Cyclooxygenase inhibitor use is associated with increased COVID-19 severity
et al., medRxiv, doi:10.1101/2021.04.13.21255438, Apr 2021
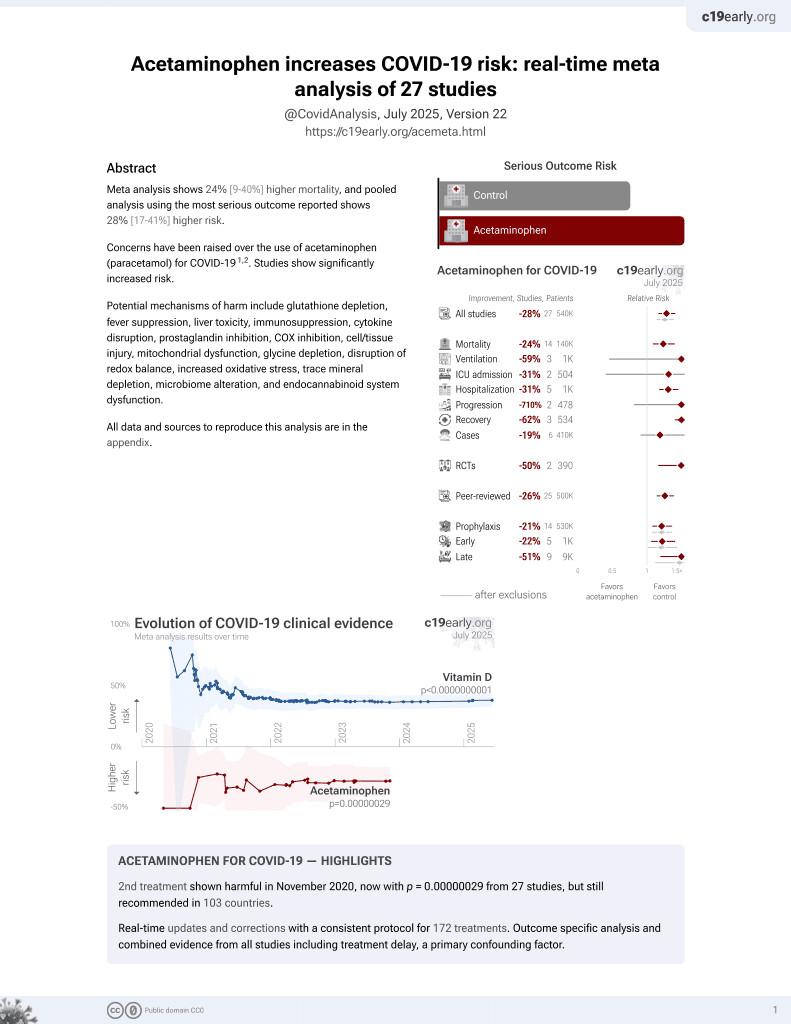
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
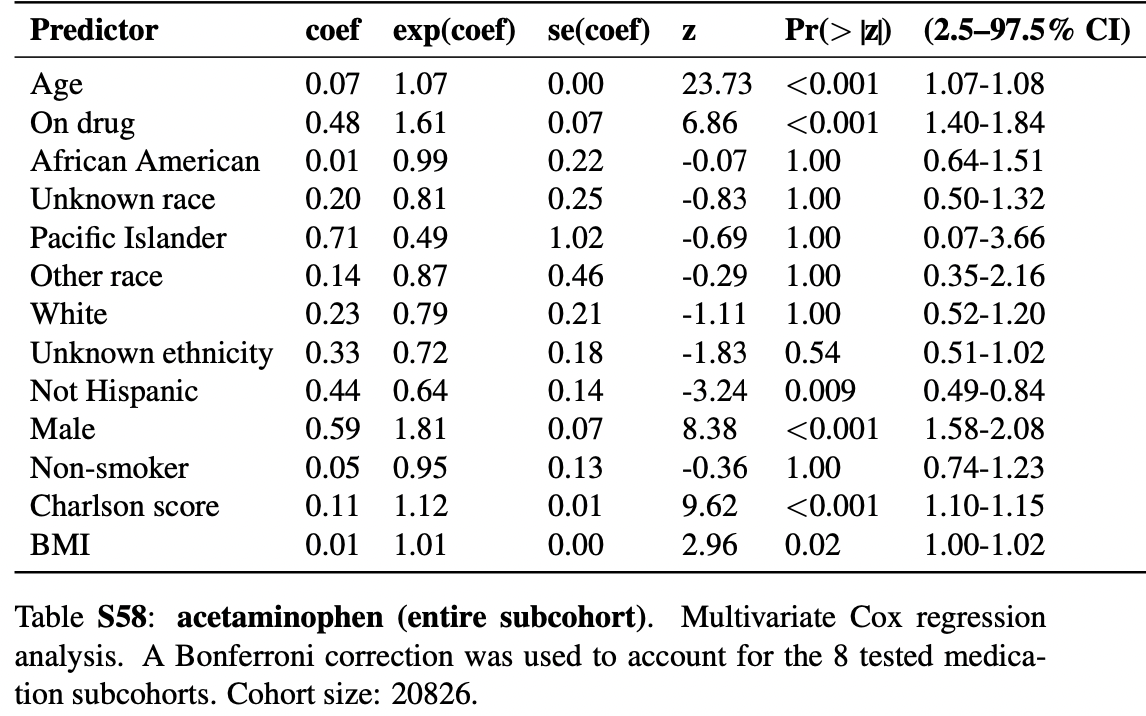
N3C retrospective 250,533 patients showing significantly higher mortality with acetaminophen use. Note that acetaminophen results were not included in the journal version or v2 of this preprint, which focuses on NSAID analysis.
Acetaminophen is also known as paracetamol, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 61.0% higher, HR 1.61, p < 0.001, treatment 20,826, control 20,826, propensity score matching, Cox proportional hazards, Table S58.
|
|
risk of severe case, 816.0% higher, OR 9.16, p < 0.001, treatment 20,826, control 20,826, propensity score matching, Table S50, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Reese et al., 20 Apr 2021, retrospective, USA, preprint, 23 authors.
Cyclooxygenase inhibitor use is associated with increased COVID-19 severity
doi:10.1101/2021.04.13.21255438
BACKGROUND Cyclooxygenase (COX) inhibitors including non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to reduce pain, fever, and inflammation but have been associated with complications in community acquired pneumonia and other respiratory tract infections (RTIs). Conclusive data are not available about potential beneficial or adverse effects of COX inhibitors on COVID-19 patients.
METHODS We conducted a retrospective, multi-center observational study by leveraging the harmonized, high-granularity electronic health record data of the National COVID Cohort Collaborative (N3C). Potential associations of eight COX inhibitors with COVID-19 severity were assessed using ordinal logistic regression (OLR) on treatment with the medication in question after matching by treatment propensity as predicted by age, race, ethnicity, gender, smoking status, comorbidities, and BMI. Cox proportional hazards analysis was used to estimate the correlation of medication use with morbidity for eight subcohorts defined by common indications for COX inhibitors.
RESULTS OLR revealed statistically significant associations between use of any of five COX inhibitors and increased severity of COVID-19. For instance, the odds ratio of aspirin use in the osteoarthritis cohort (n=2266 patients) was 3.25 (95% CI 2.76 -3.83). Aspirin and acetaminophen were associated with increased mortality.
CONCLUSIONS The association between use of COX inhibitors and COVID-19 severity was consistent across five COX inhibitors and multiple indication subcohorts. Our results align with earlier reports associating NSAID use with complications in RTI patients. Further research is needed to characterize the precise risk of individual COX inhibitors in COVID-19 patients. .
References
Amici, Caro, Ciucci, Indomethacin has a potent antiviral activity against SARS coronavirus, Antivir Ther
Austin, Wong, Uzzo, Beck, Egleston, Why Summary Comorbidity Measures Such As the Charlson Comorbidity Index and Elixhauser Score Work, Med Care
Bancos, Bernard, Topham, Phipps, Ibuprofen and other widely used nonsteroidal anti-inflammatory drugs inhibit antibody production in human cells, Cell Immunol
Bennett, Moffitt, Hajagos, The National COVID Cohort Collaborative: Clinical Characterization and Early Severity Prediction, doi:10.1101/2021.01.12.21249511
Bruce, Barlow-Pay, Short, Prior Routine Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Important Outcomes in Hospitalised Patients with COVID-19, J Clin Med Res, doi:10.3390/jcm9082586
Cannon, Cannon, Physiology. COX-2 inhibitors and cardiovascular risk, Science
Choi, Ahn, Ryu, Clinical Characteristics and Disease Progression in Early-Stage COVID-19 Patients in South Korea, J Clin Med Res, doi:10.3390/jcm9061959
Chow, Khanna, Kethireddy, Aspirin Use is Associated with Decreased Mechanical Ventilation, ICU Admission, and In-Hospital Mortality in Hospitalized Patients with COVID-19, Anesth Analg, doi:10.1213/ANE.0000000000005292
Christopher, Chute supported by U24 TR002306; Heidi Spratt supported by NIH
Christopher, Mungall Supported, None
Day, Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists, BMJ
Fitzgerald, Patrono, The coxibs, selective inhibitors of cyclooxygenase-2, N Engl J Med
Gianfrancesco, Hyrich, Al-Adely, Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry, Ann Rheum Dis
Graham, Burrell, Douglas, Debelle, Davies, Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers, J Infect Dis
Grosser, Fries, Fitzgerald, Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities, J Clin Invest
Haendel, Chute, Bennett, The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment, J Am Med Inform Assoc
Hendren, De Lemos, Ayers, Association of Body Mass Index and Age With Morbidity and Mortality in Patients Hospitalized With COVID-19: Results From the American Heart Association COVID-19 Cardiovascular Disease Registry, Circulation
Hinz, Cheremina, Brune, Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man, FASEB J
Hripcsak, Duke, Shah, Observational Health Data Sciences and Informatics (OHDSI): Opportunities for Observational Researchers, Stud Health Technol Inform
Katie ; Chan, Reese, Chute, Christopher, Tiffany et al., Bill and Melinda Gates Foundation grant to Sage Bionetworks • Icahn School of Medicine at Mount Sinai -UL1TR001433
Lund, Reilev, Hallas, Association of Nonsteroidal Anti-inflammatory Drug Use and Adverse Outcomes Among Patients Hospitalized With Influenza, JAMA Netw Open
Maier, Kapsner, Mate, Prokosch, Kraus, Patient Cohort Identification on Time Series Data Using the OMOP Common Data Model, Appl Clin Inform
Micallef, Soeiro, Society, of Pharmacology, Therapeutics (SFPT). Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection, Therapie
Nomi, Harris supported by Director
Ortiz-Prado, Simbaña-Rivera, Gómez-Barreno, Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review, Diagn Microbiol Infect Dis
Peter, Robinson, Donald, Roux Family Fund at the Jackson Laboratory Conflicts of Interest References
Pirmohamed, James, Meakin, Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients, BMJ
Rinott, Kozer, Shapira, Bar-Haim, Youngster, Ibuprofen use and clinical outcomes in COVID-19 patients, Clin Microbiol Infect
Schönthal, Direct non-cyclooxygenase-2 targets of celecoxib and their potential relevance for cancer therapy, Br J Cancer
Silverstein, Faich, Goldstein, Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study, JAMA
Suzuki, Eastwood, Bailey, Paracetamol therapy and outcome of critically ill patients: a multicenter retrospective observational study, Crit Care
Torjesen, Ibuprofen can mask symptoms of infection and might worsen outcomes, says European drugs agency, BMJ
Ursu, Holmes, Knockel, DrugCentral: online drug compendium, Nucleic Acids Res
Vaja, Chan, Ferreira, The COVID-19 ibuprofen controversy: A systematic review of NSAIDs in adult acute lower respiratory tract infections, Br J Clin Pharmacol
Vanderweele, Ding, Sensitivity Analysis in Observational Research: Introducing the E-Value, Ann Intern Med
Voiriot, Dury, Parrot, Mayaud, Fartoukh, Nonsteroidal antiinflammatory drugs may affect the presentation and course of community-acquired pneumonia, Chest
Voiriot, Philippot, Elabbadi, Elbim, Chalumeau et al., Risks Related to the Use of Non-Steroidal Anti-Inflammatory Drugs in Community-Acquired Pneumonia in Adult and Pediatric Patients, J Clin Med Res, doi:10.3390/jcm8060786
Voss, Makadia, Matcho, Feasibility and utility of applications of the common data model to multiple, disparate observational health databases, J Am Med Inform Assoc
Weiss, Murdoch, Clinical course and mortality risk of severe COVID-19
Wong, Mackenna, Morton, Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-219517
Wright, Moots, Bucknall, Edwards, Neutrophil function in inflammation and inflammatory diseases, Rheumatology
Yu, Ricciotti, Scalia, Vascular COX-2 modulates blood pressure and thrombosis in mice, Sci Transl Med
Zhang, Propensity score method: a non-parametric technique to reduce model dependence, Ann Transl Med
DOI record:
{
"DOI": "10.1101/2021.04.13.21255438",
"URL": "http://dx.doi.org/10.1101/2021.04.13.21255438",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to reduce pain, fever, and inflammation but have been associated with complications in community-acquired pneumonia. Observations shortly after the start of the COVID-19 pandemic in 2020 suggested that ibuprofen was associated with an increased risk of adverse events in COVID-19 patients, but subsequent observational studies failed to demonstrate increased risk and in one case showed reduced risk associated with NSAID use.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>A 38-center retrospective cohort study was performed that leveraged the harmonized, high-granularity electronic health record data of the National COVID Cohort Collaborative. A propensity-matched cohort of COVID-19 inpatients was constructed by matching cases (treated with NSAIDs) and controls (not treated) from 857,061 patients with COVID-19. The primary outcome of interest was COVID-19 severity in hospitalized patients, which was classified as: moderate, severe, or mortality/hospice. Secondary outcomes were acute kidney injury (AKI), extracorporeal membrane oxygenation (ECMO), invasive ventilation, and all-cause mortality at any time following COVID-19 diagnosis.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Logistic regression showed that NSAID use was not associated with increased COVID-19 severity (OR: 0.57 95% CI: 0.53-0.61). Analysis of secondary outcomes using logistic regression showed that NSAID use was not associated with increased risk of all-cause mortality (OR 0.51 95% CI: 0.47-0.56), invasive ventilation (OR: 0.59 95% CI: 0.55-0.64), AKI (OR: 0.67 95% CI: 0.63-0.72), or ECMO (OR: 0.51 95% CI: 0.36-0.7). In contrast, the odds ratios indicate reduced risk of these outcomes, but our quantitative bias analysis showed E-values of between 1.9 and 3.3 for these associations, indicating that comparatively weak or moderate confounder associations could explain away the observed associations.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Study interpretation is limited by the observational design. Recording of NSAID use may have been incomplete. Our study demonstrates that NSAID use is not associated with increased COVID-19 severity, all-cause mortality, invasive ventilation, AKI, or ECMO in COVID-19 inpatients. A conservative interpretation in light of the quantitative bias analysis is that there is no evidence that NSAID use is associated with risk of increased severity or the other measured outcomes. Our findings are the largest EHR-based analysis of the effect of NSAIDs on outcome in COVID-19 patients to date. Our results confirm and extend analogous findings in previous observational studies using a large cohort of patients drawn from 38 centers in a nationally representative multicenter database.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
12,
22
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2170-2250",
"affiliation": [],
"authenticated-orcid": false,
"family": "Reese",
"given": "Justin T.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-4422-1708",
"affiliation": [],
"authenticated-orcid": false,
"family": "Coleman",
"given": "Ben",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7463-6306",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chan",
"given": "Lauren",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4557-5492",
"affiliation": [],
"authenticated-orcid": false,
"family": "Blau",
"given": "Hannah",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8169-9049",
"affiliation": [],
"authenticated-orcid": false,
"family": "Callahan",
"given": "Tiffany J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1269-2038",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cappelletti",
"given": "Luca",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9806-3493",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fontana",
"given": "Tommaso",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9730-1808",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bradwell",
"given": "Katie Rebecca",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6315-3707",
"affiliation": [],
"authenticated-orcid": false,
"family": "Harris",
"given": "Nomi L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2024-7572",
"affiliation": [],
"authenticated-orcid": false,
"family": "Casiraghi",
"given": "Elena",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5694-3919",
"affiliation": [],
"authenticated-orcid": false,
"family": "Valentini",
"given": "Giorgio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1653-8206",
"affiliation": [],
"authenticated-orcid": false,
"family": "Karlebach",
"given": "Guy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6307-5227",
"affiliation": [],
"authenticated-orcid": false,
"family": "Deer",
"given": "Rachel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9353-5498",
"affiliation": [],
"authenticated-orcid": false,
"family": "McMurry",
"given": "Julie A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9114-8737",
"affiliation": [],
"authenticated-orcid": false,
"family": "Haendel",
"given": "Melissa A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5437-2545",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chute",
"given": "Christopher G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6840-9756",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pfaff",
"given": "Emily",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2723-5902",
"affiliation": [],
"authenticated-orcid": false,
"family": "Moffitt",
"given": "Richard",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9420-5028",
"affiliation": [],
"authenticated-orcid": false,
"family": "Spratt",
"given": "Heidi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3485-0006",
"affiliation": [],
"authenticated-orcid": false,
"family": "Singh",
"given": "Jasvinder",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6601-2165",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mungall",
"given": "Christopher J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0692-412X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Williams",
"given": "Andrew E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0736-9199",
"affiliation": [],
"authenticated-orcid": false,
"family": "Robinson",
"given": "Peter N.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
4,
21
]
],
"date-time": "2021-04-21T05:21:18Z",
"timestamp": 1618982478000
},
"deposited": {
"date-parts": [
[
2021,
12,
24
]
],
"date-time": "2021-12-24T14:05:33Z",
"timestamp": 1640354733000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
8,
25
]
],
"date-time": "2022-08-25T04:19:55Z",
"timestamp": 1661401195366
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 8,
"issued": {
"date-parts": [
[
2021,
4,
20
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.04.13.21255438",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
4,
20
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
4,
20
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2021122406050511000_2021.04.13.21255438v2.1",
"unstructured": "COVID-19 Map - Johns Hopkins Coronavirus Resource Center [Internet]. [cited 2021 Nov 15]. Available from: https://coronavirus.jhu.edu/map.html"
},
{
"DOI": "10.1016/j.diagmicrobio.2020.115094",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.2"
},
{
"DOI": "10.1016/j.ebiom.2021.103722",
"article-title": "Characterizing Long COVID: Deep Phenotype of a Complex Condition",
"doi-asserted-by": "crossref",
"first-page": "103722",
"journal-title": "EBioMedicine",
"key": "2021122406050511000_2021.04.13.21255438v2.3",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1136/bmj.329.7456.15",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.4"
},
{
"DOI": "10.1093/infdis/162.6.1277",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.5"
},
{
"DOI": "10.1016/j.cellimm.2009.03.007",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.6"
},
{
"DOI": "10.1136/bmj.m1614",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.7"
},
{
"DOI": "10.1016/j.therap.2020.05.003",
"article-title": "French Society of Pharmacology, Therapeutics (SFPT). Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "Therapie",
"key": "2021122406050511000_2021.04.13.21255438v2.8",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1378/chest.09-3102",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.9"
},
{
"DOI": "10.1111/bcp.14514",
"article-title": "The COVID-19 ibuprofen controversy: A systematic review of NSAIDs in adult acute lower respiratory tract infections",
"doi-asserted-by": "crossref",
"first-page": "776",
"journal-title": "Br J Clin Pharmacol",
"key": "2021122406050511000_2021.04.13.21255438v2.10",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13880",
"article-title": "Association of Nonsteroidal Anti-inflammatory Drug Use and Adverse Outcomes Among Patients Hospitalized With Influenza",
"doi-asserted-by": "crossref",
"first-page": "e2013880",
"journal-title": "JAMA Netw Open",
"key": "2021122406050511000_2021.04.13.21255438v2.11",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1128/JVI.00014-21",
"doi-asserted-by": "crossref",
"key": "2021122406050511000_2021.04.13.21255438v2.12",
"unstructured": "Chen Jennifer S. , Alfajaro Mia Madel , Chow Ryan D. , Wei Jin , Filler Renata B. , Eisenbarth Stephanie C. , et al. Nonsteroidal Anti-inflammatory Drugs Dampen the Cytokine and Antibody Response to SARS-CoV-2 Infection. J Virol. American Society for Microbiology; 95:e00014–21."
},
{
"DOI": "10.1056/NEJMra2026131",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.13"
},
{
"DOI": "10.1136/bmj.m1086",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.14"
},
{
"DOI": "10.3390/jcm9061959",
"doi-asserted-by": "crossref",
"key": "2021122406050511000_2021.04.13.21255438v2.15",
"unstructured": "Choi MH , Ahn H , Ryu HS , Kim B-J , Jang J , Jung M , et al. Clinical Characteristics and Disease Progression in Early-Stage COVID-19 Patients in South Korea. J Clin Med Res [Internet]. 2020;9. Available from: http://dx.doi.org/10.3390/jcm9061959"
},
{
"DOI": "10.1136/annrheumdis-2020-eular.3002",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.16"
},
{
"article-title": "Ibuprofen use and clinical outcomes in COVID-19 patients",
"first-page": "1259",
"journal-title": "Clin Microbiol Infect",
"key": "2021122406050511000_2021.04.13.21255438v2.17",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.3390/jcm9082586",
"doi-asserted-by": "crossref",
"key": "2021122406050511000_2021.04.13.21255438v2.18",
"unstructured": "Bruce E , Barlow-Pay F , Short R , Vilches-Moraga A , Price A , McGovern A , et al. Prior Routine Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Important Outcomes in Hospitalised Patients with COVID-19. J Clin Med Res [Internet]. 2020;9. Available from: http://dx.doi.org/10.3390/jcm9082586"
},
{
"DOI": "10.1371/journal.pmed.1003308",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.19"
},
{
"DOI": "10.1111/cts.12904",
"article-title": "Association Between Prescribed Ibuprofen and Severe COVID-19 Infection: A Nationwide Register-Based Cohort Study",
"doi-asserted-by": "crossref",
"first-page": "1103",
"journal-title": "Clin Transl Sci",
"key": "2021122406050511000_2021.04.13.21255438v2.20",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1007/s40121-020-00363-w",
"article-title": "Ibuprofen and NSAID Use in COVID-19 Infected Patients Is Not Associated with Worse Outcomes: A Prospective Cohort Study",
"doi-asserted-by": "crossref",
"first-page": "253",
"journal-title": "Infect Dis Ther",
"key": "2021122406050511000_2021.04.13.21255438v2.21",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1620/tjem.252.73",
"article-title": "Mortality Risk Factors among Hospitalized COVID-19 Patients in a Major Referral Center in Iran",
"doi-asserted-by": "crossref",
"first-page": "73",
"journal-title": "Tohoku J Exp Med",
"key": "2021122406050511000_2021.04.13.21255438v2.22",
"volume": "252",
"year": "2020"
},
{
"DOI": "10.1002/art.41593",
"article-title": "Nonsteroidal Antiinflammatory Drugs and Susceptibility to COVID-19",
"doi-asserted-by": "crossref",
"first-page": "731",
"journal-title": "Arthritis Rheumatol",
"key": "2021122406050511000_2021.04.13.21255438v2.23",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1007/s10072-020-04541-z",
"article-title": "Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study",
"doi-asserted-by": "crossref",
"first-page": "2317",
"journal-title": "Neurol Sci",
"key": "2021122406050511000_2021.04.13.21255438v2.24",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1111/joim.13119",
"article-title": "Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States",
"doi-asserted-by": "crossref",
"first-page": "469",
"journal-title": "J Intern Med",
"key": "2021122406050511000_2021.04.13.21255438v2.25",
"volume": "288",
"year": "2020"
},
{
"DOI": "10.1177/1358863X211012754",
"article-title": "Effect of aspirin on short-term outcomes in hospitalized patients with COVID-19",
"doi-asserted-by": "crossref",
"first-page": "626",
"journal-title": "Vasc Med",
"key": "2021122406050511000_2021.04.13.21255438v2.26",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1016/S2665-9913(21)00104-1",
"article-title": "Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: a matched, prospective cohort study",
"doi-asserted-by": "crossref",
"first-page": "e498",
"journal-title": "Lancet Rheumatol",
"key": "2021122406050511000_2021.04.13.21255438v2.27",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1136/annrheumdis-2020-219517",
"doi-asserted-by": "crossref",
"key": "2021122406050511000_2021.04.13.21255438v2.28",
"unstructured": "Wong AY , MacKenna B , Morton CE , Schultze A , Walker AJ , Bhaskaran K , et al. Use of non-steroidal anti-inflammatory drugs and risk of death from COVID-19: an OpenSAFELY cohort analysis based on two cohorts. Ann Rheum Dis [Internet]. 2021; Available from: http://dx.doi.org/10.1136/annrheumdis-2020-219517"
},
{
"DOI": "10.1093/jamia/ocaa196",
"article-title": "The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment",
"doi-asserted-by": "crossref",
"first-page": "427",
"journal-title": "J Am Med Inform Assoc",
"key": "2021122406050511000_2021.04.13.21255438v2.29",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1093/jamia/ocu023",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.30"
},
{
"DOI": "10.1101/2021.01.12.21249511",
"doi-asserted-by": "crossref",
"key": "2021122406050511000_2021.04.13.21255438v2.31",
"unstructured": "Bennett TD , Moffitt RA , Hajagos JG , Amor B , Anand A , Bissell MM , et al. The National COVID Cohort Collaborative: Clinical Characterization and Early Severity Prediction. medRxiv [Internet]. 2021; Available from: http://dx.doi.org/10.1101/2021.01.12.21249511"
},
{
"DOI": "10.1055/s-0040-1721481",
"article-title": "Patient Cohort Identification on Time Series Data Using the OMOP Common Data Model",
"doi-asserted-by": "crossref",
"first-page": "57",
"journal-title": "Appl Clin Inform",
"key": "2021122406050511000_2021.04.13.21255438v2.32",
"volume": "12",
"year": "2021"
},
{
"article-title": "Observational Health Data Sciences and Informatics (OHDSI): Opportunities for Observational Researchers",
"first-page": "574",
"journal-title": "Stud Health Technol Inform",
"key": "2021122406050511000_2021.04.13.21255438v2.33",
"volume": "216",
"year": "2015"
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.34"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.35"
},
{
"DOI": "10.7326/M16-2607",
"doi-asserted-by": "publisher",
"key": "2021122406050511000_2021.04.13.21255438v2.36"
},
{
"DOI": "10.21037/atm.2016.08.57",
"article-title": "Propensity score method: a non-parametric technique to reduce model dependence",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Ann Transl Med",
"key": "2021122406050511000_2021.04.13.21255438v2.37",
"volume": "5",
"year": "2017"
},
{
"DOI": "10.1007/s40264-021-01089-5",
"article-title": "NSAIDs and COVID-19: A Systematic Review and Meta-analysis",
"doi-asserted-by": "crossref",
"first-page": "929",
"journal-title": "Drug Saf",
"key": "2021122406050511000_2021.04.13.21255438v2.38",
"volume": "44",
"year": "2021"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.04.13.21255438"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "NSAID use and clinical outcomes in COVID-19 patients: A 38-center retrospective cohort study",
"type": "posted-content"
}