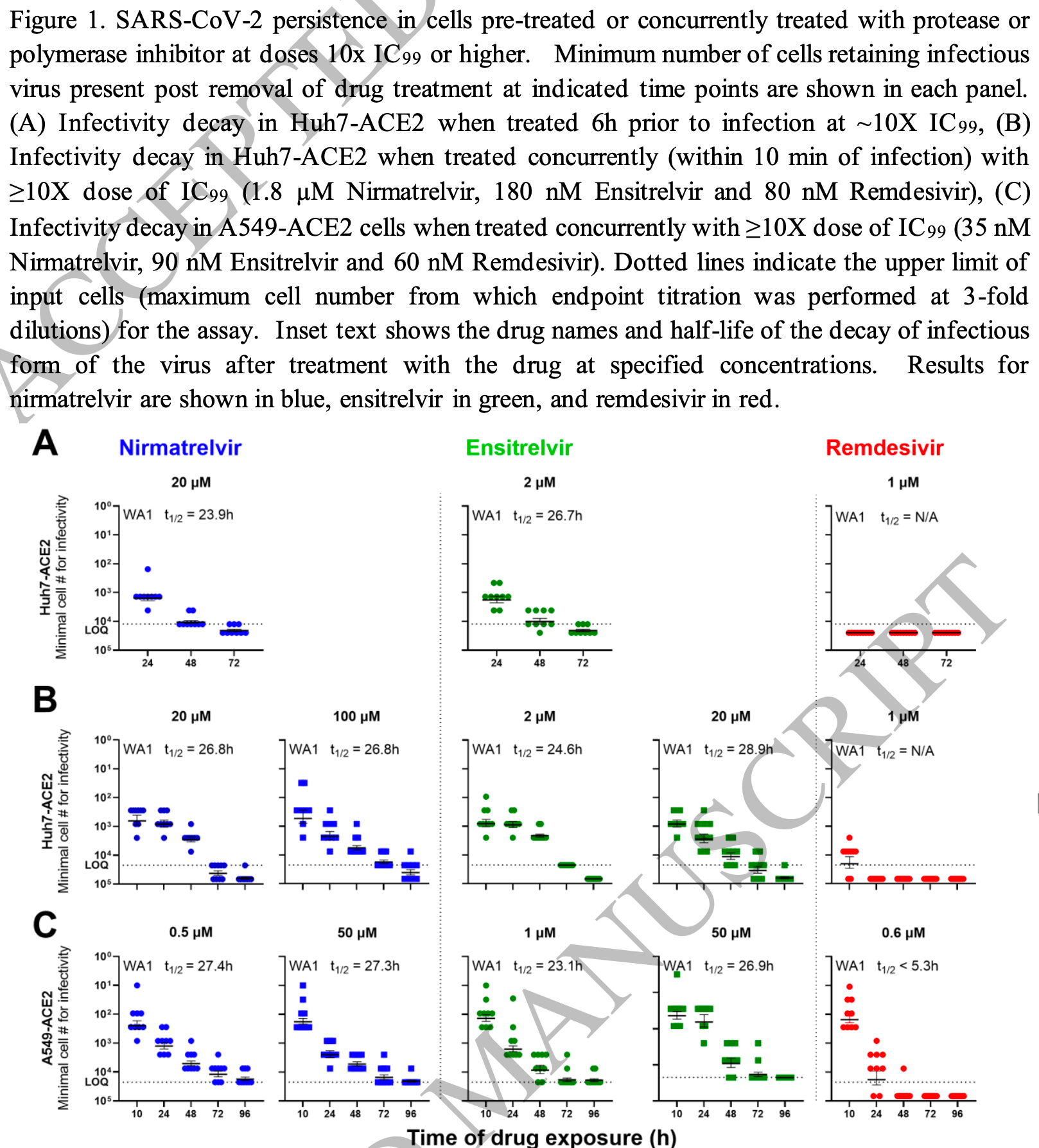
Persistence of an infectious form of SARS-CoV-2 post protease inhibitor treatment of permissive cells in vitro
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiae385, Aug 2024
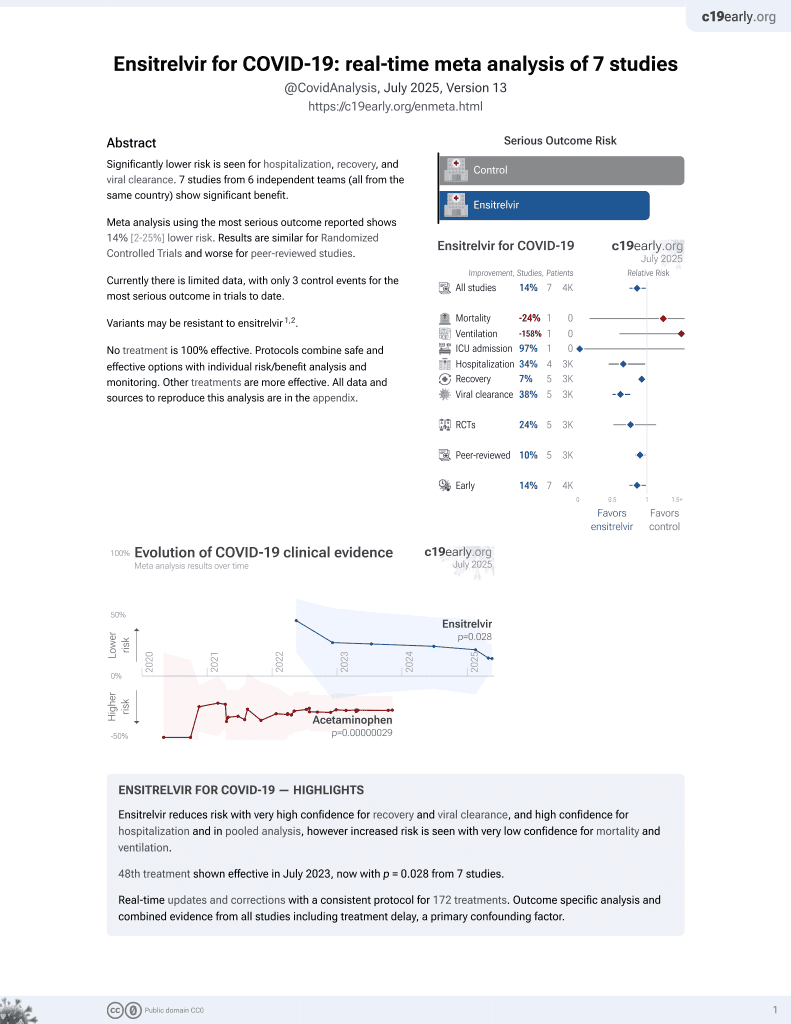
50th treatment shown to reduce risk in
July 2023, now with p = 0.015 from 8 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing the persistence of an infectious form of SARS-CoV-2 after treatment with 3CLpro inhibitors nirmatrelvir and ensitrelvir, which may explain the rebound often seen with paxlovid.
3CLpro is crucial for processing viral polyproteins into functional viral proteins necessary for the assembly of new virus particles. This study suggests that despite 3CLpro inhibition, viral ribonucleoprotein complexes or replicating forms of the virus may still persist intracellularly. These persistent forms might not require immediate protease activity to survive in the short term and can potentially reinitiate replication once the drug is removed. This persistence could explain why the virus can cause a rebound after the cessation of paxlovid treatment.
5 preclinical studies support the efficacy of ensitrelvir for COVID-19:
Study covers paxlovid and ensitrelvir.
1.
Nair et al., Persistence of an infectious form of SARS-CoV-2 post protease inhibitor treatment of permissive cells in vitro, The Journal of Infectious Diseases, doi:10.1093/infdis/jiae385.
2.
Moghadasi et al., Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors, Science Advances, doi:10.1126/sciadv.ade8778.
a.
VeroE6/TMPRSS2 is a Vero E6 cell line engineered to express the human serine protease TMPRSS2, enabling SARS-CoV-2 S protein priming and entry.
b.
HEK293T/ACE2-TMPRSS2 is a human embryonic kidney cell line engineered to express human ACE2 and TMPRSS2, making it highly susceptible to SARS-CoV-2 infection.
c.
MucilAir cells are primary human nasal epithelial cells that mimic the structure and physiology of the human airway epithelium.
d.
A mouse model commonly used in infectious disease and cancer research due to higher immune response and susceptibility to infection.
e.
A rodent model used in SARS-CoV-2 research that replicates key aspects of human infection including efficient replication in the upper and lower respiratory tract.
f.
The original SARS-CoV-2 strain that emerged in Wuhan, China in late 2019. Also referred to as wild-type.
g.
A variant of concern first identified in India in late 2020, delta (B.1.617.2) transmitted more efficiently than previous variants. It contains spike mutations including L452R which increases binding to the ACE2 receptor.
h.
A highly transmissible variant of concern first detected in South Africa in late 2021. Omicron possesses many spike mutations which confer partial immune evasion, including deletions near the furin cleavage site.
Nair et al., 12 Aug 2024, USA, peer-reviewed, 5 authors.
Contact: dh2994@cumc.columbia.edu, ys2581@cumc.columbia.edu.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Persistence of an infectious form of SARS-CoV-2 post protease inhibitor treatment of permissive cells in vitro
doi:10.1093/infdis/jiae3851
4 Lead contact Reports have described SARS-CoV-2 rebound in COVID-19 patients treated with nirmatrelvir, a 3CL protease inhibitor. The cause remains a mystery, although drug resistance, re-infection, and lack of adequate immune responses have been excluded. We now present virologic findings that provide a clue to the cause of viral rebound, which occurs in ~20% of the treated cases. Persistence of infectious SARS-CoV-2 was experimentally documented in vitro after treatment with nirmatrelvir or another 3CL protease inhibitor, but not with a polymerase inhibitor, remdesivir. This infectious form decayed slowly with a half-life of ~1 day, suggesting that its persistence could outlive the treatment course to re-ignite SARS-CoV-2 infection as the drug is eliminated. Notably, extending nirmatrelvir treatment beyond 8 days abolished viral rebound in vitro. Our findings point in a particular direction for future investigation of virus persistence and offer a specific treatment recommendation that should be tested clinically.
SUPPLEMENTARY DATA Supplementary materials are available at The Journal of Infectious Diseases online.
NOTES
AUTHOR NOTES: Potential conflict of interest: All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. Authors do not report any potential conflict of interest.
References
Anderson, Caubel, Rusnak, Investigators, Nirmatrelvir-Ritonavir and Viral Load Rebound in Covid-19, N Engl J Med
Boldogh, Albrecht, Porter, Persistent Viral Infections
Boucau, Uddin, Marino, Characterization of Virologic Rebound Following Nirmatrelvir-Ritonavir Treatment for Coronavirus Disease 2019 (COVID-19), Clin Infect Dis
Cao, Wang, Lu, Oral Simnotrelvir for Adult Patients with Mild -to-Moderate Covid-19, N Engl J Med
Carlin, Clark, Chaillon, Virologic and Immunologic Characterization of Coronavirus Disease 2019 Recrudescence After Nirmatrelvir/Ritonavir Treatment, Clin Infect Dis
Charness, Gupta, Stack, Rebound of SARS-CoV-2 Infection after Nirmatrelvir-Ritonavir Treatment, N Engl J Med
Choi, Choudhary, Regan, Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host, N Engl J Med
Deo, Choudhary, Moser, Symptom and Viral Rebound in Untreated SARS-CoV-2 Infection, Ann Intern Med
Ducharme, COVID-19 Antiviral Drugs Promise Speedier Recoveries
Edelstein, Boucau, Uddin, SARS-CoV-2 Virologic Rebound With Nirmatrelvir-Ritonavir Therapy : An Observational Study, Ann Intern Med
Epling, Rocco, Boswell, Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment, Clin Infect Dis
Fu, Ye, Feng, Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease, Nat Commun
Ghafari, Hall, Golubchik, Prevalence of persistent SARS-CoV-2 in a large community surveillance study, Nature
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
Hay, Kissler, Fauver, Quantifying the impact of immune history and variant on SARS-CoV-2 viral kinetics and infection rebound: A retrospective cohort study, Elife
Kochan, Wawro, Kasza, Simultaneous detection of mRNA and protein in single cells using immunofluorescence-combined single-molecule RNA FISH, Biotechniques
Li, Choudhary, Regan, SARS-CoV-2 viral clearance and evolution varies by type and severity of immunodeficiency, Sci Transl Med
Lieber, Kang, Sobolik, Efficacy of late-onset antiviral treatment in immunecompromised hosts with persistent SARS-CoV-2 infection, bioRxiv
Mukae, Yotsuyanagi, Ohmagari, Efficacy and safety of ensitrelvir in patients with mild-to-moderate COVID-19: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study, Clin Infect Dis
Owen, Allerton, Anderson, An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19, Science
Pandit, Radin, Chiang, The COVID-19 Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences in Participants Treated with Nirmatrelvir Plus Ritonavir Versus Untreated Controls, Clin Infect Dis
Puhach, Adea, Hulo, Infectious viral load in unvaccinated and vaccinated individuals infected with ancestral, Delta or Omicron SARS-CoV-2, Nat Med
Ranganath, Horo, Challener, Rebound Phenomenon After Nirmatrelvir/Ritonavir Treatment of Coronavirus Disease 2019 (COVID-19) in High-Risk Persons, Clin Infect Dis
Rubin, From Positive to Negative to Positive Again-The Mystery of Why COVID-19 Rebounds in Some Patients Who Take Paxlovid, JAMA
Se, Biddle, Talbot, Symptoms, Viral Loads, and Rebound Among COVID-19 Outpatients Treated With Nirmatrelvir/Ritonavir Compared With Propensity Score-Matched Untreated Individuals, Clin Infect Dis
Unoh, Uehara, Nakahara, Discovery of S-217622, a Noncovalent Oral SARS-CoV-2 3CL Protease Inhibitor Clinical Candidate for Treating COVID-19, J Med Chem
Wong, Yip, Lai, Wong, Hui et al., Incidence of Viral Rebound After Treatment With Nirmatrelvir-Ritonavir and Molnupiravir, JAMA Netw Open
Xie, Muruato, Lokugamage, An Infectious cDNA Clone of SARS-CoV-2, Cell Host Microbe
Zuo, He, Liang, The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: a cross-sectional cohort study in China, Lancet Infect Dis
DOI record:
{
"DOI": "10.1093/infdis/jiae385",
"ISSN": [
"0022-1899",
"1537-6613"
],
"URL": "http://dx.doi.org/10.1093/infdis/jiae385",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Reports have described SARS-CoV-2 rebound in COVID-19 patients treated with nirmatrelvir, a 3CL protease inhibitor. The cause remains a mystery, although drug resistance, re-infection, and lack of adequate immune responses have been excluded. We now present virologic findings that provide a clue to the cause of viral rebound, which occurs in ∼20% of the treated cases. Persistence of infectious SARS-CoV-2 was experimentally documented in vitro after treatment with nirmatrelvir or another 3CL protease inhibitor, but not with a polymerase inhibitor, remdesivir. This infectious form decayed slowly with a half-life of ∼1 day, suggesting that its persistence could outlive the treatment course to re-ignite SARS-CoV-2 infection as the drug is eliminated. Notably, extending nirmatrelvir treatment beyond 8 days abolished viral rebound in vitro. Our findings point in a particular direction for future investigation of virus persistence and offer a specific treatment recommendation that should be tested clinically.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5994-3957",
"affiliation": [
{
"name": "Aaron Diamond AIDS Research Center, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
},
{
"name": "Division of Infectious Diseases, Department of Medicine, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
}
],
"authenticated-orcid": false,
"family": "Nair",
"given": "Manoj S",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Aaron Diamond AIDS Research Center, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
},
{
"name": "Division of Infectious Diseases, Department of Medicine, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
}
],
"family": "Luck",
"given": "Maria I",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Aaron Diamond AIDS Research Center, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
},
{
"name": "Division of Infectious Diseases, Department of Medicine, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
}
],
"family": "Huang",
"given": "Yaoxing",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4973-8247",
"affiliation": [
{
"name": "Aaron Diamond AIDS Research Center, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
},
{
"name": "Division of Infectious Diseases, Department of Medicine, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
}
],
"authenticated-orcid": false,
"family": "Sabo",
"given": "Yosef",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1627-149X",
"affiliation": [
{
"name": "Aaron Diamond AIDS Research Center, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
},
{
"name": "Division of Infectious Diseases, Department of Medicine, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
},
{
"name": "Department of Microbiology and Immunology, Columbia University Vagelos College of Physicians and Surgeons , New York, NY, 10032 , USA"
},
{
"name": "Lead contact"
}
],
"authenticated-orcid": false,
"family": "Ho",
"given": "David D",
"sequence": "additional"
}
],
"container-title": "The Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
8,
9
]
],
"date-time": "2024-08-09T10:29:09Z",
"timestamp": 1723199349000
},
"deposited": {
"date-parts": [
[
2024,
8,
12
]
],
"date-time": "2024-08-12T07:24:10Z",
"timestamp": 1723447450000
},
"indexed": {
"date-parts": [
[
2024,
8,
12
]
],
"date-time": "2024-08-12T11:40:05Z",
"timestamp": 1723462805883
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
8,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
12
]
],
"date-time": "2024-08-12T00:00:00Z",
"timestamp": 1723420800000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiae385/58797791/jiae385.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiae385/58797791/jiae385.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2024,
8,
12
]
]
},
"published-online": {
"date-parts": [
[
2024,
8,
12
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiae385/7731564"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Persistence of an infectious form of SARS-CoV-2 post protease inhibitor treatment of permissive cells in vitro",
"type": "journal-article"
}
nair