The impact of COVID-19 monoclonal antibodies on clinical outcomes: A retrospective cohort study
et al., American Journal of Health-System Pharmacy, doi:10.1093/ajhp/zxac295, Oct 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
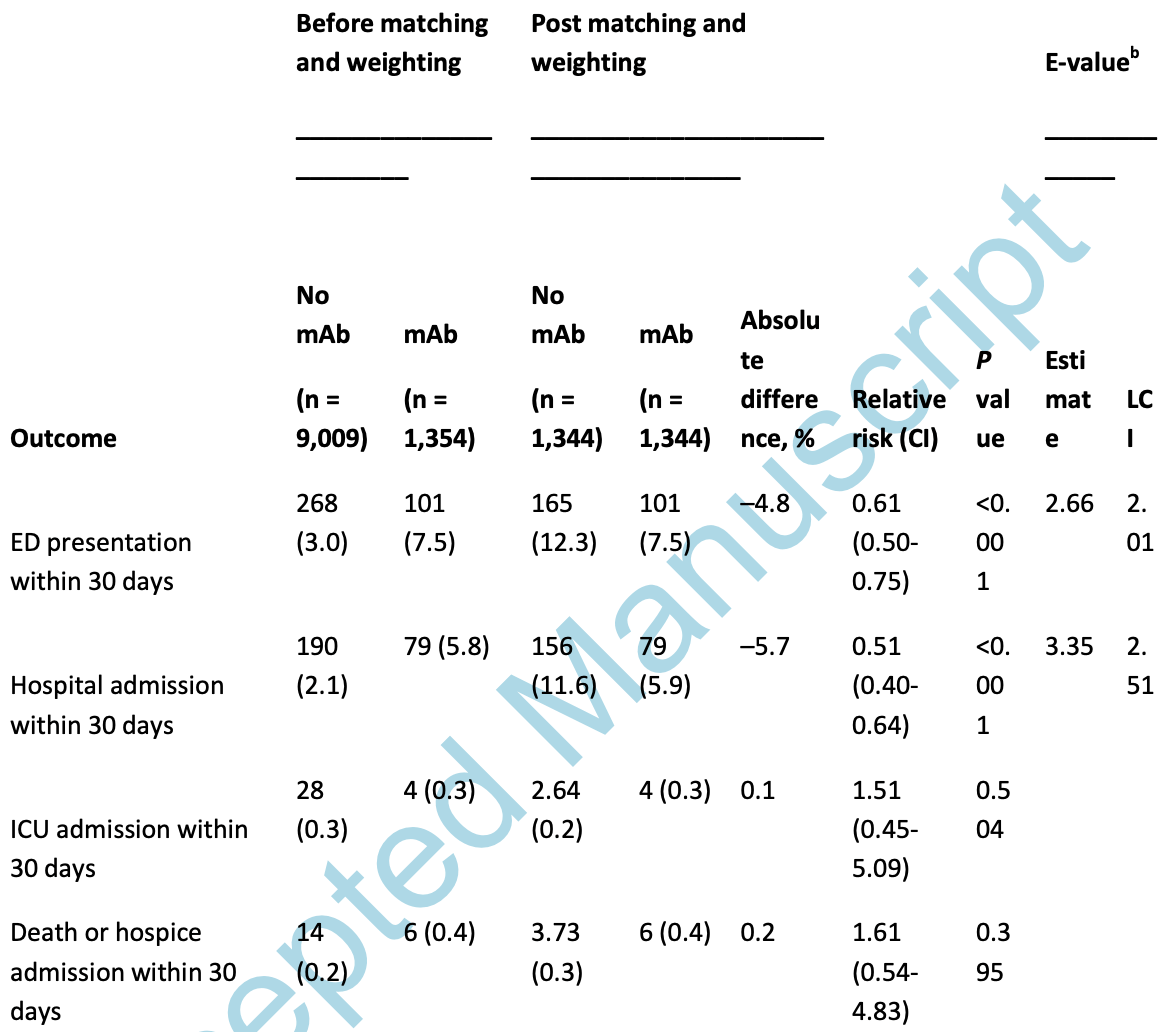
PSM retrospective 1,344 patients in the USA, showing lower hospitalization with monoclonal antibody treatment. Authors combine patients treated with bamlanivimab, bamlanivimab/etesevimab, or casirivimab/imdevimab.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This study is excluded in meta-analysis:
results are only provided for use of one or more treatments within a class of treatments, results for each treatment are not provided.
Study covers casirivimab/imdevimab and bamlanivimab/etesevimab.
|
risk of death, 61.0% higher, RR 1.61, p = 0.40, treatment 1,344, control 1,344, propensity score matching.
|
|
risk of ICU admission, 51.0% higher, RR 1.51, p = 0.50, treatment 1,344, control 1,344, propensity score matching.
|
|
risk of hospitalization, 49.0% lower, RR 0.51, p < 0.001, treatment 1,344, control 1,344, propensity score matching.
|
|
risk of progression, 39.0% lower, RR 0.61, p < 0.001, treatment 1,344, control 1,344, ER visit, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Nagler et al., 15 Oct 2022, retrospective, USA, peer-reviewed, mean age 59.5, 14 authors, study period 24 November, 2020 - 15 May, 2021.
Contact: arielle.nagler@nyumc.org.
Abstract: The impact of COVID-19 monoclonal antibodies on clinical outcomes: A retrospective
cohort study
Arielle R. Nagler, MD, The Ronald O. Perelman Department of Dermatology, NYU Grossman
t
us
cr
ip
Leora I. Horwitz, MD, MHS, Department of Medicine and Department of Population Health,
NYU Grossman School of Medicine, New York, NY, USA
Simon Jones, PhD, Department of Population Health, NYU Grossman School of Medicine,
M
an
New York, NY, USA
d
Christopher M. Petrilli, MD, NYU Grossman School of Medicine, New York, NY, USA
ce
York, NY, USA
pt
e
Eduardo Iturrate, MD, Department of Medicine, NYU Grossman School of Medicine, New
Ac
Jennifer L. Lighter, MD, Division of Pediatric Infectious Diseases, Department of Pediatrics,
NYU Grossman School of Medicine, New York, NY, USA
Michael Phillips, MD, Division of Infectious Disease, Department of Medicine, NYU
Grossman School of Medicine, New York, NY, USA
© American Society of Health-System Pharmacists 2022. All rights reserved. For permissions, please
e-mail: journals.permissions@oup.com.
School of Medicine, New York, NY, USA
Brian P. Bosworth, MD, Department of Medicine, NYU Grossman School of Medicine, New
York, NY, and NYU Langone Health, New York, NY, USA
Bruce Polsky, MD, Department of Medicine, NYU Long Island School of Medicine, New York,
t
us
cr
ip
Frank M. Volpicelli, MD, Department of Medicine, NYU Grossman School of Medicine, New
York, NY, USA
an
Isaac Dapkins, MD, Family Health Centers at NYU Langone, Brooklyn, NY, USA
M
Anand Viswanathan, MD, Department of Medicine, NYU Grossman School of Medicine,
pt
e
d
New York, NY, USA
Fritz François, MD, Department of Medicine, NYU Grossman School of Medicine, New York,
ce
NY, and NYU Langone Health, New York, NY, USA
Ac
Gary Kalkut, MD, MPH, Division of Infectious Disease, Department of Medicine, NYU
Grossman School of Medicine, New York, NY, and NYU Langone Health, New York, NY, USA
Address correspondence to Dr. Nagler (Arielle.Nagler@nyumc.org).
Twitter: @ArielleNaglerMD
NY, USA
Purpose: Despite progress in the treatment of coronavirus disease 2019 (COVID-19),
including the development of monoclonal antibodies (mAbs), more clinical data to support
the use of mAbs in outpatients with COVID-19 is needed. This study is designed to
determine the impact of bamlanivimab, bamlanivimab/etesevimab, or
t
Methods: A retrospective cohort study was conducted at a single academic medical center
us
cr
ip
with 3 campuses in Manhattan, Brooklyn, and Long Island, NY. Patients 12 years of age or
older who tested positive for COVID-19 or were treated with a COVID-19–specific therapy,
including COVID-19 mAb therapies, at the study site between November 24, 2020, and May
15, 2021, were included. The primary outcomes included rates of emergency department
M
from the date of COVID-19 diagnosis.
an
(ED) visit, inpatient admission, intensive care unit (ICU) admission, or death within 30 days
Results: A total of 1,344 mAb-treated patients were propensity matched to 1,344 patients
d
with COVID-19 patients who were not treated with mAb therapy. Within 30 days of
pt
e
diagnosis, among the patients who received mAb therapy, 101 (7.5%) presented to the ED
and 79 (5.9%) were admitted...
DOI record:
{
"DOI": "10.1093/ajhp/zxac295",
"ISSN": [
"1079-2082",
"1535-2900"
],
"URL": "http://dx.doi.org/10.1093/ajhp/zxac295",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Disclaimer</jats:title>\n <jats:p>In an effort to expedite the publication of articles, AJHP is posting manuscripts online as soon as possible after acceptance. Accepted manuscripts have been peer-reviewed and copyedited, but are posted online before technical formatting and author proofing. These manuscripts are not the final version of record and will be replaced with the final article (formatted per AJHP style and proofed by the authors) at a later time.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Purpose</jats:title>\n <jats:p>Despite progress in the treatment of coronavirus disease 2019 (COVID-19), including the development of monoclonal antibodies (mAbs), more clinical data to support the use of mAbs in outpatients with COVID-19 is needed. This study is designed to determine the impact of bamlanivimab, bamlanivimab/etesevimab, or casirivimab/imdevimab on clinical outcomes within 30 days of COVID-19 diagnosis.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>A retrospective cohort study was conducted at a single academic medical center with 3 campuses in Manhattan, Brooklyn, and Long Island, NY. Patients 12 years of age or older who tested positive for COVID-19 or were treated with a COVID-19–specific therapy, including COVID-19 mAb therapies, at the study site between November 24, 2020, and May 15, 2021, were included. The primary outcomes included rates of emergency department (ED) visit, inpatient admission, intensive care unit (ICU) admission, or death within 30 days from the date of COVID-19 diagnosis.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 1,344 mAb-treated patients were propensity matched to 1,344 patients with COVID-19 patients who were not treated with mAb therapy. Within 30 days of diagnosis, among the patients who received mAb therapy, 101 (7.5%) presented to the ED and 79 (5.9%) were admitted. Among the patients who did not receive mAb therapy, 165 (12.3%) presented to the ED and 156 (11.6%) were admitted (relative risk [RR], 0.61 [95% CI, 0.50-0.75] and 0.51 [95% CI, 0.40-0.64], respectively). Four mAb patients (0.3%) and 2.64 control patients (0.2%) were admitted to the ICU (RR, 01.51; 95% CI, 0.45-5.09). Six mAb-treated patients (0.4%) and 3.37 controls (0.3%) died and/or were admitted to hospice (RR, 1.61; 95% CI, 0.54-4.83). mAb therapy in ambulatory patients with COVID-19 decreases the risk of ED presentation and hospital admission within 30 days of diagnosis.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "The Ronald O. Perelman Department of Dermatology, NYU Grossman School of Medicine, New York , NY, USA"
}
],
"family": "Nagler",
"given": "Arielle R",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medicine and Department of Population Health, NYU Grossman School of Medicine, New York , NY, USA"
}
],
"family": "Horwitz",
"given": "Leora I",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Population Health, NYU Grossman School of Medicine, New York , NY, USA"
}
],
"family": "Jones",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "NYU Grossman School of Medicine, New York , NY, USA"
}
],
"family": "Petrilli",
"given": "Christopher M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York , NY, USA"
}
],
"family": "Iturrate",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pediatric Infectious Diseases, Department of Pediatrics, NYU Grossman School of Medicine, New York , NY, USA"
}
],
"family": "Lighter",
"given": "Jennifer L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, NYU Grossman School of Medicine, New York , NY, USA"
}
],
"family": "Phillips",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York , NY, andNYU Langone Health, New York, NY, USA"
}
],
"family": "Bosworth",
"given": "Brian P",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Long Island School of Medicine, New York , NY, USA"
}
],
"family": "Polsky",
"given": "Bruce",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York , NY, USA"
}
],
"family": "Volpicelli",
"given": "Frank M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Family Health Centers at NYU Langone, Brooklyn , NY, USA"
}
],
"family": "Dapkins",
"given": "Isaac",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York , NY, USA"
}
],
"family": "Viswanathan",
"given": "Anand",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York , NY, and NYU Langone Health, New York, NY, USA"
}
],
"family": "François",
"given": "Fritz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, NYU Grossman School of Medicine, New York , NY, and NYU Langone Health, New York, NY, USA"
}
],
"family": "Kalkut",
"given": "Gary",
"sequence": "additional"
}
],
"container-title": "American Journal of Health-System Pharmacy",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
15
]
],
"date-time": "2022-10-15T17:06:42Z",
"timestamp": 1665853602000
},
"deposited": {
"date-parts": [
[
2022,
10,
15
]
],
"date-time": "2022-10-15T17:06:43Z",
"timestamp": 1665853603000
},
"indexed": {
"date-parts": [
[
2022,
10,
15
]
],
"date-time": "2022-10-15T17:42:43Z",
"timestamp": 1665855763377
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
15
]
]
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/pages/standard-publication-reuse-rights",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
15
]
],
"date-time": "2022-10-15T00:00:00Z",
"timestamp": 1665792000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ajhp/advance-article-pdf/doi/10.1093/ajhp/zxac295/46539445/zxac295.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ajhp/advance-article-pdf/doi/10.1093/ajhp/zxac295/46539445/zxac295.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
10,
15
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
15
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ajhp/advance-article/doi/10.1093/ajhp/zxac295/6761664"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Health Policy",
"Pharmacology"
],
"subtitle": [],
"title": "The impact of COVID-19 monoclonal antibodies on clinical outcomes: A retrospective cohort study",
"type": "journal-article"
}
nagler
