Vitamin D and COVID-19 Severity in Hospitalized Older Patients: Potential Benefit of Prehospital Vitamin D Supplementation
et al., Nutrients, doi:10.3390/nu14081641, NCT04877509, Apr 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
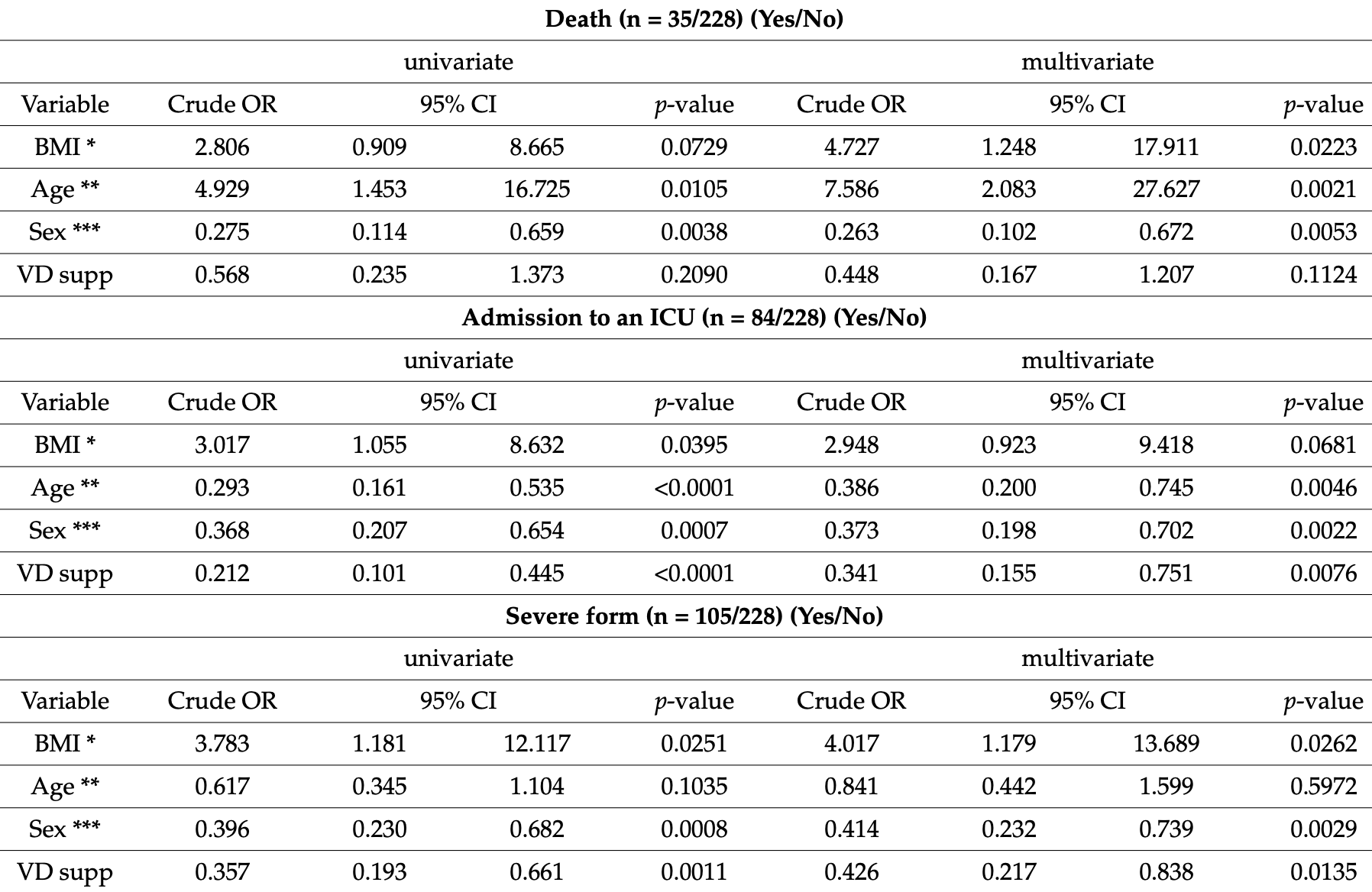
Retrospective 228 hospitalized COVID-19 patients, median age 78, showing significantly lower risk of ICU admission and severe cases with vitamin D prophylaxis. NCT04877509 (history).
This is the 76th of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
|
risk of death, 50.5% lower, RR 0.50, p = 0.11, treatment 7 of 66 (10.6%), control 28 of 162 (17.3%), adjusted per study, odds ratio converted to relative risk, multivariable.
|
|
risk of ICU admission, 51.2% lower, RR 0.49, p = 0.008, treatment 10 of 66 (15.2%), control 74 of 162 (45.7%), NNT 3.3, adjusted per study, odds ratio converted to relative risk, multivariable.
|
|
risk of severe case, 38.7% lower, RR 0.61, p = 0.01, treatment 19 of 66 (28.8%), control 86 of 162 (53.1%), NNT 4.1, adjusted per study, odds ratio converted to relative risk, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Parant et al., 14 Apr 2022, retrospective, France, peer-reviewed, median age 78.0, 12 authors, study period 1 March, 2020 - 30 June, 2020, dosage varies, trial NCT04877509 (history).
Vitamin D and COVID-19 Severity in Hospitalized Older Patients: Potential Benefit of Prehospital Vitamin D Supplementation
Nutrients, doi:10.3390/nu14081641
Studies involving the associations between vitamin D supplementation taken before the onset of COVID-19 infection and the clinical outcomes are still scarce and this issue remains controversial. This study aimed to assess the relationships between vitamin D (VitD) status and supplementation and coronavirus disease 2019 (COVID-19) severity in older adults (average age of 78 years) hospitalized for COVID-19. We conducted an observational retrospective cohort study with 228 older hospitalized patients during the first wave of the COVID-19 pandemic. The outcomes were in-hospital mortality secondary to COVID-19 or critically severe COVID-19. A logistic regression analysis was conducted to test whether pre-hospital VitD supplementation was independently associated with severity. In this study, 46% of patients developed a severe form and the overall in-hospital mortality was 15%. Sixty-six (29%) patients received a VitD supplement during the 3 months preceding the infection onset. Additionally, a VitD supplement was associated with fewer severe COVID-19 forms (OR = 0.426, p = 0.0135) and intensive care unit (ICU) admissions (OR = 0.341, p = 0.0076). As expected, age > 70 years, male gender and BMI ≥ 35 kg/m 2 were independent risk factors for severe forms of COVID-19. No relationship between serum 25(OH)D levels and the severity of the COVID-19 was identified. VitD supplementation taken during the 3 months preceding the infection onset may have a protective effect on the development of severe COVID-19 forms in older adults. Randomized controlled trials and large-scale cohort studies are necessary to strengthen this observation.
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki, and approved by the ethical committee of the Hospices Civils de Lyon (protocol code 21_5067, the 30 December 2021). Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Data Availability Statement: The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical considerations, regarding personal information and respecting what was written in the generic information sheet dedicated to COVID-19 research at the Hospices Civils de Lyon (Lyon, France).
Conflicts of Interest: The authors declare that they have no conflicts of interest regarding this manuscript.
References
Annweiler, Beaudenon, Gautier, Simon, Dubée, COvid-19 and High-Dose VITamin D Supplementation TRIAL in High-Risk Older Patients (COVIT-TRIAL): Study Protocol for a Randomized Controlled Trial, Trials, doi:10.1186/s13063-020-04928-5
Annweiler, Corvaisier, Gautier, Dubée, Legrand et al., Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study, Nutrients, doi:10.3390/nu12113377
Baeke, Takiishi, Korf, Gysemans, Mathieu et al., Modulator of the Immune System, Curr. Opin. Pharmacol, doi:10.1016/j.coph.2010.04.001
Bajaj, Gadi, Spihlman, Wu, Choi et al., Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections?, Front. Physiol, doi:10.3389/fphys.2020.571416
Barassi, Pezzilli, Mondoni, Rinaldo, Davì et al., in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Patients with Non-Invasive Ventilation Support, Panminerva Med, doi:10.23736/S0031-0808.21.04277-4
Basit, Vitamin D in Health and Disease: A Literature Review, Br. J. Biomed. Sci, doi:10.1080/09674845.2013.11669951
Bassatne, Basbous, Chakhtoura, El Zein, Rahme et al., The Link between COVID-19 and VItamin D (VIVID): A Systematic Review and Meta-Analysis, Metab. Clin. Exp, doi:10.1016/j.metabol.2021.154753
Benhamou, Souberbielle, Cortet, La Vitamine D Chez l'adulte: Recommandations Du GRIO, Presse Med
Bilezikian, Bikle, Hewison, Lazaretti-Castro, Formenti et al., MECHANISMS IN ENDOCRINOLOGY: Vitamin D and COVID-19, Eur. J. Endocrinol
Bonafè, Prattichizzo, Giuliani, Storci, Sabbatinelli et al., Inflamm-Aging: Why Older Men Are the Most Susceptible to SARS-CoV-2 Complicated Outcomes, Cytokine Growth Factor Rev, doi:10.1016/j.cytogfr.2020.04.005
Bouillon, Carmeliet, Vitamin D Insufficiency: Definition, Diagnosis and Management, Best Pract. Res. Clin. Endocrinol. Metab, doi:10.1016/j.beem.2018.09.014
Bouillon, Comparative Analysis of Nutritional Guidelines for Vitamin D, Nat. Rev. Endocrinol, doi:10.1038/nrendo.2017.31
Breysse, Guillot, Berrut, Étude de La Supplémentation En Vitamine D Chez Les Personnes de plus de 65 Ans En Médecine Générale, Geriatr. Psychol. Neuropsychiatr. Vieil, doi:10.1684/pnv.2015.0530
Cashman, Dowling, Skrabáková, Gonzalez-Gross, Valtueña et al., Vitamin D Deficiency in Europe: Pandemic? 1,2, Am. J. Clin. Nutr, doi:10.3945/ajcn.115.120873
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study, Lancet, doi:10.1016/S0140-6736(20)30211-7
Conseil, de la Santé Publique. Avis Relatif à L'actualisation de la Liste des Facteurs de Risque de Forme Grave de COVID-19
Damayanthi, Prabani, Nutritional Determinants and COVID-19 Outcomes of Older Patients with COVID-19: A Systematic Review, Arch. Gerontol. Geriatr, doi:10.1016/j.archger.2021.104411
Daneshkhah, Agrawal, Eshein, Subramanian, Roy et al., Evidence for Possible Association of Vitamin D Status with Cytokine Storm and Unregulated Inflammation in COVID-19 Patients, Aging Clin. Exp. Res, doi:10.1007/s40520-020-01677-y
El Rais, Aflak-Kattar, Bleistein, Parcours Hospitaliers Des Patients Atteints de La COVID-19 de Mars 2020 à Janvier 2021, Les Dossiers de la DREES
Fakhoury, Kvietys, Shakir, Shams, Grant et al., Lung-Centric Inflammation of COVID-19: Potential Modulation by Vitamin D, Nutrients, doi:10.3390/nu13072216
Fassio, Adami, Rossini, Giollo, Caimmi et al., Pharmacokinetics of Oral Cholecalciferol in Healthy Subjects with Vitamin D Deficiency: A Randomized, Nutrients, doi:10.3390/nu12061553
Ferrari, Locatelli, Briguglio, Lombardi, Is There a Link between Vitamin D Status, SARS-CoV-2 Infection Risk and COVID-19 Severity?, Cell Biochem. Funct, doi:10.1002/cbf.3597
Greiller, Martineau, Modulation of the Immune Response to Respiratory Viruses by Vitamin D, Nutrients, doi:10.3390/nu7064240
Griffin, Hewison, Hopkin, Kenny, Quinton et al., Vitamin D and COVID-19: Evidence and Recommendations for Supplementation, R. Soc. Open Sci, doi:10.1098/rsos.201912
Horst, Jaeger, Smeekens, Oosting, Swertz et al., Host and Environmental Factors Influencing Individual Human Cytokine Responses, Cell, doi:10.1016/j.cell.2016.10.018
Jolliffe, Camargo, Sluyter, Aglipay, Aloia et al., Vitamin D Supplementation to Prevent Acute Respiratory Infections: A Systematic Review and Meta-Analysis of Aggregate Data from Randomised Controlled Trials, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(21)00051-6
Kara, Ekiz, Ricci, Kara, Chang et al., Scientific Strabismus" or Two Related Pandemics: Coronavirus Disease and Vitamin D Deficiency, Br. J. Nutr, doi:10.1017/S0007114520001749
Leaf, Ginde, Vitamin D3 to Treat COVID-19: Different Disease, Same Answer, JAMA, doi:10.1001/jama.2020.26850
Martineau, Forouhi, Vitamin D for COVID-19: A Case to Answer?, Lancet Diabetes Endocrinol
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data, BMJ, doi:10.1136/bmj.i6583
Meehan, Penckofer, The Role of Vitamin D in the Aging Adult, J. Aging Gerontol, doi:10.12974/2309-6128.2014.02.02.1
Mueller, Mcnamara, Sinclair, Why Does COVID-19 Disproportionately Affect Older People?, Aging, doi:10.18632/aging.103344
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a Single High Dose of Vitamin D3on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.26848
Pereira, Dantas Damascena, Galvão Azevedo, De Almeida Oliveira, Da Mota Santana, Vitamin D Deficiency Aggravates COVID-19: Systematic Review and Meta-Analysis, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2020.1841090
Pouw, De Maat, Van Veerman, Oever, Ten Janssen et al., Clinical Characteristics and Outcomes of 952 Hospitalized COVID-19 Patients in the Netherlands: A Retrospective Cohort Study, PLoS ONE, doi:10.1371/journal.pone.0248713
Prietl, Treiber, Pieber, Amrein, Vitamin D and Immune Function, Nutrients, doi:10.3390/nu5072502
Rhodes, Subramanian, Laird, Griffin, Kenny, Perspective: Vitamin D Deficiency and COVID-19 Severity-Plausibly Linked by Latitude, Ethnicity, Impacts on Cytokines, ACE2 and Thrombosis, J. Intern. Med
Richardson, Lovegrove, Nutritional Status of Micronutrients as a Possible and Modifiable Risk Factor for COVID-19: A UK Perspective, Br. J. Nutr, doi:10.1017/S000711452000330X
Saadatian-Elahi, Picot, Hénaff, Pradel, Escuret et al., Protocol for a Prospective, Observational, Hospital-Based Multicentre Study of Nosocomial SARS-CoV-2 Transmission: NOSO-COR Project, BMJ Open, doi:10.1136/bmjopen-2020-039088
Silva, Furlanetto, Does Serum 25-Hydroxyvitamin D Decrease during Acute-Phase Response? A Systematic Review, Nutr. Res, doi:10.1016/j.nutres.2014.12.008
Teshome, Adane, Girma, Mekonnen, The Impact of Vitamin D Level on COVID-19 Infection: Systematic Review and Meta-Analysis, Front. Public Heal, doi:10.3389/fpubh.2021.624559
Williamson, Walker, Bhaskaran, Bacon, Bates et al., Factors Associated with COVID-19-Related Death Using OpenSAFELY, Nature, doi:10.1038/s41586-020-2521-4
DOI record:
{
"DOI": "10.3390/nu14081641",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu14081641",
"abstract": "<jats:p>Studies involving the associations between vitamin D supplementation taken before the onset of COVID-19 infection and the clinical outcomes are still scarce and this issue remains controversial. This study aimed to assess the relationships between vitamin D (VitD) status and supplementation and coronavirus disease 2019 (COVID-19) severity in older adults (average age of 78 years) hospitalized for COVID-19. We conducted an observational retrospective cohort study with 228 older hospitalized patients during the first wave of the COVID-19 pandemic. The outcomes were in-hospital mortality secondary to COVID-19 or critically severe COVID-19. A logistic regression analysis was conducted to test whether pre-hospital VitD supplementation was independently associated with severity. In this study, 46% of patients developed a severe form and the overall in-hospital mortality was 15%. Sixty-six (29%) patients received a VitD supplement during the 3 months preceding the infection onset. Additionally, a VitD supplement was associated with fewer severe COVID-19 forms (OR = 0.426, p = 0.0135) and intensive care unit (ICU) admissions (OR = 0.341, p = 0.0076). As expected, age > 70 years, male gender and BMI ≥ 35 kg/m2 were independent risk factors for severe forms of COVID-19. No relationship between serum 25(OH)D levels and the severity of the COVID-19 was identified. VitD supplementation taken during the 3 months preceding the infection onset may have a protective effect on the development of severe COVID-19 forms in older adults. Randomized controlled trials and large-scale cohort studies are necessary to strengthen this observation.</jats:p>",
"alternative-id": [
"nu14081641"
],
"author": [
{
"affiliation": [],
"family": "Parant",
"given": "François",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7805-0778",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bouloy",
"given": "Justin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9109-5604",
"affiliation": [],
"authenticated-orcid": false,
"family": "Haesebaert",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bendim’red",
"given": "Lamia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldet",
"given": "Karine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vanhems",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Henaff",
"given": "Laetitia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8270-7605",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gilbert",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cuerq",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blond",
"given": "Emilie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bost",
"given": "Muriel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonnefoy",
"given": "Marc",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T05:03:01Z",
"timestamp": 1650344581000
},
"deposited": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T06:19:08Z",
"timestamp": 1650349148000
},
"funder": [
{
"DOI": "10.13039/501100006451",
"award": [
"NCT04877509"
],
"doi-asserted-by": "publisher",
"name": "Hospices Civils de Lyon"
}
],
"indexed": {
"date-parts": [
[
2024,
2,
28
]
],
"date-time": "2024-02-28T15:22:20Z",
"timestamp": 1709133740804
},
"is-referenced-by-count": 10,
"issue": "8",
"issued": {
"date-parts": [
[
2022,
4,
14
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
14
]
],
"date-time": "2022-04-14T00:00:00Z",
"timestamp": 1649894400000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/14/8/1641/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1641",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
4,
14
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
14
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41586-020-2521-4",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.18632/aging.103344",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.3389/fphys.2020.571416",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1016/j.cytogfr.2020.04.005",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1016/j.archger.2021.104411",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1017/S000711452000330X",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1530/EJE-20-0665",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1098/rsos.201912",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1111/joim.13149",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1080/09674845.2013.11669951",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.3390/nu7064240",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.3390/nu5072502",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1136/bmj.i6583",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1016/S2213-8587(21)00051-6",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1017/S0007114520001749",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"key": "ref16"
},
{
"DOI": "10.1016/S2213-8587(20)30268-0",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1186/s13063-020-04928-5",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1002/cbf.3597",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.beem.2018.09.014",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1038/nrendo.2017.31",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.12974/2309-6128.2014.02.02.1",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.3945/ajcn.115.120873",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1684/pnv.2015.0530",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.3390/nu12113377",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1136/bmjopen-2020-039088",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1016/j.lpm.2011.04.001",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1016/j.nutres.2014.12.008",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"key": "ref29",
"unstructured": "Avis Relatif à L’actualisation de la Liste des Facteurs de Risque de Forme Grave de COVID-19http://Www.Rpfc.Fr/Espacepro/Wp-Content/Pdf/Avis HCSP Grippe et Antiviraux Patients Extra-Hospitalier-09-11-12.Pdf"
},
{
"article-title": "Parcours Hospitaliers Des Patients Atteints de La COVID-19 de Mars 2020 à Janvier 2021",
"author": "El Rais",
"first-page": "1",
"journal-title": "Les Dossiers de la DREES",
"key": "ref30",
"volume": "79",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1371/journal.pone.0248713",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1007/s40520-020-01677-y",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1016/j.coph.2010.04.001",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.3390/nu13072216",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1016/j.cell.2016.10.018",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.3389/fpubh.2021.624559",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1080/10408398.2020.1841090",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.23736/S0031-0808.21.04277-4",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1016/j.metabol.2021.154753",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.3390/nu12061553",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1001/jama.2020.26848",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1001/jama.2020.26850",
"doi-asserted-by": "publisher",
"key": "ref43"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/14/8/1641"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Food Science",
"Nutrition and Dietetics"
],
"subtitle": [],
"title": "Vitamin D and COVID-19 Severity in Hospitalized Older Patients: Potential Benefit of Prehospital Vitamin D Supplementation",
"type": "journal-article",
"volume": "14"
}
