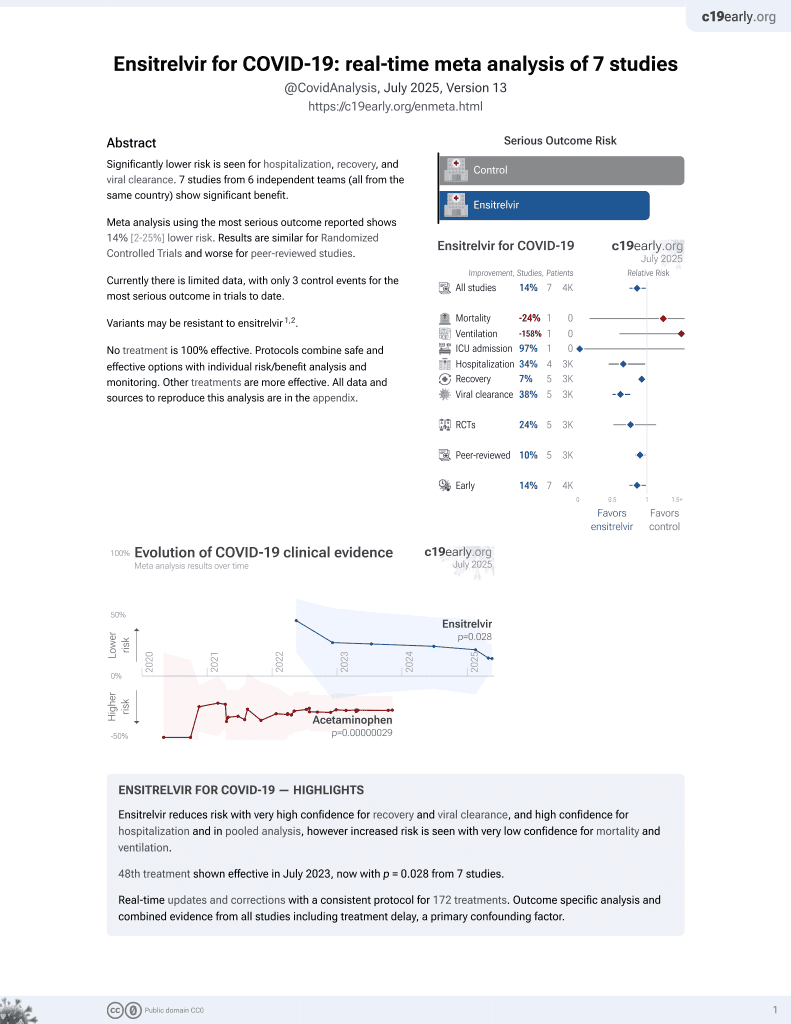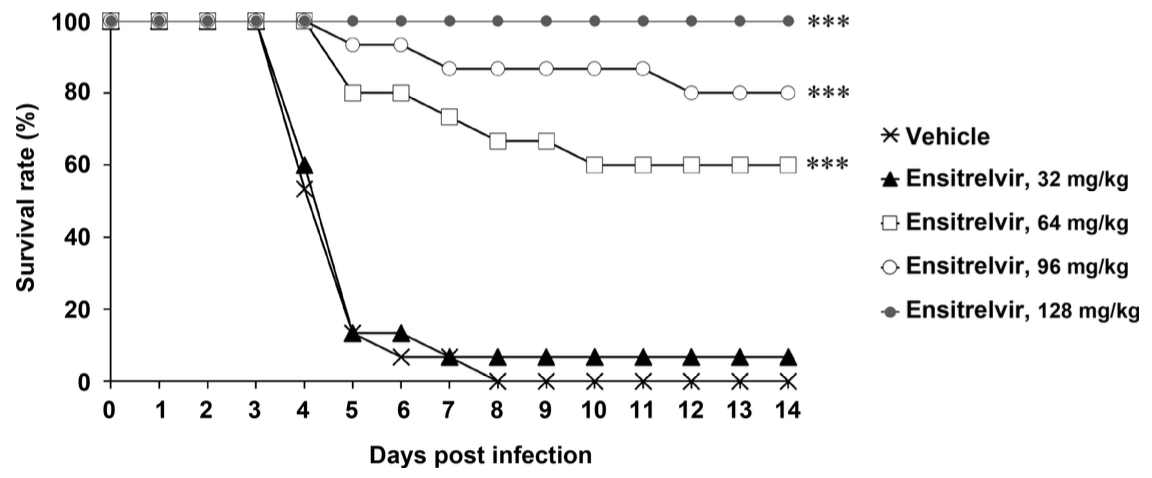
Prophylactic effect of ensitrelvir in mice infected with SARS-CoV-2
et al., Antiviral Research, doi:10.1016/j.antiviral.2024.105852, Feb 2024
50th treatment shown to reduce risk in
July 2023, now with p = 0.015 from 8 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Mouse study showing protective effects against SARS-CoV-2 infection in aged mice with the 3CL protease inhibitor ensitrelvir. A single subcutaneous dose of ensitrelvir at 64, 96, or 128 mg/kg given 24 hours before a lethal SARS-CoV-2 challenge significantly increased 14-day survival rates and reduced body weight loss compared to control mice. Ensitrelvir plasma concentrations of 2.99 μg/mL or greater at the time of infection conferred significant protection. Viral titers were suppressed in the lungs of ensitrelvir-treated mice, suggesting ensitrelvir exerts its prophylactic effect by inhibiting viral replication.
5 preclinical studies support the efficacy of ensitrelvir for COVID-19:
1.
Nair et al., Persistence of an infectious form of SARS-CoV-2 post protease inhibitor treatment of permissive cells in vitro, The Journal of Infectious Diseases, doi:10.1093/infdis/jiae385.
2.
Moghadasi et al., Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors, Science Advances, doi:10.1126/sciadv.ade8778.
a.
VeroE6/TMPRSS2 is a Vero E6 cell line engineered to express the human serine protease TMPRSS2, enabling SARS-CoV-2 S protein priming and entry.
b.
HEK293T/ACE2-TMPRSS2 is a human embryonic kidney cell line engineered to express human ACE2 and TMPRSS2, making it highly susceptible to SARS-CoV-2 infection.
c.
MucilAir cells are primary human nasal epithelial cells that mimic the structure and physiology of the human airway epithelium.
d.
A mouse model commonly used in infectious disease and cancer research due to higher immune response and susceptibility to infection.
e.
A rodent model used in SARS-CoV-2 research that replicates key aspects of human infection including efficient replication in the upper and lower respiratory tract.
f.
The original SARS-CoV-2 strain that emerged in Wuhan, China in late 2019. Also referred to as wild-type.
g.
A variant of concern first identified in India in late 2020, delta (B.1.617.2) transmitted more efficiently than previous variants. It contains spike mutations including L452R which increases binding to the ACE2 receptor.
h.
A highly transmissible variant of concern first detected in South Africa in late 2021. Omicron possesses many spike mutations which confer partial immune evasion, including deletions near the furin cleavage site.
Nobori et al., 29 Feb 2024, Japan, peer-reviewed, 10 authors.
Contact: haruaki.nobori@shionogi.co.jp, keiko.baba@shionogi.co.jp, takayuki.kuroda@shionogi.co.jp, shinpei.yoshida@shionogi.co.jp, ryosuke.watari@shionogi.co.jp, yuki.tachibana@shionogi.co.jp, teruhisa.kato@shionogi.co.jp, keita.fukao@shionogi.co.jp, kaoru.baba@shionogi.co.jp, kazumi.matsumoto@shionogi.co.jp.
Prophylactic effect of ensitrelvir in mice infected with SARS-CoV-2
Antiviral Research, doi:10.1016/j.antiviral.2024.105852
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiological cause of coronavirus disease 2019 and continues to be a major health concern worldwide. Strategies to protect individuals at high risk of COVID-19 are critical but are currently a largely unmet need. We evaluated the oral antiviral drug ensitrelvir, which specifically targets the SARS-CoV-2 3CL protease, for its efficacy as a pre-exposure prophylactic treatment. Aged BALB/c mice were subcutaneously treated with various doses of ensitrelvir 24 h prior to a lethal SARS-CoV-2 challenge infection. Mouse body weight changes, survival rates, and viral titers in the lungs were evaluated, and plasma concentrations of ensitrelvir were determined. A single subcutaneous administration of ensitrelvir at 64 mg/kg or greater 24 h prior to SARS-CoV-2 challenge infection significantly protected aged mice against lethality and inhibited body weight loss. Pharmacokinetic analysis of ensitrelvir in the aged mice suggested that plasma concentrations ≥ 2.99 µg/mL resulted in a significant prophylactic effect against SARS-CoV-2 infection. In the aged mouse prophylaxis model, SARS-CoV-2 titers were suppressed in the lungs of mice treated with ensitrelvir 24 h prior to challenge infection, suggesting that the prophylactic administration of ensitrelvir exerted its prophylactic effect by suppressing viral proliferation. These findings suggest that ensitrelvir is a candidate drug for pre-exposure prophylactic treatment of individuals at high risk of COVID-19.
Effect of prophylactic ensitrelvir on body weight in aged mice infected with SARS-CoV-2 The prophylactic effect of S-217622 against lethal infection with the SARS-CoV-2 MA-P10 strain in mice was evaluated by monitoring body weight loss for 14 d post-challenge infection. A single dose of ensitrelvir administered subcutaneously at 64, 96, or 128 mg/kg 24 h prior to infection significantly suppressed body weight loss due to SARS-CoV-2 infection (Fig. 3 and Supplementary Figure S2 ). A single subcutaneous dose of 32 mg/kg ensitrelvir failed to provide significant prophylactic protection compared to that of vehicle (P ≥ 0.3039). The 64 mg/kg and 96 mg/kg ensitrelvir treatment provided significant protection from weight loss compared to that of vehicle from day 3 through day 7 post-infection (P < 0.05 -P < 0.001). Significant protection was also observed for ensitrelvir doses 128 mg/kg from day 2 through day 7 post-infection (P < 0.001).
Effect of prophylactic ensitrelvir on viral load in the lungs of aged mice infected with SARS-
CoV-2 To evaluate the prophylactic effect of ensitrelvir against lethal infection with the SARS-CoV-2 MA-P10 strain, lung virus titers were measured at 1, 2, 4, and 9 d post-challenge infection. A single prophylactic subcutaneous administration of 64 or 128 mg/kg ensitrelvir 24 h before infection significantly suppressed the lung viral load in mice (Fig. 4 , Supplementary Table S1 ). The lung viral titers peaked at 2 d post-infection and..
References
Alpizar, Accini, Anderson, Eysa, Medina-Piñón et al., Molnupiravir for intra-household prevention of COVID-19: The MOVe-AHEAD randomized, placebo-controlled trial, J. Infect, doi:10.1016/j.jinf.2023.08.016
Ando, Noshi, Sato, Ishibashi, Yoshida et al., Pharmacokinetic and pharmacodynamic analysis of baloxavir marboxil, a novel cap-dependent endonuclease inhibitor, in a murine model of influenza virus infection, J. Antimicrob. Chemother, doi:10.1093/jac/dkaa393
Fukao, Nobori, Kuroda, Baba, Matsumoto et al., Pharmacokinetic and pharmacodynamic analysis of the 3CL protease inhibitor ensitrelvir in a SARS-CoV-2 infection mouse model, Viruses, doi:10.3390/v15102052
Harrison, Lin, Wang, Mechanisms of SARS-CoV-2 transmission and pathogenesis, Trends Immunol, doi:10.1016/j.it.2020.10.004
Hayden, Gubareva, Monto, Klein, Elliot et al., Inhaled zanamivir for the prevention of influenza in families. Zanamivir Family Study Group, N. Engl. J. Med, doi:10.1056/NEJM200011023431801
Ikematsu, Hayden, Kawaguchi, Kinoshita, De Jong et al., Baloxavir marboxil for prophylaxis against influenza in household contacts, N. Engl. J. Med, doi:10.1056/NEJMoa1915341
Kashiwagi, Watanabe, Ikematsu, Awamura, Okamoto et al., Laninamivir octanoate for post-exposure prophylaxis of influenza in household contacts: a randomized double blind placebo controlled trial, J. Infect. Chemother, doi:10.1007/s10156-013-0622-9
Kawashima, Matsui, Adachi, Morikawa, Inoue et al., Ensitrelvir is effective against SARS-CoV-2 3CL protease mutants circulating globally, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2023.01.040
Kuroda, Nobori, Fukao, Baba, Matsumoto et al., Efficacy comparison of 3CL protease inhibitors ensitrelvir and nirmatrelvir against SARS-CoV-2 in vitro and in vivo, J. Antimicrob. Chemother, doi:10.1093/jac/dkad027
Martins-Filho, Ferreira, Heimfarth, De Souza Araújo, Quintans-Júnior, Efficacy and safety of hydroxychloroquine as pre-and post-exposure prophylaxis and J o u r n a l P r e -p r o o f treatment of COVID-19: A systematic review and meta-analysis of blinded, placebocontrolled, randomized clinical trials, Lancet Reg. Health Am, doi:10.1016/j.lana.2021.100062
Matsuyama, Nao, Shirato, Kawase, Saito et al., Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells, Proc. Natl. Acad. Sci. U. S. A, doi:10.1073/pnas.2002589117
Mukae, Yotsuyanagi, Ohmagari, Doi, Imamura et al., A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part, Antimicrob. Agents Chemother, doi:10.1128/aac.00697-22
Mukae, Yotsuyanagi, Ohmagari, Doi, Sakaguchi et al., Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebocontrolled, phase 2/3 study, Clin. Infect. Dis, doi:10.1101/2022.06.22.22276792
Nobori, Fukao, Kuroda, Anan, Tashima et al., Efficacy of ensitrelvir against SARS-CoV-2 in a delayed-treatment mouse model, J. Antimicrob. Chemother, doi:10.1093/jac/dkac257
Ouyang, Zaongo, Harypursat, Li, Routy et al., SARS-CoV-2 pre-exposure prophylaxis: A potential COVID-19 preventive strategy for high-risk populations, including healthcare workers, immunodeficient individuals, and poor vaccine responders, Front. Public Health, doi:10.3389/fpubh.2022.945448
Pfizer, Pfizer Shares Top-Line Results from Phase 2/3 EPIC-PEP Study of PAXLOVID™ for Post-Exposure Prophylactic Use
Shimizu, Sonoyama, Fukuhara, Kuwata, Matsuo et al., A phase 1 study of ensitrelvir fumaric acid tablets evaluating the safety, pharmacokinetics and food effect in healthy adult populations, Clin. Drug Investig, doi:10.1007/s40261-023-01309-z
Uemura, Nobori, Sato, Toba, Kusakabe et al., 2-Thiouridine is a broad-spectrum antiviral nucleoside analogue against positive-strand RNA viruses, Proc. Natl. Acad. Sci. U. S. A, doi:10.1073/pnas.2304139120
Ullrich, Nitsche, The SARS-CoV-2 main protease as drug target, Bioorg. Med. Chem. Lett, doi:10.1016/j.bmcl.2020.127377
Unoh, Uehara, Nakahara, Nobori, Yamatsu et al., Discovery of S-217622, a Noncovalent Oral SARS-J o u r n a l P r e -p r o o f CoV-2 3CL Protease Inhibitor Clinical Candidate for Treating COVID-19, J. Med. Chem, doi:10.1021/acs.jmedchem.2c00117
Vangeel, Chiu, De Jonghe, Maes, Slechten et al., Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res, doi:10.1016/j.antiviral.2022.105252
Welliver, Monto, Carewicz, Schatteman, Hassman et al., Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial, doi:10.1001/jama.285.6.748
DOI record:
{
"DOI": "10.1016/j.antiviral.2024.105852",
"ISSN": [
"0166-3542"
],
"URL": "http://dx.doi.org/10.1016/j.antiviral.2024.105852",
"alternative-id": [
"S0166354224000603"
],
"article-number": "105852",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Prophylactic effect of ensitrelvir in mice infected with SARS-CoV-2"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Antiviral Research"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.antiviral.2024.105852"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Authors. Published by Elsevier B.V."
}
],
"author": [
{
"affiliation": [],
"family": "Nobori",
"given": "Haruaki",
"sequence": "first"
},
{
"affiliation": [],
"family": "Baba",
"given": "Keiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kuroda",
"given": "Takayuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baba",
"given": "Kaoru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matsumoto",
"given": "Kazumi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0004-5845",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yoshida",
"given": "Shinpei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watari",
"given": "Ryosuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tachibana",
"given": "Yuki",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5041-0110",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kato",
"given": "Teruhisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fukao",
"given": "Keita",
"sequence": "additional"
}
],
"container-title": "Antiviral Research",
"container-title-short": "Antiviral Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
2,
28
]
],
"date-time": "2024-02-28T10:55:24Z",
"timestamp": 1709117724000
},
"deposited": {
"date-parts": [
[
2024,
2,
29
]
],
"date-time": "2024-02-29T16:28:24Z",
"timestamp": 1709224104000
},
"indexed": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:44:11Z",
"timestamp": 1709253851517
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T00:00:00Z",
"timestamp": 1706745600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 28,
"start": {
"date-parts": [
[
2024,
2,
29
]
],
"date-time": "2024-02-29T00:00:00Z",
"timestamp": 1709164800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0166354224000603?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0166354224000603?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "105852",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
2
]
]
},
"published-print": {
"date-parts": [
[
2024,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.jinf.2023.08.016",
"article-title": "Molnupiravir for intra-household prevention of COVID-19: the MOVe-AHEAD randomized, placebo-controlled trial",
"author": "Alpizar",
"doi-asserted-by": "crossref",
"first-page": "392",
"journal-title": "J. Infect.",
"key": "10.1016/j.antiviral.2024.105852_bib1",
"volume": "87",
"year": "2023"
},
{
"DOI": "10.1093/jac/dkaa393",
"article-title": "Pharmacokinetic and pharmacodynamic analysis of baloxavir marboxil, a novel cap-dependent endonuclease inhibitor, in a murine model of influenza virus infection",
"author": "Ando",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "J. Antimicrob. Chemother.",
"key": "10.1016/j.antiviral.2024.105852_bib2",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.3390/v15102052",
"article-title": "Pharmacokinetic and pharmacodynamic analysis of the 3CL protease inhibitor ensitrelvir in a SARS-CoV-2 infection mouse model",
"author": "Fukao",
"doi-asserted-by": "crossref",
"first-page": "2052",
"journal-title": "Viruses",
"key": "10.1016/j.antiviral.2024.105852_bib3",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1016/j.it.2020.10.004",
"article-title": "Mechanisms of SARS-CoV-2 transmission and pathogenesis",
"author": "Harrison",
"doi-asserted-by": "crossref",
"first-page": "1100",
"journal-title": "Trends Immunol.",
"key": "10.1016/j.antiviral.2024.105852_bib4",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1056/NEJM200011023431801",
"article-title": "Inhaled zanamivir for the prevention of influenza in families. Zanamivir Family Study Group",
"author": "Hayden",
"doi-asserted-by": "crossref",
"first-page": "1282",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.antiviral.2024.105852_bib5",
"volume": "343",
"year": "2000"
},
{
"DOI": "10.1056/NEJMoa1915341",
"article-title": "Baloxavir marboxil for prophylaxis against influenza in household contacts",
"author": "Ikematsu",
"doi-asserted-by": "crossref",
"first-page": "309",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.antiviral.2024.105852_bib6",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1007/s10156-013-0622-9",
"article-title": "Laninamivir octanoate for post-exposure prophylaxis of influenza in household contacts: a randomized double blind placebo controlled trial",
"author": "Kashiwagi",
"doi-asserted-by": "crossref",
"first-page": "740",
"journal-title": "J. Infect. Chemother.",
"key": "10.1016/j.antiviral.2024.105852_bib7",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.1016/j.bbrc.2023.01.040",
"article-title": "Ensitrelvir is effective against SARS-CoV-2 3CL protease mutants circulating globally",
"author": "Kawashima",
"doi-asserted-by": "crossref",
"first-page": "132",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "10.1016/j.antiviral.2024.105852_bib8",
"volume": "645",
"year": "2023"
},
{
"DOI": "10.1093/jac/dkad027",
"article-title": "Efficacy comparison of 3CL protease inhibitors ensitrelvir and nirmatrelvir against SARS-CoV-2 in vitro and in vivo",
"author": "Kuroda",
"doi-asserted-by": "crossref",
"first-page": "946",
"journal-title": "J. Antimicrob. Chemother.",
"key": "10.1016/j.antiviral.2024.105852_bib9",
"volume": "78",
"year": "2023"
},
{
"article-title": "Efficacy and safety of hydroxychloroquine as pre-and post-exposure prophylaxis and treatment of COVID-19: a systematic review and meta-analysis of blinded, placebo-controlled, randomized clinical trials",
"author": "Martins-Filho",
"journal-title": "Lancet Reg. Health Am.",
"key": "10.1016/j.antiviral.2024.105852_bib10",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2002589117",
"article-title": "Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells",
"author": "Matsuyama",
"doi-asserted-by": "crossref",
"first-page": "7001",
"journal-title": "Proc. Natl. Acad. Sci. U. S. A.",
"key": "10.1016/j.antiviral.2024.105852_bib11",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1128/aac.00697-22",
"article-title": "A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part",
"author": "Mukae",
"doi-asserted-by": "crossref",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "10.1016/j.antiviral.2024.105852_bib12",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac933",
"article-title": "Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study",
"author": "Mukae",
"doi-asserted-by": "crossref",
"first-page": "1403",
"journal-title": "Clin. Infect. Dis.",
"key": "10.1016/j.antiviral.2024.105852_bib13",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.1093/jac/dkac257",
"article-title": "Efficacy of ensitrelvir against SARS-CoV-2 in a delayed-treatment mouse model",
"author": "Nobori",
"doi-asserted-by": "crossref",
"first-page": "2984",
"journal-title": "J. Antimicrob. Chemother.",
"key": "10.1016/j.antiviral.2024.105852_bib14",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.3389/fpubh.2022.945448",
"article-title": "SARS-CoV-2 pre-exposure prophylaxis: a potential COVID-19 preventive strategy for high-risk populations, including healthcare workers, immunodeficient individuals, and poor vaccine responders",
"author": "Ouyang",
"doi-asserted-by": "crossref",
"journal-title": "Front. Public Health",
"key": "10.1016/j.antiviral.2024.105852_bib15",
"volume": "10",
"year": "2022"
},
{
"author": "Pfizer",
"key": "10.1016/j.antiviral.2024.105852_bib16",
"series-title": "Pfizer Shares Top-Line Results from Phase 2/3 EPIC-PEP Study of PAXLOVID™ for Post-Exposure Prophylactic Use",
"year": "2022"
},
{
"DOI": "10.1007/s40261-023-01309-z",
"article-title": "A phase 1 study of ensitrelvir fumaric acid tablets evaluating the safety, pharmacokinetics and food effect in healthy adult populations",
"author": "Shimizu",
"doi-asserted-by": "crossref",
"first-page": "785",
"journal-title": "Clin. Drug Invest.",
"key": "10.1016/j.antiviral.2024.105852_bib17",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.1073/pnas.2304139120",
"article-title": "2-Thiouridine is a broad-spectrum antiviral nucleoside analogue against positive-strand RNA viruses",
"author": "Uemura",
"doi-asserted-by": "crossref",
"journal-title": "Proc. Natl. Acad. Sci. U. S. A.",
"key": "10.1016/j.antiviral.2024.105852_bib18",
"volume": "120",
"year": "2023"
},
{
"DOI": "10.1016/j.bmcl.2020.127377",
"article-title": "The SARS-CoV-2 main protease as drug target",
"author": "Ullrich",
"doi-asserted-by": "crossref",
"journal-title": "Bioorg. Med. Chem. Lett.",
"key": "10.1016/j.antiviral.2024.105852_bib19",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1021/acs.jmedchem.2c00117",
"article-title": "Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19",
"author": "Unoh",
"doi-asserted-by": "crossref",
"first-page": "6499",
"journal-title": "J. Med. Chem.",
"key": "10.1016/j.antiviral.2024.105852_bib20",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"article-title": "Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern",
"author": "Vangeel",
"doi-asserted-by": "crossref",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.antiviral.2024.105852_bib21",
"volume": "198",
"year": "2022"
},
{
"DOI": "10.1001/jama.285.6.748",
"article-title": "Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial",
"author": "Welliver",
"doi-asserted-by": "crossref",
"first-page": "748",
"journal-title": "JAMA",
"key": "10.1016/j.antiviral.2024.105852_bib22",
"volume": "285",
"year": "2001"
},
{
"key": "10.1016/j.antiviral.2024.105852_bib23",
"series-title": "Organization Weekly Epidemiological Update on COVID-19",
"year": "2023"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0166354224000603"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Pharmacology"
],
"subtitle": [],
"title": "Prophylactic effect of ensitrelvir in mice infected with SARS-CoV-2",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}