Clinical efficacy of casirivimab and imdevimab in preventing COVID-19 in the Omicron BA.5 subvariant epidemic: a retrospective study
et al., Journal of Pharmaceutical Health Care and Sciences, doi:10.1186/s40780-025-00501-x, May 2025 (preprint)
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective study of 52 hospitalized patients showing significantly lower COVID-19 incidence with casirivimab/imdevimab for post-exposure prophylaxis during a period when Omicron BA.5 was dominant.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of case, 87.9% lower, OR 0.12, p = 0.02, treatment 14, control 38, adjusted per study, multivariable, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Ohtani et al., 13 May 2025, retrospective, Japan, peer-reviewed, 13 authors, study period October 2022 - December 2022.
Contact: mariko.ootani@med.toho-u.ac.jp.
Clinical efficacy of casirivimab and imdevimab in preventing COVID-19 in the Omicron BA.5 subvariant epidemic: a retrospective study
Journal of Pharmaceutical Health Care and Sciences, doi:10.1186/s40780-025-00501-x
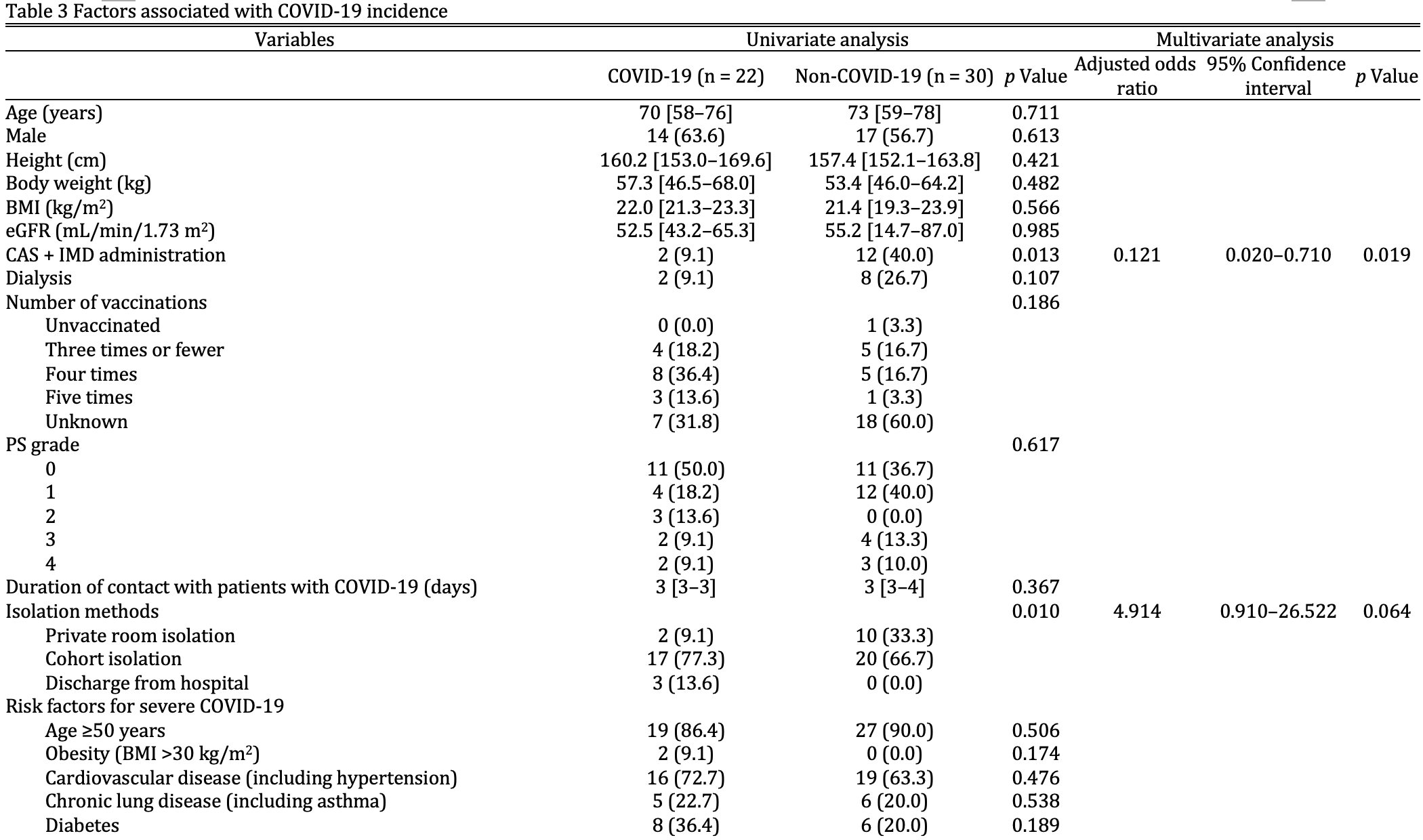
Background The neutralizing monoclonal antibody combination of casirivimab and imdevimab (CAS + IMD) is the only therapy approved for preventing coronavirus disease 2019 following exposure to severe acute respiratory syndrome coronavirus 2. However, the efficacy of CAS + IMD against Omicron variants remains uncertain, with in vitro studies indicating reduced neutralizing activity. This study aimed to evaluate the clinical efficacy of CAS + IMD in preventing COVID-19 among uninfected hospitalized contacts of patients with COVID-19. Methods A retrospective chart review was conducted on 154 inpatients exposed to patients with COVID-19 between October and December 2022. Fifty-two uninfected participants who were unvaccinated or immunosuppressed and had risk factors for severe COVID-19 were included. The primary endpoint was the COVID-19 incidence rate. Statistical analyses included the chi-square test, Fisher's exact test, and Mann-Whitney U test, as appropriate. Factors associated with COVID-19 incidence (p < 0.05) in univariate analysis were included in the multivariate logistic regression. Statistical significance was set at p < 0.05.
Results Among the 52 participants, 14 and 38 were included in the CAS + IMD and non-CAS + IMD groups, respectively. The COVID-19 incidence rate was significantly lower in the CAS + IMD group than in the non-CAS + IMD group (14.3% vs. 52.6%, p = 0.013). Multivariate analysis identified CAS + IMD administration as significantly associated with reduced COVID-19 incidence (adjusted odds ratio [OR], 0.121; 95% confidence interval [CI], 0.020-0.710; p = 0.019), whereas long-term use of immunosuppressive therapy was associated with increased incidence (adjusted OR, 4.320; 95% CI,; p = 0.037). Conclusions CAS + IMD may be effective for post-exposure prophylaxis of COVID-19 during the Omicron BA.5 subvariant epidemic. However, prudent clinical use should consider the circulating variant profile. Further research is warranted to validate CAS + IMD's role in COVID-19 post-exposure prophylaxis.
Declarations Ethics approval and consent to participate This study adhered to the "Ethical Guidelines for Medical and Biological Research Involving Human Subjects" and was approved by the Ethics Committee of Toho University Omori Medical Center (approval number: M24037 22285). Patients were informed of their option to opt out, with details clearly outlined on the institutional website.
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Carabelli, Peacock, Thorne, Harvey, Hughes et al., SARS-CoV-2 variant biology: immune escape, transmission and fitness, Nat Rev Microbiol
Deeks, Casirivimab/imdevimab: first approval, Drugs
Gershengorn, Patel, Ferreira, Das, Parekh et al., The clinical effectiveness of REGEN-COV in SARS-CoV-2 infection with Omicron versus Delta variants, PLoS ONE
Hagihara, Hayashi, Nakashima, Imai, Nakano et al., Clinical efficacy of imdevimab/casirivimab for persistent Omicron SARS-CoV-2 infection in patients with hematological malignancies, Intern Med
Herman, Brien, Forleo-Neto, Sarkar, Isa et al., Efficacy and safety of a single dose of casirivimab and imdevimab for the prevention of COVID-19 over an 8-month period: a randomised, double-blind, placebocontrolled trial, Lancet Infect Dis
Imai, Ito, Kiso, Yamayoshi, Uraki et al., Efficacy of antiviral agents against Omicron subvariants BQ.1.1 and XBB, N Engl J Med
Kamegai, Iwamoto, Ishikane, Yamamoto, Horii et al., A novel protocol for de-isolating moderately and severely immunocompromised COVID-19 patients, Glob Health Med
O'brien, Forleo-Neto, Musser, Chan, Sarkar, Subcutaneous REGEN-COV antibody combination to prevent Covid-19, N Engl J Med
Planas, Bruel, Staropoli, Guivel-Benhassine, Porrot et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6, and BQ.1.1 to neutralizing antibodies, Nat Commun
Syed, Ciling, Taha, Chen, Khalid et al., Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles, Proc Natl Acad Sci
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2, N Engl J Med
Takashita, Yamayoshi, Simon, Van Bakel, Sordillo et al., Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants, N Engl J Med
Walinjkar, Kumbhar, Shinde, Chaurasia, Real-world effect of casirivimab and imdevimab cocktail in patients infected with SARS-CoV-2 delta and omicron variants, J Infect Dev Ctries
Wang, Golubov, Oswald, Poon, Wei et al., Potential immunomodulatory effects of CAS+IMD monoclonal antibody cocktail in hospitalized patients with COVID-19, EBioMedicine
Wang, Iketani, Li, Liu, Guo et al., Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants, Cell
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med
DOI record:
{
"DOI": "10.1186/s40780-025-00501-x",
"ISSN": [
"2055-0294"
],
"URL": "http://dx.doi.org/10.1186/s40780-025-00501-x",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The neutralizing monoclonal antibody combination of casirivimab and imdevimab (CAS + IMD) is the only therapy approved for preventing coronavirus disease 2019 (COVID-19) following exposure to severe acute respiratory syndrome coronavirus 2. However, the efficacy of CAS + IMD against Omicron variants remains uncertain, with in vitro studies indicating reduced neutralizing activity. This study aimed to evaluate the clinical efficacy of CAS + IMD in preventing COVID-19 among uninfected hospitalized contacts of patients with COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>\n A retrospective chart review was conducted on 154 inpatients exposed to patients with COVID-19 between October and December 2022. Fifty-two uninfected participants who were unvaccinated or immunosuppressed and had risk factors for severe COVID-19 were included. The primary endpoint was the COVID-19 incidence rate. Statistical analyses included the chi-square test, Fisher's exact test, and Mann–Whitney U test, as appropriate. Factors associated with COVID-19 incidence (\n <jats:italic>p</jats:italic>\n < 0.05) in univariate analysis were included in the multivariate logistic regression. Statistical significance was set at\n <jats:italic>p</jats:italic>\n < 0.05.\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>\n Among the 52 participants, 14 and 38 were included in the CAS + IMD and non-CAS + IMD groups, respectively. The COVID-19 incidence rate was significantly lower in the CAS + IMD group than in the non-CAS + IMD group (14.3% vs. 52.6%,\n <jats:italic>p</jats:italic>\n = 0.013). Multivariate analysis identified CAS + IMD administration as significantly associated with reduced COVID-19 incidence (adjusted odds ratio [OR], 0.121; 95% confidence interval [CI], 0.020–0.710;\n <jats:italic>p</jats:italic>\n = 0.019), whereas long-term use of immunosuppressive therapy was associated with increased incidence (adjusted OR, 4.320; 95% CI, 1.090–17.126;\n <jats:italic>p</jats:italic>\n = 0.037).\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>CAS + IMD may be effective for post-exposure prophylaxis of COVID-19 during the Omicron BA.5 subvariant epidemic. However, prudent clinical use should consider the circulating variant profile. Further research is warranted to validate CAS + IMD’s role in COVID-19 post-exposure prophylaxis.</jats:p>\n </jats:sec>",
"alternative-id": [
"501"
],
"article-number": "94",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "3 May 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "30 September 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "27 October 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study adhered to the “Ethical Guidelines for Medical and Biological Research Involving Human Subjects” and was approved by the Ethics Committee of Toho University Omori Medical Center (approval number: M24037 22285). Patients were informed of their option to opt out, with details clearly outlined on the institutional website."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Ohtani",
"given": "Mariko",
"sequence": "first"
},
{
"affiliation": [],
"family": "Yokoo",
"given": "Takuya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miyazaki",
"given": "Taito",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yasuda",
"given": "Hiroshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nishikawa",
"given": "Eriko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomida",
"given": "Manabu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsukada",
"given": "Mayumi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sato",
"given": "Emi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hirayama",
"given": "Shinobu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murakami",
"given": "Hinako",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yoshizawa",
"given": "Sadako",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matsumoto",
"given": "Takahiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tateda",
"given": "Kazuhiro",
"sequence": "additional"
}
],
"container-title": "Journal of Pharmaceutical Health Care and Sciences",
"container-title-short": "J Pharm Health Care Sci",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
10,
27
]
],
"date-time": "2025-10-27T13:11:17Z",
"timestamp": 1761570677000
},
"deposited": {
"date-parts": [
[
2025,
10,
27
]
],
"date-time": "2025-10-27T13:11:18Z",
"timestamp": 1761570678000
},
"indexed": {
"date-parts": [
[
2025,
10,
27
]
],
"date-time": "2025-10-27T14:17:19Z",
"timestamp": 1761574639498,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
10,
27
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
27
]
],
"date-time": "2025-10-27T00:00:00Z",
"timestamp": 1761523200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
27
]
],
"date-time": "2025-10-27T00:00:00Z",
"timestamp": 1761523200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40780-025-00501-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s40780-025-00501-x/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40780-025-00501-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
10,
27
]
]
},
"published-online": {
"date-parts": [
[
2025,
10,
27
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"author": "AM Carabelli",
"first-page": "162",
"journal-title": "Nat Rev Microbiol",
"key": "501_CR1",
"unstructured": "Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J, COVID-19 Genomics UK Consortium, et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21:162–77.",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2109682",
"author": "MP O’Brien",
"doi-asserted-by": "publisher",
"first-page": "1184",
"journal-title": "N Engl J Med",
"key": "501_CR2",
"unstructured": "O’Brien MP, Forleo-Neto E, Musser BJ, Isa F, Chan KC, Sarkar N, et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med. 2021;385:1184–95.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(22)00416-9",
"author": "GA Herman",
"doi-asserted-by": "publisher",
"first-page": "1444",
"journal-title": "Lancet Infect Dis",
"key": "501_CR3",
"unstructured": "Herman GA, O’Brien MP, Forleo-Neto E, Sarkar N, Isa F, Hou P, et al. Efficacy and safety of a single dose of casirivimab and imdevimab for the prevention of COVID-19 over an 8-month period: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2022;22:1444–54.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"author": "E Takashita",
"doi-asserted-by": "publisher",
"first-page": "1475",
"journal-title": "N Engl J Med",
"key": "501_CR4",
"unstructured": "Takashita E, Kinoshita N, Yamayoshi S, Sakai-Tagawa Y, Fujisaki S, Ito M, et al. Efficacy of antiviral agents against the SARS-CoV-2 Omicron subvariant BA.2. N Engl J Med. 2022;386:1475–7.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2207519",
"author": "E Takashita",
"doi-asserted-by": "publisher",
"first-page": "468",
"journal-title": "N Engl J Med",
"key": "501_CR5",
"unstructured": "Takashita E, Yamayoshi S, Simon V, van Bakel H, Sordillo EM, Pekosz A, et al. Efficacy of antibodies and antiviral drugs against Omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med. 2022;387:468–70.",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2214302",
"author": "M Imai",
"doi-asserted-by": "publisher",
"first-page": "89",
"journal-title": "N Engl J Med",
"key": "501_CR6",
"unstructured": "Imai M, Ito M, Kiso M, Yamayoshi S, Uraki R, Fukushi S, et al. Efficacy of antiviral agents against Omicron subvariants BQ.1.1 and XBB. N Engl J Med. 2023;388:89–91.",
"volume": "388",
"year": "2023"
},
{
"DOI": "10.1073/pnas.2200592119",
"author": "AM Syed",
"doi-asserted-by": "publisher",
"first-page": "e2200592119",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "501_CR7",
"unstructured": "Syed AM, Ciling A, Taha TY, Chen IP, Khalid MM, Sreekumar B, et al. Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. Proc Natl Acad Sci U S A. 2022;119:e2200592119.",
"volume": "119",
"year": "2022"
},
{
"DOI": "10.1038/s41467-023-36561-6",
"author": "D Planas",
"doi-asserted-by": "publisher",
"first-page": "824",
"journal-title": "Nat Commun",
"key": "501_CR8",
"unstructured": "Planas D, Bruel T, Staropoli I, Guivel-Benhassine F, Porrot F, Maes P, et al. Resistance of Omicron subvariants BA.2.75.2, BA.4.6, and BQ.1.1 to neutralizing antibodies. Nat Commun. 2023;14:824.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.cell.2022.12.018",
"author": "Q Wang",
"doi-asserted-by": "publisher",
"first-page": "279",
"journal-title": "Cell",
"key": "501_CR9",
"unstructured": "Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186:279-86.e8.",
"volume": "186",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2108163",
"author": "DM Weinreich",
"doi-asserted-by": "publisher",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "501_CR10",
"unstructured": "Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. N Engl J Med. 2021;385:e81.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.35772/ghm.2023.01053",
"author": "K Kamegai",
"doi-asserted-by": "publisher",
"first-page": "366",
"journal-title": "Glob Health Med",
"key": "501_CR11",
"unstructured": "Kamegai K, Iwamoto N, Ishikane M, Yamamoto K, Horii K, Kubota S, et al. A novel protocol for de-isolating moderately and severely immunocompromised COVID-19 patients. Glob Health Med. 2023;5:366–71.",
"volume": "5",
"year": "2023"
},
{
"key": "501_CR12",
"unstructured": "Japan Institute for Health Security. The infectious disease information website. https://id-info.jihs.go.jp/diseases/sa/covid-19/170/cepr-topics-log.html. Accessed 3 Apr 2025."
},
{
"DOI": "10.1007/s40265-021-01620-z",
"author": "ED Deeks",
"doi-asserted-by": "publisher",
"first-page": "2047",
"journal-title": "Drugs",
"key": "501_CR13",
"unstructured": "Deeks ED. Casirivimab/imdevimab: first approval. Drugs. 2021;81:2047–55.",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.3855/jidc.17039",
"author": "R Subhash Walinjkar",
"doi-asserted-by": "publisher",
"first-page": "293",
"journal-title": "J Infect Dev Ctries",
"key": "501_CR14",
"unstructured": "Subhash Walinjkar R, Kumbhar M, Harihar Shinde R, Chaurasia E. Real-world effect of casirivimab and imdevimab cocktail in patients infected with SARS-CoV-2 delta and omicron variants. J Infect Dev Ctries. 2023;17:293–301.",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1371/journal.pone.0278770",
"author": "HB Gershengorn",
"doi-asserted-by": "publisher",
"first-page": "e0278770",
"journal-title": "PLoS ONE",
"key": "501_CR15",
"unstructured": "Gershengorn HB, Patel S, Ferreira T, Das S, Parekh DJ, Shukla B. The clinical effectiveness of REGEN-COV in SARS-CoV-2 infection with Omicron versus Delta variants. PLoS ONE. 2022;17:e0278770.",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.2169/internalmedicine.2900-23",
"author": "M Hagihara",
"doi-asserted-by": "publisher",
"first-page": "2283",
"journal-title": "Intern Med",
"key": "501_CR16",
"unstructured": "Hagihara M, Hayashi H, Nakashima S, Imai Y, Nakano H, Uchida T, et al. Clinical efficacy of imdevimab/casirivimab for persistent Omicron SARS-CoV-2 infection in patients with hematological malignancies. Intern Med. 2024;63:2283–7.",
"volume": "63",
"year": "2024"
},
{
"DOI": "10.1016/j.ebiom.2024.105334",
"author": "B Wang",
"doi-asserted-by": "publisher",
"journal-title": "EBioMedicine",
"key": "501_CR17",
"unstructured": "Wang B, Golubov J, Oswald EM, Poon P, Wei Q, Lett C, et al. Potential immunomodulatory effects of CAS+IMD monoclonal antibody cocktail in hospitalized patients with COVID-19. EBioMedicine. 2024;108:105334.",
"volume": "108",
"year": "2024"
},
{
"key": "501_CR18",
"unstructured": "Ministry of Health, Labour and Welfare. Vaccination and vaccine information. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/yobou-sesshu/syukeihou_00002.html. Accessed 17 Sep 2025."
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://jphcs.biomedcentral.com/articles/10.1186/s40780-025-00501-x"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Clinical efficacy of casirivimab and imdevimab in preventing COVID-19 in the Omicron BA.5 subvariant epidemic: a retrospective study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "11"
}
