Efficacy of colchicine in patients with moderate COVID-19: A double-blinded, randomized, placebo-controlled trial
et al., PLOS ONE, doi:10.1371/journal.pone.0277790, NCT04527562, Nov 2022
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 300 patients in Bangladesh, published 2 years after completion, showing significantly lower mortality with treatment at 28 days (not significant at 14 days). 1.2mg colchicine on day 1 followed by 0.6mg for 13 days.
|
risk of death, 71.0% lower, HR 0.29, p = 0.04, treatment 4 of 146 (2.7%), control 13 of 146 (8.9%), NNT 16, Cox proportional hazards, day 28.
|
|
risk of progression, 71.0% lower, HR 0.29, p = 0.04, treatment 4 of 146 (2.7%), control 13 of 146 (8.9%), NNT 16, 2 point deterioration, Cox proportional hazards, day 28.
|
|
risk of death, 61.0% lower, HR 0.39, p = 0.26, treatment 2 of 146 (1.4%), control 5 of 146 (3.4%), NNT 49, Cox proportional hazards, day 14.
|
|
risk of mechanical ventilation, 51.0% lower, HR 0.49, p = 0.41, treatment 2 of 146 (1.4%), control 4 of 146 (2.7%), NNT 73, Cox proportional hazards, day 14.
|
|
risk of progression, 56.0% lower, HR 0.44, p = 0.17, treatment 4 of 146 (2.7%), control 9 of 146 (6.2%), NNT 29, 2 point deterioration, Cox proportional hazards, day 14, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rahman et al., 16 Nov 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Bangladesh, peer-reviewed, 14 authors, study period June 2020 - November 2020, dosage 1.2mg day 1, 0.6mg days 2-14, trial NCT04527562 (history).
Contact: ponkajdatta@yahoo.com.
Efficacy of colchicine in patients with moderate COVID-19: A double-blinded, randomized, placebo-controlled trial
PLOS ONE, doi:10.1371/journal.pone.0277790
Background Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may cause severe life-threatening diseases called acute respiratory distress syndrome (ARDS) owing to cytokine storms. The mortality rate of COVID-19-related ARDS is as high as 40% to 50%. However, effective treatment for the extensive release of acute inflammatory mediators induced by hyperactive and inappropriate immune responses is very limited. Many antiinflammatory drugs with variable efficacies have been investigated. Colchicine inhibits interleukin 1 beta (IL-1β) and its subsequent inflammatory cascade by primarily blocking pyrin and nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3 (NLRP3) activation. Therefore, this cheap, widely available, oral drug might provide an added benefit in combating the cytokine storm in COVID-19. Here, we sought to determine whether adding colchicine to other standards of care could be beneficial for moderate COVID-19 pneumonia in terms of the requirement for advanced respiratory support and mortality.
Methods and findings This blinded placebo-controlled drug trial was conducted at the Dhaka Medical College Hospital, Dhaka, Bangladesh. A total of 300 patients with moderate COVID-19 based on a positive RT-PCR result were enrolled based on strict selection criteria from June 2020 to November 2020. Patients were randomly assigned to either treatment group in a 1:1 ratio. Patients were administered 1.2 mg of colchicine on day 1 followed by daily treatment with 0.6 mg of colchicine for 13 days or placebo along with the standard of care. The primary outcome was the time to clinical deterioration from randomization to two or more points on a seven-category ordinal scale within the 14 days post-randomization. Clinical outcomes
Subgroup analysis Subgroup analysis of sex (male vs. female), age (18-40 years, 41-60 years, > 60 years), comorbidities (diabetes mellitus, hypertension, asthma, and COPD), and duration of symptoms before enrolment (10 days or less vs. more than 10 days) did not reveal any credible subgroup effects (S1 Fig).
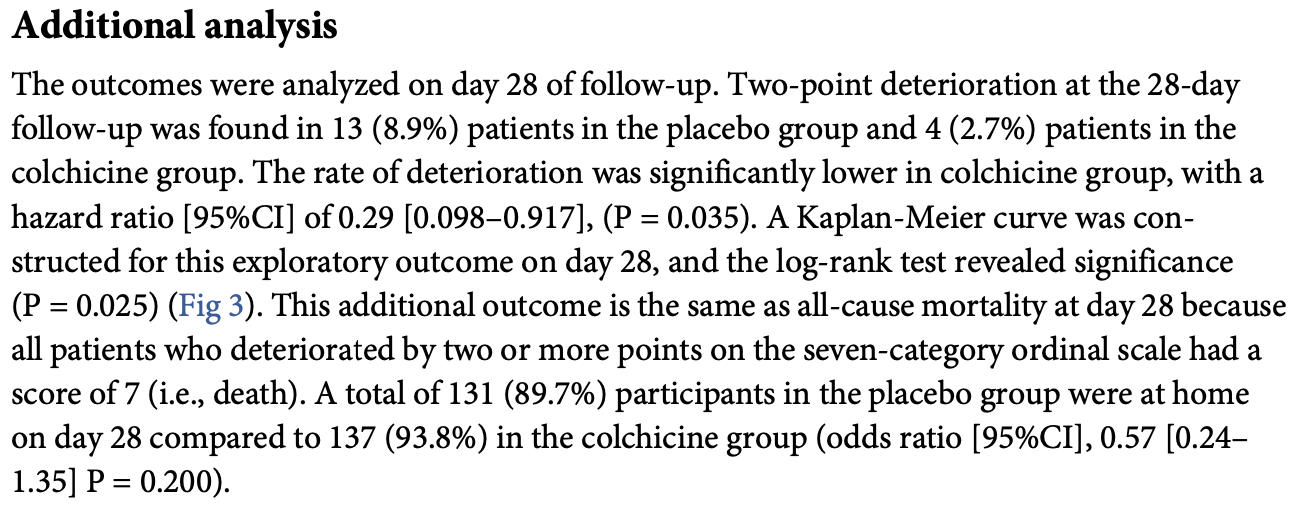
Additional analysis The outcomes were analyzed on day 28 of follow-up. Two-point deterioration at the 28-day follow-up was found in 13 (8.9%) patients in the placebo group and 4 (2.7%) patients in the colchicine group. The rate of deterioration was significantly lower in colchicine group, with a hazard ratio [95%CI] of 0.29 [0.098-0.917], (P = 0.035). A Kaplan-Meier curve was constructed for this exploratory outcome on day 28, and the log-rank test revealed significance (P = 0.025) (Fig 3 ). This additional outcome is the same as all-cause mortality at day 28 because all patients who deteriorated by two or more points on the seven-category ordinal scale had a score of 7 (i.e., death). A total of 131 (89.7%) participants in the placebo group were at home on day 28 compared to 137 (93.8%) in the colchicine group (odds ratio [95%CI], 0.57 [0.24-1.35] P = 0.200).
Safety outcome We actively searched for three well-known side effects of colchicine, including diarrhea, nausea/vomiting, and abdominal pain. Further, we collected self-reported adverse events
References
Cao, Wang, Wen, A Trial of Lopinavir Ritonavir in Adults Hospitalized with Severe Covid-19, N Engl J Med, doi:10.1056/NEJMoa2001282
Cecconi, Piovani, Brunetta, Aghemo, Greco et al., Early Predictors of Clinical Deterioration in a Cohort of 239 Patients Hospitalized for Covid-19 Infection in Lombardy, Italy, Journal of Clinical Medicine, doi:10.3390/jcm9051548
Chiu, Chow, Chiu, Colchicine use in patients with COVID-19: a systematic review and meta-analysis, medRxiv, doi:10.1101/2021.02.02.21250960
Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.13136
Della-Torre, Della-Torre, Kusanovic, Treating COVID-19 with colchicine in community healthcare setting, Clin Immunol, doi:10.1016/j.clim.2020.108490
Horby, Lim, Dexamethasone in hospitalized patients with covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Huang, Su, Theron, An interferon-gamma-related cytokine storm in SARS patients, J Med Virol, doi:10.1002/jmv.20255
Islam, Haque, Hasan, Haque, Faruq et al., Formal analysis: Motlabur Rahman
Jamilloux, Henry, Belot, Viel, Fauter et al., Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions, Autoimmun Rev, doi:10.1016/j.autrev.2020.102567
Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial, RMD Open, doi:10.1136/rmdopen-2020-001455
Manenti, Maggiore, Fiaccadori, Meschi, Antoni et al., Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study, PLoS ONE, doi:10.1371/journal.pone.0248276
Mikolajewska, Fischer, Piechotta, Mueller, Metzendorf et al., Colchicine for the treatment of COVID-19, Cochrane Database of Systematic Reviews, doi:10.1002/14651858.CD015045
Rahman, Datta, Hasan, Haque, Abul et al., None
Rahman, Rahman, Datta, Islam, Hasan et al., Efficacy of colchicine in moderate symptomatic COVID-19 patients: a study protocol for a double-blind, randomized, placebo-controlled trial, Int J Clin Trials, doi:10.18203/2349-3259.ijct2021xxxx
Sakpal, Sample Size Estimation in Clinical Trial, Perspect Clin Res
Scarsi, Piantoni, Colombo, Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-217712
Sinha, Matthay, Calfee, Is a "Cytokine Storm" Relevant to COVID-19?, JAMA Intern Med, doi:10.1001/jamainternmed.2020.3313
Tardif, Bouabdallaoui, Efficacy of Colchicine in Non-Hospitalized Patients with COVID-19, doi:10.1101/2021.01.26.21250494
Wang, Fan, Salam, Horby, Hayden et al., Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection, J Infect Dis, doi:10.1093/infdis/jiz656
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review, JAMA, doi:10.1001/jama.2020.12839
DOI record:
{
"DOI": "10.1371/journal.pone.0277790",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0277790",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may cause severe life-threatening diseases called acute respiratory distress syndrome (ARDS) owing to cytokine storms. The mortality rate of COVID-19-related ARDS is as high as 40% to 50%. However, effective treatment for the extensive release of acute inflammatory mediators induced by hyperactive and inappropriate immune responses is very limited. Many anti-inflammatory drugs with variable efficacies have been investigated. Colchicine inhibits interleukin 1 beta (IL-1β) and its subsequent inflammatory cascade by primarily blocking pyrin and nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3 (NLRP3) activation. Therefore, this cheap, widely available, oral drug might provide an added benefit in combating the cytokine storm in COVID-19. Here, we sought to determine whether adding colchicine to other standards of care could be beneficial for moderate COVID-19 pneumonia in terms of the requirement for advanced respiratory support and mortality.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods and findings</jats:title>\n<jats:p>This blinded placebo-controlled drug trial was conducted at the Dhaka Medical College Hospital, Dhaka, Bangladesh. A total of 300 patients with moderate COVID-19 based on a positive RT-PCR result were enrolled based on strict selection criteria from June 2020 to November 2020. Patients were randomly assigned to either treatment group in a 1:1 ratio. Patients were administered 1.2 mg of colchicine on day 1 followed by daily treatment with 0.6 mg of colchicine for 13 days or placebo along with the standard of care. The primary outcome was the time to clinical deterioration from randomization to two or more points on a seven-category ordinal scale within the 14 days post-randomization. Clinical outcomes were also recorded on day 28. The primary endpoint was met by 9 (6.2%) patients in the placebo group and 4 (2.7%) patients in the colchicine group (P = 0.171), which corresponds to a hazard ratio (95% CI) of 0.44 (0.13–1.43). Additional analysis of the outcomes on day 28 revealed significantly lower clinical deterioration (defined as a decrease by two or more points) in the colchicine group, with a hazard ratio [95%CI] of 0.29 [0.098–0.917], (P = 0.035). Despite a 56% reduction in the need for mechanical ventilation and death with colchicine treatment on day 14, the reduction was not statistically significant. On day 28, colchicine significantly reduced clinical deterioration measured as the need for mechanical ventilation and all-cause mortality.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Conclusion</jats:title>\n<jats:p>Colchicine was not found to have a significant beneficial effect on reducing mortality and the need for mechanical ventilation. However, a delayed beneficial effect was observed. Therefore, further studies should be conducted to evaluate the late benefits of colchicine.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Clinical trial registration</jats:title>\n<jats:p><jats:bold>Clinical trial registration no:</jats:bold> ClinicalTrials.gov Identifier: NCT04527562 <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://www.google.com/search?client=firefox-b-d&q=NCT04527562\" xlink:type=\"simple\">https://www.google.com/search?client=firefox-b-d&q=NCT04527562</jats:ext-link>.</jats:p>\n</jats:sec>",
"author": [
{
"affiliation": [],
"family": "Rahman",
"given": "Motlabur",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-4508-4549",
"affiliation": [],
"authenticated-orcid": true,
"family": "Datta",
"given": "Ponkaj K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Islam",
"given": "Khairul",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2796-2560",
"affiliation": [],
"authenticated-orcid": true,
"family": "Haque",
"given": "Mahfuzul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahmud",
"given": "Reaz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mallik",
"given": "Uzzwal",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1765-8477",
"affiliation": [],
"authenticated-orcid": true,
"family": "Hasan",
"given": "Pratyay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haque",
"given": "Manjurul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faruq",
"given": "Imtiaz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharif",
"given": "Mohiuddin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ratul",
"given": "Rifat H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Azad",
"given": "Khan Abul Kalam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miah",
"given": "Titu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4381-1511",
"affiliation": [],
"authenticated-orcid": true,
"family": "Rahman",
"given": "Md. Mujibur",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2022,
11,
16
]
],
"date-time": "2022-11-16T18:34:11Z",
"timestamp": 1668623651000
},
"deposited": {
"date-parts": [
[
2022,
11,
16
]
],
"date-time": "2022-11-16T18:34:38Z",
"timestamp": 1668623678000
},
"editor": [
{
"affiliation": [],
"family": "Mockridge",
"given": "James",
"sequence": "first"
}
],
"indexed": {
"date-parts": [
[
2022,
11,
17
]
],
"date-time": "2022-11-17T06:12:29Z",
"timestamp": 1668665549830
},
"is-referenced-by-count": 0,
"issue": "11",
"issued": {
"date-parts": [
[
2022,
11,
16
]
]
},
"journal-issue": {
"issue": "11",
"published-online": {
"date-parts": [
[
2022,
11,
16
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
16
]
],
"date-time": "2022-11-16T00:00:00Z",
"timestamp": 1668556800000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0277790",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0277790",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2022,
11,
16
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
16
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"key": "pone.0277790.ref001",
"volume-title": "World Health Organization",
"year": "2020"
},
{
"key": "pone.0277790.ref002",
"volume-title": "World Health Organization",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.12839",
"article-title": "Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review",
"author": "WJ Wiersinga",
"doi-asserted-by": "crossref",
"first-page": "782",
"issue": "8",
"journal-title": "JAMA",
"key": "pone.0277790.ref003",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.3313",
"article-title": "Is a “Cytokine Storm” Relevant to COVID-19?",
"author": "P Sinha",
"doi-asserted-by": "crossref",
"first-page": "1152",
"issue": "9",
"journal-title": "JAMA Intern Med",
"key": "pone.0277790.ref004",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1002/jmv.20255",
"article-title": "An interferon-gamma-related cytokine storm in SARS patients",
"author": "KJ Huang",
"doi-asserted-by": "crossref",
"first-page": "185",
"issue": "2",
"journal-title": "J Med Virol",
"key": "pone.0277790.ref005",
"volume": "75",
"year": "2005"
},
{
"DOI": "10.1016/j.autrev.2020.102567",
"article-title": "Should we stimulate or suppress immune responses in COVID- 19? Cytokine and anti-cytokine interventions",
"author": "Y Jamilloux",
"doi-asserted-by": "crossref",
"first-page": "102567",
"issue": "7",
"journal-title": "Autoimmun Rev",
"key": "pone.0277790.ref006",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with covid-19",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "pone.0277790.ref007",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.18203/2349-3259.ijct20210144",
"article-title": "Efficacy of colchicine in moderate symptomatic COVID-19 patients: a study protocol for a double-blind, randomized, placebo-controlled trial",
"author": "M Rahman",
"doi-asserted-by": "crossref",
"first-page": "xxx",
"issue": "1",
"journal-title": "Int J Clin Trials",
"key": "pone.0277790.ref008",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.clim.2020.108490",
"article-title": "Treating COVID-19 with colchicine in community healthcare setting",
"author": "E Della-Torre",
"doi-asserted-by": "crossref",
"first-page": "108490",
"journal-title": "Clin Immunol",
"key": "pone.0277790.ref009",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0248276",
"article-title": "Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study",
"author": "L Manenti",
"doi-asserted-by": "crossref",
"first-page": "e0248276",
"issue": "3",
"journal-title": "PLoS ONE",
"key": "pone.0277790.ref010",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"article-title": "Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial",
"author": "SG Deftereos",
"doi-asserted-by": "crossref",
"first-page": "e2013136",
"journal-title": "JAMA Netw Open",
"key": "pone.0277790.ref011",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"article-title": "Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome",
"author": "M Scarsi",
"doi-asserted-by": "crossref",
"first-page": "1286",
"journal-title": "Ann Rheum Dis",
"key": "pone.0277790.ref012",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"article-title": "Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial",
"author": "MI Lopes",
"doi-asserted-by": "crossref",
"first-page": "e001455",
"journal-title": "RMD Open",
"key": "pone.0277790.ref013",
"volume": "7",
"year": "2021"
},
{
"article-title": "Colchicine for the treatment of COVID-19",
"author": "A Mikolajewska",
"issue": "10",
"journal-title": "Cochrane Database of Systematic Reviews",
"key": "pone.0277790.ref014",
"year": "2021"
},
{
"key": "pone.0277790.ref015",
"unstructured": "National Guidelines on Clinical Management of Coronavirus Disease 2019 (COVID-19).Version 7.0 28 May, 2020, DGHS, MOHFW, Government of the People’s Republic of Bangladesh."
},
{
"author": "World Health Organization, WHO",
"journal-title": "Clinical Management of COVID-19, Interim Guidance",
"key": "pone.0277790.ref016",
"year": "2021"
},
{
"key": "pone.0277790.ref017",
"unstructured": "World Health Organization,WHO. Coronavirus disease (COVID-2019) R&D. Available at: https://www.who.int/blueprint/priority-diseases/ key action/novel-coronavirus/en/. [Accessed on: 25 March 2020]."
},
{
"DOI": "10.1093/infdis/jiz656",
"article-title": "Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection",
"author": "Y Wang",
"doi-asserted-by": "crossref",
"first-page": "1688",
"issue": "10",
"journal-title": "J Infect Dis",
"key": "pone.0277790.ref018",
"volume": "221",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of Lopinavir Ritonavir in Adults Hospitalized with Severe Covid- 19",
"author": "B Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N Engl J Med",
"key": "pone.0277790.ref019",
"volume": "382",
"year": "2020"
},
{
"article-title": "Sample Size Estimation in Clinical Trial",
"author": "TV Sakpal",
"first-page": "67",
"issue": "2",
"journal-title": "Perspect Clin Res",
"key": "pone.0277790.ref020",
"volume": "1",
"year": "2010"
},
{
"DOI": "10.3390/jcm9051548",
"article-title": "Early Predictors of Clinical Deterioration in a Cohort of 239 Patients Hospitalized for Covid-19 Infection in Lombardy, Italy",
"author": "M Cecconi",
"doi-asserted-by": "crossref",
"first-page": "1548",
"issue": "5",
"journal-title": "Journal of Clinical Medicine",
"key": "pone.0277790.ref021",
"volume": "9",
"year": "2020"
},
{
"author": "JC Tardif",
"journal-title": "Efficacy of Colchicine in Non-Hospitalized Patients with COVID-19",
"key": "pone.0277790.ref022",
"year": "2021"
},
{
"article-title": "Colchicine use in patients with COVID-19: a systematic review and meta-analysis",
"author": "L Chiu",
"journal-title": "medRxiv",
"key": "pone.0277790.ref023",
"year": "2021"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0277790"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Efficacy of colchicine in patients with moderate COVID-19: A double-blinded, randomized, placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "17"
}
