high-risk for complications: the TOGETHER master trial protocol, Gates Open Res, doi:10.12688/gatesopenres.13304.1
Reis, Silva, Medeiros Silva, Thabane, Singh et al., Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the TOGETHER randomized clinical trial, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2021.6468
Reis, Silva, Silva, Thabane, Milagres et al., Effect of early treatment with ivermectin among patients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2115869
Schandelmaier, Briel, Varadhan, Schmid, Devasenapathy et al., Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses, CMAJ, doi:10.1503/cmaj.200077
Selmi, Leung, Fischer, German, Yang et al., The effects of spirulina on anemia and immune function in senior citizens, Cell. Mol. Immunol, doi:10.1038/cmi.2010.76
Shenoy, Mohanty, Suganthy, Manavalan, Alexander, Utility of biochemical markers in predicting severe COVID-19: experience from a tertiary hospital in South India, EJIFCC
DOI record:
{
"DOI": "10.1016/j.ajcnut.2024.06.016",
"ISSN": [
"0002-9165"
],
"URL": "http://dx.doi.org/10.1016/j.ajcnut.2024.06.016",
"alternative-id": [
"S0002916524005884"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Effect of spirulina on risk of hospitalization among patients with COVID-19: the TOGETHER randomized trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The American Journal of Clinical Nutrition"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ajcnut.2024.06.016"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 Published by Elsevier Inc. on behalf of American Society for Nutrition."
}
],
"author": [
{
"affiliation": [],
"family": "Reis",
"given": "Gilmar",
"sequence": "first"
},
{
"affiliation": [],
"family": "Augusto dos Santos Moreira Silva",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carla Medeiros Silva",
"given": "Daniela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thabane",
"given": "Lehana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santiago Ferreira",
"given": "Thiago",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vitor Quirino dos Santos",
"given": "Castilho",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paula Figueiredo Guimaraes Almeida",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cançado Monteiro Savassi",
"given": "Leonardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dias de Figueiredo Neto",
"given": "Adhemar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lanna França Reis",
"given": "Luiza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Helena de Souza Campos",
"given": "Vitoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bitarães",
"given": "Carina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diniz Callegari",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Izabel Campos Simplicio",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barra Ribeiro",
"given": "Luciene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oliveira",
"given": "Rosemary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harari",
"given": "Ofir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Forrest",
"given": "Jamie I",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lat",
"given": "Prince Kumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dron",
"given": "Louis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thorlund",
"given": "Kristian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mills",
"given": "Edward J",
"sequence": "additional"
}
],
"container-title": "The American Journal of Clinical Nutrition",
"container-title-short": "The American Journal of Clinical Nutrition",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
8,
15
]
],
"date-time": "2024-08-15T19:49:06Z",
"timestamp": 1723751346000
},
"deposited": {
"date-parts": [
[
2024,
8,
15
]
],
"date-time": "2024-08-15T19:49:19Z",
"timestamp": 1723751359000
},
"indexed": {
"date-parts": [
[
2024,
8,
16
]
],
"date-time": "2024-08-16T00:26:03Z",
"timestamp": 1723767963117
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2024,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
1
]
],
"date-time": "2024-08-01T00:00:00Z",
"timestamp": 1722470400000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
1
]
],
"date-time": "2024-08-01T00:00:00Z",
"timestamp": 1722470400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0002916524005884?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0002916524005884?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
8
]
]
},
"published-print": {
"date-parts": [
[
2024,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.nut.2020.111089",
"article-title": "Prospective options of algae-derived nutraceuticals as supplements to combat COVID-19 and human coronavirus diseases",
"author": "Ratha",
"doi-asserted-by": "crossref",
"journal-title": "Nutrition",
"key": "10.1016/j.ajcnut.2024.06.016_bib1",
"volume": "83",
"year": "2021"
},
{
"DOI": "10.1038/cmi.2010.76",
"article-title": "The effects of spirulina on anemia and immune function in senior citizens",
"author": "Selmi",
"doi-asserted-by": "crossref",
"first-page": "248",
"issue": "3",
"journal-title": "Cell. Mol. Immunol.",
"key": "10.1016/j.ajcnut.2024.06.016_bib2",
"volume": "8",
"year": "2011"
},
{
"DOI": "10.1016/S1567-5769(01)00166-7",
"article-title": "Activation of the human innate immune system by spirulina: augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis",
"author": "Hirahashi",
"doi-asserted-by": "crossref",
"first-page": "423",
"issue": "4",
"journal-title": "Int. Immunopharmacol.",
"key": "10.1016/j.ajcnut.2024.06.016_bib3",
"volume": "2",
"year": "2002"
},
{
"article-title": "Chemical and biological characterization of spirulina protein hydrolysates: focus on ACE and DPP-IV activities modulation",
"author": "Aiello",
"journal-title": "J. Funct. Foods.",
"key": "10.1016/j.ajcnut.2024.06.016_bib4",
"volume": "63",
"year": "2019"
},
{
"DOI": "10.1038/s41598-020-58896-6",
"article-title": "Spirulina maxima extract prevents activation of the NLRP3 inflammasome by inhibiting ERK signaling",
"author": "Chei",
"doi-asserted-by": "crossref",
"first-page": "2075",
"issue": "1",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.ajcnut.2024.06.016_bib5",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1038/s41598-022-22148-6",
"article-title": "In vitro inhibition of SARS-CoV-2 infection by dry algae powders",
"author": "Garcia-Ruiz",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.ajcnut.2024.06.016_bib6",
"volume": "12",
"year": "2022"
},
{
"author": "Javid",
"key": "10.1016/j.ajcnut.2024.06.016_bib7",
"series-title": "The effects of Spirulina platensis supplementation on COVID-19 severity in critically ill patients: a randomized clinical trial",
"year": "2022"
},
{
"DOI": "10.12688/gatesopenres.13304.2",
"article-title": "A multi-center, adaptive, randomized, platform trial to evaluate the effect of repurposed medicines in outpatients with early coronavirus disease 2019 (COVID-19) and high-risk for complications: the TOGETHER master trial protocol",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "117",
"journal-title": "Gates Open Res",
"key": "10.1016/j.ajcnut.2024.06.016_bib8",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"article-title": "Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "e42",
"issue": "1",
"journal-title": "Lancet Glob. Health",
"key": "10.1016/j.ajcnut.2024.06.016_bib9",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.6468",
"article-title": "Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the TOGETHER randomized clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "JAMA Netw. Open",
"key": "10.1016/j.ajcnut.2024.06.016_bib10",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2115869",
"article-title": "Effect of early treatment with ivermectin among patients with COVID-19",
"author": "Reis",
"doi-asserted-by": "crossref",
"first-page": "1721",
"issue": "18",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.ajcnut.2024.06.016_bib11",
"volume": "386",
"year": "2022"
},
{
"article-title": "Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial",
"author": "Reis",
"journal-title": "Lancet Reg. Health Am",
"key": "10.1016/j.ajcnut.2024.06.016_bib12",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"doi-asserted-by": "crossref",
"first-page": "e192",
"issue": "8",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.ajcnut.2024.06.016_bib13",
"volume": "20",
"year": "2020"
},
{
"article-title": "Rasch analysis of the WURSS-21 dimensional validation and assessment of invariance",
"author": "Brown",
"issue": "2",
"journal-title": "J. Lung Pulm. Respir. Res.",
"key": "10.1016/j.ajcnut.2024.06.016_bib14",
"volume": "3",
"year": "2016"
},
{
"DOI": "10.1007/s11136-011-9903-x",
"article-title": "Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L)",
"author": "Herdman",
"doi-asserted-by": "crossref",
"first-page": "1727",
"issue": "10",
"journal-title": "Qual. Life Res.",
"key": "10.1016/j.ajcnut.2024.06.016_bib15",
"volume": "20",
"year": "2011"
},
{
"DOI": "10.1503/cmaj.200077",
"article-title": "Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses",
"author": "Schandelmaier",
"doi-asserted-by": "crossref",
"first-page": "E901",
"issue": "32",
"journal-title": "CMAJ",
"key": "10.1016/j.ajcnut.2024.06.016_bib17",
"volume": "192",
"year": "2020"
},
{
"article-title": "Utility of biochemical markers in predicting severe COVID-19: experience from a tertiary hospital in South India",
"author": "Shenoy",
"first-page": "131",
"issue": "2",
"journal-title": "EJIFCC",
"key": "10.1016/j.ajcnut.2024.06.016_bib18",
"volume": "33",
"year": "2022"
},
{
"DOI": "10.1177/20503121221108613",
"article-title": "Potential role of biochemical markers in the prognosis of COVID-19 patients",
"author": "Niraula",
"doi-asserted-by": "crossref",
"journal-title": "SAGE Open Med",
"key": "10.1016/j.ajcnut.2024.06.016_bib19",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1001/jama.2023.3651",
"article-title": "Heterogeneous treatment effects of therapeutic-dose heparin in patients hospitalized for COVID-19",
"author": "Goligher",
"doi-asserted-by": "crossref",
"first-page": "1066",
"issue": "13",
"journal-title": "JAMA",
"key": "10.1016/j.ajcnut.2024.06.016_bib20",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1038/s41598-023-33425-3",
"article-title": "Causal Bayesian machine learning to assess treatment effect heterogeneity by dexamethasone dose for patients with COVID-19 and severe hypoxemia",
"author": "Blette",
"doi-asserted-by": "crossref",
"first-page": "6570",
"issue": "1",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.ajcnut.2024.06.016_bib21",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1016/j.bbi.2021.12.020",
"article-title": "Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis",
"author": "Ceban",
"doi-asserted-by": "crossref",
"first-page": "93",
"journal-title": "Brain Behav Immun",
"key": "10.1016/j.ajcnut.2024.06.016_bib22",
"volume": "101",
"year": "2022"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0002916524005884"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effect of spirulina on risk of hospitalization among patients with COVID-19: the TOGETHER randomized trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}