Povidone-iodine nasal spray (Nasodine®) for the common cold: a randomized, controlled, double-blind, Phase III clinical trial
et al., Frontiers in Medicine, doi:10.3389/fmed.2025.1565069, ACTRN12619000764134, Jun 2025
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Non-COVID-19 RCT of 260 outpatients with early common-cold symptoms comparing 0.5% povidone-iodine (PVP-I) nasal spray and saline spray four-times daily for five days. PVP-I showed lower Global Severity Score (GSS) and improved quality-of-life, with larger effects for patients treated within 24 hours of onset (≈40% GSS reduction). The trial compares two treatments, efficacy of PVP-I versus no treatment may be larger.
Polasek et al., 5 Jun 2025, Double Blind Randomized Controlled Trial, Australia, peer-reviewed, mean age 32.4, 2 authors, trial ACTRN12619000764134.
Contact: tom.polasek@monash.edu.
Povidone-iodine nasal spray (Nasodine®) for the common cold: a randomized, controlled, double-blind, Phase III clinical trial
Frontiers in Medicine, doi:10.3389/fmed.2025.1565069
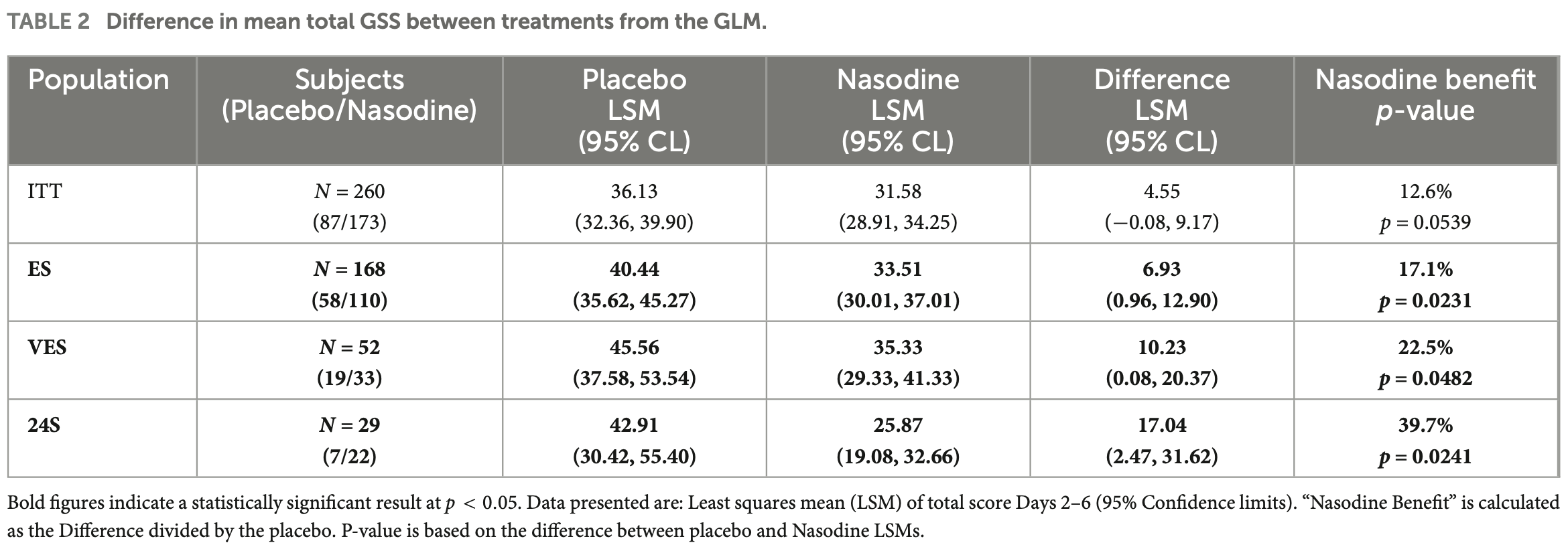
Aim: To determine the safety and efficacy of a 0.5% povidone-iodine nasal spray (Nasodine) as a treatment for the common cold (ACTRN12619000764134). Methods: A multi-center, randomized, controlled, double-blind Phase III study was conducted to assess the impact of Nasodine on the common cold. Two hundred and sixty (260) euthyroid adults with qualifying cold symptoms and meeting inclusion/exclusion criteria were randomized 2:1 to Nasodine or matching saline nasal spray (SNS), each applied 4 times daily for 5 days. Cold severity was reported using the WURSS-21 survey. The primary endpoint was impact on nasal symptoms (4-item scale), with the validated 19-item Global Severity Score (GSS) as the key secondary endpoint. Results: All cold severity outcomes pointed in favor of Nasodine over SNS. In the ITT (n = 260), the Nasodine benefit over SNS on nasal symptoms was 8.4% (p = 0.217). For GSS, the benefit was 12.6% (p = 0.054) in the ITT population. Post hoc subset analyses showed markedly improved benefits of Nasodine: In subjects with stronger symptoms at enrollment (ES), the GSS benefit was 17.1% (p = 0.023); for those with confirmed viral infection (VES), GSS benefit was 23.0% (p = 0.048); and for those enrolled within 24 h of symptom onset (24S), GSS benefit was 39.7% (p = 0.024). In terms of functional impairment, the Nasodine benefit was greater in all subsets, with 16.1% (p = 0.041) benefit in ITT, 22.2% in ES (p = 0.012), 32.1% in VES (p = 0.023) and 37.1% in 24S (p = 0.093). Nasodine was well tolerated, with mild transient nasopharyngeal discomfort being a common adverse effect.
Conclusion: Nasodine treatment had a consistently positive and clinically meaningful benefit on overall cold severity when compared with saline nasal spray. Early treatment after symptom onset is an important efficacy factor.
Ethics statement The studies involving humans were approved by the Bellberry Human Research Ethics Committees. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions TP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing -original draft, Writing -review and editing. PF: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Supervision, Writingoriginal draft, Writing -review and editing.
Conflict of interest TP was the Principal Investigator for the study and was employed by Fusion Clinical Research Pty Ltd (Adelaide, South Australia), the Clinical Research Organization that was contracted by the sponsor, Firebrick Pharma Ltd., to conduct the study and act as the primary recruiting site. PF is an Ear Nose and Throat Specialist who was the Medical Monitor for the study and is a consultant to the sponsor, Firebrick Pharma Ltd.
Generative AI statement The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this..
References
Alves, Gryson, Hajjar, Lepelletier, Reners et al., Role of antiseptics in the prevention and treatment of infections in nursing homes, J Hosp Infect, doi:10.1016/j.jhin.2022.09.021
Arefin, Rumi, Uddin, Banu, Khan et al., Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: An open-label randomized clinical trial, Indian J Otolaryngol Head Neck Surg, doi:10.1007/s12070-021-02616-7
Cameron, Povidone iodine as a low cost therapeutic against the SARS-CoV-2 virus and its potential for refugee health, J Hum Virol Retrovirol, doi:10.15406/jhvre.2022.09.00241
Capriotti, Capriotti, Topical iodophor preparations: Chemistry, microbiology, and clinical utility, Dermatol Online J, doi:10.5070/D39RP912J2
Castelain, Girardin, Moumane, Aubin, Pelletier, Anaphylactic reaction to povidone in a skin antiseptic, Contact Dermatitis, doi:10.1111/cod.12473
Daroudi, Sari, Nahvijou, Faramarzi, Cost per DALY averted in low, middle-and high-income countries: Evidence from the global burden of disease study to estimate the cost-effectiveness thresholds, Cost Eff Resour Alloc, doi:10.1186/s12962-021-00260-0
Esneau, Duff, Bartlett, Understanding rhinovirus circulation and impact on illness, Viruses, doi:10.3390/v14010141
Fendrick, Monto, Nightengale, Sarnes, The economic burden of noninfluenza-related viral respiratory tract infection in the United States, Arch Intern Med, doi:10.1001/archinte.163.4.487
Fernández-Rodríguez, Cho, Chisari, Citardi, Parvizi, Nasal microbiome and the effect of nasal decolonization with a novel povidone-iodine antiseptic solution: A prospective and randomized clinical trial, Sci Rep, doi:10.1038/s41598-023-46792-8
Frank, Capriotti, Brown, Tessema, Povidone-Iodine use in sinonasal and oral cavities: A review of safety in the COVID-19 era, Ear Nose Throat J, doi:10.1177/0145561320932318
Freeman, Duan, Kessler, Molecular iodine is not responsible for cytotoxicity in iodophors, J Hosp Infect, doi:10.1016/j.jhin.2022.01.015
Friedland, Nasal disinfection as a front-line defence in future pandemics, J Military Veterans' Health
Friedland, Polasek, Topliss, Phase 1 study of the iodine absorption, safety, and tolerability of a 0.5% povidone-iodine nasal spray (Nasodine), Int Forum Allergy Rhinol, doi:10.1002/alr.23389
Friedland, Tucker, Goodall, Julander, Mendenhall et al., In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray, Aust J Otolaryngol, doi:10.21037/ajo-21-40
Friedland, Tucker, Phase II trial of the impact 0.5% povidone-iodine nasal spray (Nasodine R ) on shedding of SARS-CoV-2, Laryngoscope, doi:10.1002/lary.31430
Goodall, Molloy, Treatment and Prevention of the Common Cold Using Povidone-Iodine, U.S. Patent
Hinkle, Wykoff, Lim, Hahn, Kim et al., Iodine Allergy" and the Use of Povidone Iodine for Endophthalmitis Prophylaxis, J Vitreoretin Dis, doi:10.1177/2474126419865991
Jackson, Dowling, Spiesman, Boand, Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity, AMA Arch Intern Med, doi:10.1001/archinte.1958.00260140099015
Jin, Ren, Li, Gao, Zhang et al., Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100986
Kawana, Kitamura, Nakagomi, Matsumoto, Arita et al., Inactivation of human viruses by povidone-iodine in comparison with other antiseptics, Dermatology, doi:10.1159/000246027
King, Mitchell, Williams, Spurling, Saline nasal irrigation for acute upper respiratory tract infections, Cochrane Database Syst Rev, doi:10.1002/14651858.CD006821.pub3
Kluzek, Dean, Wartolowska, Patient-reported outcome measures (PROMs) as proof of treatment efficacy, BMJ Evid Based Med, doi:10.1136/bmjebm-2020-111573
König, Reimer, Fleischer, König, Effects of betaisodona on parameters of host defense, Dermatology, doi:10.1159/000246029
Lachapelle, Casado, Antiseptics in the era of bacterial resistance: a focus on povidone iodine, Future Med, doi:10.2217/cpr.13.50
Lepelletier, Maillard, Pozzetto, Simon, Povidone iodine: Properties, mechanisms of action, and role in infection control and staphylococcus aureus decolonization, Antimicrob Agents Chemother, doi:10.1128/AAC.00682-20
Molloy, Goodall, Prevention of Infection by Highly Pathogenic Viruses Using Topical Application of Povidone-Iodine on Mucous Membranes, U.S. Patent
Mäkelä, Puhakka, Ruuskanen, Leinonen, Saikku et al., Viruses and bacteria in the etiology of the common cold, J Clin Microbiol, doi:10.1128/JCM.36.2.539-542.1998
O'neill, Secondary endpoints cannot be validly analyzed if the primary endpoint does not demonstrate clear statistical significance, Control Clin Trials, doi:10.1016/s0197-2456(97)00075-5
Polasek, None
Ramalingam, Graham, Dove, Morrice, Sheikh, A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold, Sci Rep, doi:10.1038/s41598-018-37703-3
Ramezanpour, Smith, Psaltis, Wormald, Vreugde, In vitro safety evaluation of a povidone-iodine solution applied to human nasal epithelial cells, Int Forum Allergy Rhinol, doi:10.1002/alr.22575
Sauerbrei, Bactericidal and virucidal activity of ethanol and povidone-iodine, Microbiologyopen, doi:10.1002/mbo3.1097
Sirijatuphat, Leelarasamee, Puangpet, Thitithanyanont, Pilot study of 0.4% povidone-iodine nasal spray to eradicate SARS-CoV-2 in the nasopharynx, Infect Drug Resist, doi:10.2147/IDR.S391630
Slapak, Skoupá, Strnad, Horník, Efficacy of isotonic nasal wash (seawater) in the treatment and prevention of rhinitis in children, Arch Otolaryngol Head Neck Surg, doi:10.1001/archoto.2007.19
Sriwilaijaroen, Wilairat, Hiramatsu, Takahashi, Suzuki et al., Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: Its effects on hemagglutination and sialidase activities, Virol J, doi:10.1186/1743-422X-6-124
Stewart, Doctor i have an iodine allergy, Ophthalmol Ther, doi:10.1007/s40123-022-00502-1
Tano, Tano, A daily nasal spray with saline prevents symptoms of rhinitis, Acta Otolaryngol, doi:10.1080/00016480410017657
Van Driel, Scheire, Deckx, Gevaert, Sutter, What treatments are effective for common cold in adults and children?, BMJ, doi:10.1136/bmj.k3786
Who, World Health Organization Model List of Essential Medicines, 21st List 2019
Wulf, Schmitz, Choi, Kapusnik-Uner, Iodine allergy: Common misperceptions, Am J Health Syst Pharm, doi:10.1093/ajhp/zxab033
DOI record:
{
"DOI": "10.3389/fmed.2025.1565069",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2025.1565069",
"abstract": "<jats:sec><jats:title>Aim</jats:title><jats:p>To determine the safety and efficacy of a 0.5% povidone-iodine nasal spray (Nasodine) as a treatment for the common cold (ACTRN12619000764134).</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>A multi-center, randomized, controlled, double-blind Phase III study was conducted to assess the impact of Nasodine on the common cold. Two hundred and sixty (260) euthyroid adults with qualifying cold symptoms and meeting inclusion/exclusion criteria were randomized 2:1 to Nasodine or matching saline nasal spray (SNS), each applied 4 times daily for 5 days. Cold severity was reported using the WURSS-21 survey. The primary endpoint was impact on nasal symptoms (4-item scale), with the validated 19-item Global Severity Score (GSS) as the key secondary endpoint.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>All cold severity outcomes pointed in favor of Nasodine over SNS. In the ITT (<jats:italic>n</jats:italic> = 260), the Nasodine benefit over SNS on nasal symptoms was 8.4% (<jats:italic>p</jats:italic> = 0.217). For GSS, the benefit was 12.6% (<jats:italic>p</jats:italic> = 0.054) in the ITT population. <jats:italic>Post hoc</jats:italic> subset analyses showed markedly improved benefits of Nasodine: In subjects with stronger symptoms at enrollment (ES), the GSS benefit was 17.1% (<jats:italic>p</jats:italic> = 0.023); for those with confirmed viral infection (VES), GSS benefit was 23.0% (<jats:italic>p</jats:italic> = 0.048); and for those enrolled within 24 h of symptom onset (24S), GSS benefit was 39.7% (<jats:italic>p</jats:italic> = 0.024). In terms of functional impairment, the Nasodine benefit was greater in all subsets, with 16.1% (<jats:italic>p</jats:italic> = 0.041) benefit in ITT, 22.2% in ES (<jats:italic>p</jats:italic> = 0.012), 32.1% in VES (<jats:italic>p</jats:italic> = 0.023) and 37.1% in 24S (<jats:italic>p</jats:italic> = 0.093). Nasodine was well tolerated, with mild transient nasopharyngeal discomfort being a common adverse effect.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Nasodine treatment had a consistently positive and clinically meaningful benefit on overall cold severity when compared with saline nasal spray. Early treatment after symptom onset is an important efficacy factor.</jats:p></jats:sec><jats:sec><jats:title>Clinical trial registration</jats:title><jats:p><jats:uri>https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=377353&amp;isReview=true</jats:uri>, identifier ACTRN12619000764134.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2025.1565069"
],
"author": [
{
"affiliation": [],
"family": "Polasek",
"given": "Thomas M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Friedland",
"given": "Peter L.",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2025,
6,
5
]
],
"date-time": "2025-06-05T04:10:28Z",
"timestamp": 1749096628000
},
"deposited": {
"date-parts": [
[
2025,
6,
5
]
],
"date-time": "2025-06-05T04:10:29Z",
"timestamp": 1749096629000
},
"indexed": {
"date-parts": [
[
2025,
6,
6
]
],
"date-time": "2025-06-06T04:02:31Z",
"timestamp": 1749182551189,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
6,
5
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
6,
5
]
],
"date-time": "2025-06-05T00:00:00Z",
"timestamp": 1749081600000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2025.1565069/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2025,
6,
5
]
]
},
"published-online": {
"date-parts": [
[
2025,
6,
5
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1128/AAC.00682-20",
"article-title": "Povidone iodine: Properties, mechanisms of action, and role in infection control and staphylococcus aureus decolonization.",
"author": "Lepelletier",
"doi-asserted-by": "publisher",
"first-page": "e682",
"journal-title": "Antimicrob Agents Chemother.",
"key": "B1",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.2217/cpr.13.50",
"article-title": "Antiseptics in the era of bacterial resistance: a focus on povidone iodine.",
"author": "Lachapelle",
"doi-asserted-by": "publisher",
"first-page": "579",
"journal-title": "Future Med.",
"key": "B2",
"volume": "10",
"year": "2013"
},
{
"DOI": "10.1159/000246027",
"article-title": "Inactivation of human viruses by povidone-iodine in comparison with other antiseptics.",
"author": "Kawana",
"doi-asserted-by": "publisher",
"first-page": "29",
"journal-title": "Dermatology.",
"key": "B3",
"volume": "195",
"year": "1997"
},
{
"journal-title": "World Health Organization Model List of Essential Medicines, 21st List 2019.",
"key": "B4",
"year": "2019"
},
{
"DOI": "10.1002/mbo3.1097",
"article-title": "Bactericidal and virucidal activity of ethanol and povidone-iodine.",
"author": "Sauerbrei",
"doi-asserted-by": "publisher",
"first-page": "e1097",
"journal-title": "Microbiologyopen.",
"key": "B5",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.jhin.2022.01.015",
"article-title": "Molecular iodine is not responsible for cytotoxicity in iodophors.",
"author": "Freeman",
"doi-asserted-by": "publisher",
"first-page": "194",
"journal-title": "J Hosp Infect.",
"key": "B6",
"volume": "122",
"year": "2022"
},
{
"DOI": "10.5070/D39RP912J2",
"article-title": "Topical iodophor preparations: Chemistry, microbiology, and clinical utility.",
"author": "Capriotti",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Dermatol Online J.",
"key": "B7",
"volume": "18",
"year": "2012"
},
{
"DOI": "10.1007/s12070-021-02616-7",
"article-title": "Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: An open-label randomized clinical trial.",
"author": "Arefin",
"doi-asserted-by": "publisher",
"first-page": "2963",
"journal-title": "Indian J Otolaryngol Head Neck Surg.",
"key": "B8",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.15406/jhvre.2022.09.00241",
"article-title": "Povidone iodine as a low cost therapeutic against the SARS-CoV-2 virus and its potential for refugee health.",
"author": "Cameron",
"doi-asserted-by": "publisher",
"first-page": "1525",
"journal-title": "J Hum Virol Retrovirol.",
"key": "B9",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.2147/IDR.S391630",
"article-title": "Pilot study of 0.4% povidone-iodine nasal spray to eradicate SARS-CoV-2 in the nasopharynx.",
"author": "Sirijatuphat",
"doi-asserted-by": "publisher",
"first-page": "7529",
"journal-title": "Infect Drug Resist.",
"key": "B10",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1177/0145561320932318",
"article-title": "Povidone-Iodine use in sinonasal and oral cavities: A review of safety in the COVID-19 era.",
"author": "Frank",
"doi-asserted-by": "publisher",
"first-page": "586",
"journal-title": "Ear Nose Throat J.",
"key": "B11",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.1002/lary.31430",
"article-title": "Phase II trial of the impact 0.5% povidone-iodine nasal spray (Nasodine®) on shedding of SARS-CoV-2.",
"author": "Friedland",
"doi-asserted-by": "publisher",
"first-page": "3947",
"journal-title": "Laryngoscope.",
"key": "B12",
"volume": "134",
"year": "2024"
},
{
"DOI": "10.1128/JCM.36.2.539-542.1998",
"article-title": "Viruses and bacteria in the etiology of the common cold.",
"author": "Mäkelä",
"doi-asserted-by": "publisher",
"first-page": "539",
"journal-title": "J Clin Microbiol.",
"key": "B13",
"volume": "36",
"year": "1998"
},
{
"DOI": "10.1016/j.eclinm.2021.100986",
"article-title": "Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019.",
"author": "Jin",
"doi-asserted-by": "publisher",
"first-page": "100986",
"journal-title": "EClinicalMedicine.",
"key": "B14",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1001/archinte.163.4.487",
"article-title": "The economic burden of non-influenza-related viral respiratory tract infection in the United States.",
"author": "Fendrick",
"doi-asserted-by": "publisher",
"first-page": "487",
"journal-title": "Arch Intern Med.",
"key": "B15",
"volume": "163",
"year": "2003"
},
{
"DOI": "10.3390/v14010141",
"article-title": "Understanding rhinovirus circulation and impact on illness.",
"author": "Esneau",
"doi-asserted-by": "publisher",
"first-page": "141",
"journal-title": "Viruses.",
"key": "B16",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1186/s12962-021-00260-0",
"article-title": "Cost per DALY averted in low, middle- and high-income countries: Evidence from the global burden of disease study to estimate the cost-effectiveness thresholds.",
"author": "Daroudi",
"doi-asserted-by": "publisher",
"first-page": "10",
"journal-title": "Cost Eff Resour Alloc.",
"key": "B17",
"volume": "19",
"year": "2021"
},
{
"author": "Goodall",
"journal-title": "Treatment and Prevention of the Common Cold Using Povidone-Iodine. U.S. Patent No 11,000,542",
"key": "B18",
"year": "2021"
},
{
"DOI": "10.1159/000246029",
"article-title": "Effects of betaisodona on parameters of host defense.",
"author": "König",
"doi-asserted-by": "publisher",
"first-page": "42",
"journal-title": "Dermatology.",
"key": "B19",
"volume": "195",
"year": "1997"
},
{
"DOI": "10.1186/1743-422X-6-124",
"article-title": "Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: Its effects on hemagglutination and sialidase activities.",
"author": "Sriwilaijaroen",
"doi-asserted-by": "publisher",
"first-page": "124",
"journal-title": "Virol J.",
"key": "B20",
"volume": "6",
"year": "2009"
},
{
"DOI": "10.1002/alr.22575",
"article-title": "In vitro safety evaluation of a povidone-iodine solution applied to human nasal epithelial cells.",
"author": "Ramezanpour",
"doi-asserted-by": "publisher",
"first-page": "1141",
"journal-title": "Int Forum Allergy Rhinol.",
"key": "B21",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1002/alr.23389",
"article-title": "Phase 1 study of the iodine absorption, safety, and tolerability of a 0.5% povidone-iodine nasal spray (Nasodine).",
"author": "Friedland",
"doi-asserted-by": "publisher",
"first-page": "1525",
"journal-title": "Int Forum Allergy Rhinol.",
"key": "B22",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.21037/ajo-21-40",
"article-title": "In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray.",
"author": "Friedland",
"doi-asserted-by": "publisher",
"first-page": "2",
"journal-title": "Aust J Otolaryngol.",
"key": "B23",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1001/archinte.1958.00260140099015",
"article-title": "Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity.",
"author": "Jackson",
"doi-asserted-by": "publisher",
"first-page": "267",
"journal-title": "AMA Arch Intern Med.",
"key": "B24",
"volume": "101",
"year": "1958"
},
{
"DOI": "10.1136/bmjebm-2020-111573",
"article-title": "Patient-reported outcome measures (PROMs) as proof of treatment efficacy.",
"author": "Kluzek",
"doi-asserted-by": "publisher",
"first-page": "153",
"journal-title": "BMJ Evid Based Med.",
"key": "B25",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1002/14651858.CD006821.pub3",
"article-title": "Saline nasal irrigation for acute upper respiratory tract infections.",
"author": "King",
"doi-asserted-by": "publisher",
"first-page": "CD006821",
"journal-title": "Cochrane Database Syst Rev.",
"key": "B26",
"volume": "2015",
"year": "2015"
},
{
"DOI": "10.1136/bmj.k3786",
"article-title": "What treatments are effective for common cold in adults and children?",
"author": "van Driel",
"doi-asserted-by": "publisher",
"first-page": "k3786",
"journal-title": "BMJ.",
"key": "B27",
"volume": "363",
"year": "2018"
},
{
"DOI": "10.1080/00016480410017657",
"article-title": "A daily nasal spray with saline prevents symptoms of rhinitis.",
"author": "Tano",
"doi-asserted-by": "publisher",
"first-page": "1059",
"journal-title": "Acta Otolaryngol.",
"key": "B28",
"volume": "124",
"year": "2004"
},
{
"DOI": "10.1001/archoto.2007.19",
"article-title": "Efficacy of isotonic nasal wash (seawater) in the treatment and prevention of rhinitis in children.",
"author": "Slapak",
"doi-asserted-by": "publisher",
"first-page": "67",
"journal-title": "Arch Otolaryngol Head Neck Surg.",
"key": "B29",
"volume": "134",
"year": "2008"
},
{
"DOI": "10.1038/s41598-018-37703-3",
"article-title": "A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold.",
"author": "Ramalingam",
"doi-asserted-by": "publisher",
"first-page": "1015",
"journal-title": "Sci Rep.",
"key": "B30",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1016/s0197-2456(97)00075-5",
"article-title": "Secondary endpoints cannot be validly analyzed if the primary endpoint does not demonstrate clear statistical significance.",
"author": "O’Neill",
"doi-asserted-by": "publisher",
"first-page": "550",
"journal-title": "Control Clin Trials.",
"key": "B31",
"volume": "18",
"year": "1997"
},
{
"DOI": "10.1093/ajhp/zxab033",
"article-title": "Iodine allergy: Common misperceptions.",
"author": "Wulf",
"doi-asserted-by": "publisher",
"first-page": "781",
"journal-title": "Am J Health Syst Pharm.",
"key": "B32",
"volume": "78",
"year": "2021"
},
{
"DOI": "10.1007/s40123-022-00502-1",
"article-title": "Doctor i have an iodine allergy.",
"author": "Stewart",
"doi-asserted-by": "publisher",
"first-page": "931",
"journal-title": "Ophthalmol Ther.",
"key": "B33",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1111/cod.12473",
"article-title": "Anaphylactic reaction to povidone in a skin antiseptic.",
"author": "Castelain",
"doi-asserted-by": "publisher",
"first-page": "55",
"journal-title": "Contact Dermatitis.",
"key": "B34",
"volume": "74",
"year": "2016"
},
{
"DOI": "10.1016/j.jhin.2022.09.021",
"article-title": "Role of antiseptics in the prevention and treatment of infections in nursing homes.",
"author": "Alves",
"doi-asserted-by": "publisher",
"first-page": "58",
"journal-title": "J Hosp Infect.",
"key": "B35",
"volume": "131",
"year": "2023"
},
{
"DOI": "10.1177/2474126419865991",
"article-title": "“Iodine Allergy” and the Use of Povidone Iodine for Endophthalmitis Prophylaxis.",
"author": "Hinkle",
"doi-asserted-by": "publisher",
"first-page": "65",
"journal-title": "J Vitreoretin Dis.",
"key": "B36",
"volume": "4",
"year": "2020"
},
{
"author": "Molloy",
"journal-title": "Prevention of Infection by Highly Pathogenic Viruses Using Topical Application of Povidone-Iodine on Mucous Membranes. U.S. Patent No 11,246,887",
"key": "B37",
"year": "2022"
},
{
"article-title": "Nasal disinfection as a front-line defence in future pandemics.",
"author": "Friedland",
"first-page": "62",
"journal-title": "J Military Veterans’ Health.",
"key": "B38",
"volume": "31",
"year": "2023"
},
{
"DOI": "10.1038/s41598-023-46792-8",
"article-title": "Nasal microbiome and the effect of nasal decolonization with a novel povidone-iodine antiseptic solution: A prospective and randomized clinical trial.",
"author": "Fernández-Rodríguez",
"doi-asserted-by": "publisher",
"first-page": "16739",
"journal-title": "Sci Rep.",
"key": "B39",
"volume": "14",
"year": "2024"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2025.1565069/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Povidone-iodine nasal spray (Nasodine®) for the common cold: a randomized, controlled, double-blind, Phase III clinical trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.3389/crossmark-policy",
"volume": "12"
}
