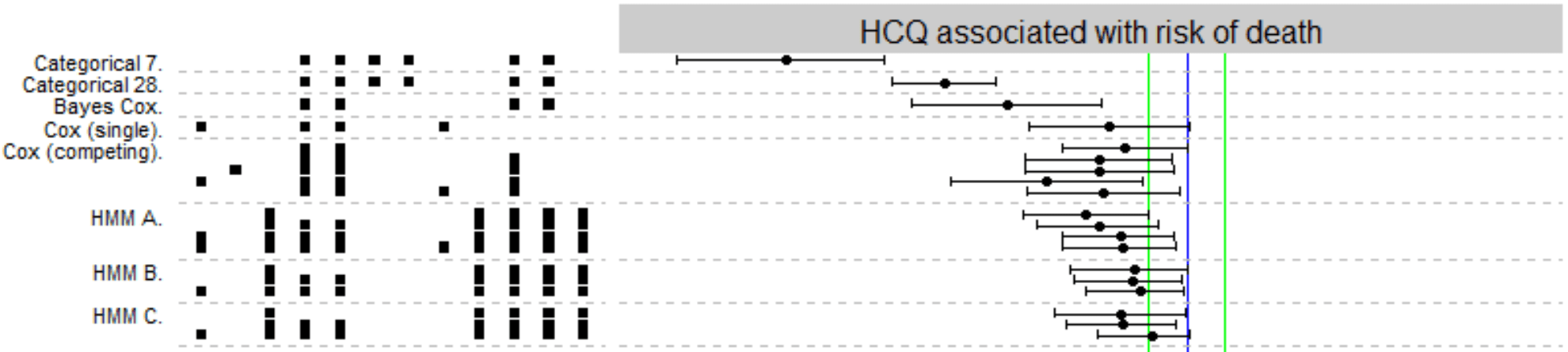
Detailed disease progression of 213 patients hospitalized with Covid-19 in the Czech Republic: An exploratory analysis
et al., medRxiv, doi:10.1101/2020.12.03.20239863, Dec 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 213 hospitalized patients in Czech Republic showing lower mortality with HCQ. Subject to confounding by indication.
|
risk of death, 59.0% lower, RR 0.41, p = 0.04, treatment 108, control 105, Cox (single).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Modrák et al., 4 Dec 2020, retrospective, Czech Republic, preprint, 27 authors.
Detailed disease progression of 213 patients hospitalized with Covid-19 in the Czech Republic: An exploratory analysis
doi:10.1101/2020.12.03.20239863
We collected a multi-centric retrospective dataset of patients (N = 213) who were admitted to ten hospitals in Czech Republic and tested positive for SARS-CoV-2. The dataset contains baseline patient characteristics, breathing support required, pharmacological treatment received and multiple markers on daily resolution. Patients in the dataset were treated with hydroxychloroquine (N = 108), azithromycin (N = 72), favipiravir (N = 9), convalescent plasma (N = 7), dexamethasone (N = 4) and remdesivir (N = 3), often in combination. Most patients were admitted during the first wave of the epidemic. To explore association between treatments and patient outcomes we performed multiverse analysis, observing how the conclusions change between defensible choices of statistical model, predictors included in the model and other analytical degrees of freedom. Weak evidence to constrain the potential efficacy of azithromycin and favipiravir can be extracted from the data. Additionally, we performed external validation of several proposed prognostic models for Covid-19 severity showing that they mostly perform unsatisfactorily on our dataset.
Conflict of Interest The authors declare that there is no conflict of interest.
References
Agarwal, Mukherjee, Kumar, Convalescent plasma in the management of moderate COVID-19 in India: An open-label parallel-arm phase II multicentre randomized controlled trial (PLACID Trial). medRxiv, doi:10.1101/2020.09.03.20187252
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, New England Journal of Medicine, doi:10.1056/NEJMoa2007764
Bello-Chavolla, Bahena-López, Ne, Predicting Mortality Due to SARS-CoV-2: A Mechanistic Score Relating Obesity and Diabetes to COVID-19 Outcomes in Mexico, J Clin Endocrinol Metab, doi:10.1210/clinem/dgaa346
Boulware, Pullen, Bangdiwala, A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2016638
Brat, Weber, Gehlenborg, International electronic health recordderived COVID-19 clinical course profiles: the 4CE consortium, npj Digital Medicine, doi:10.1038/s41746-020-00308-0
Bürkner, Advanced Bayesian Multilevel Modeling with the R Package brms, The R Journal, doi:10.32614/RJ-2018-017
Cap, Tocilizumab announcement
Caramelo, Ferreira, Oliveiros, Estimation of risk factors for COVID-19 mortality -preliminary results. medRxiv, doi:10.1101/2020.02.24.20027268
Castelnuovo, Costanzo, Antinori, Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study, European Journal of Internal Medicine, doi:10.1016/j.ejim.2020.08.019
Catteau, Dauby, Montourcy, Low-dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID-19: a nationwide observational study of 8075 participants, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106144
Cg, Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on Lopinavir-Ritonavir
Chen, Liu, Early prediction of mortality risk among severe COVID-19 patients using machine learning, doi:10.1101/2020.04.13.20064329
Chen, Nirula, Heller, SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N Engl J Med. Published online, doi:10.1056/NEJMoa2029849
Chen, Zhang, Huang, Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv, doi:10.1101/2020.03.17.20037432
Core, R: A Language and Environment for Statistical Computing
Dauby, Van Praet, Vanhomwegen, Veliziotis, Konopnicki et al., Tolerability of favipiravir therapy in critically ill patients with COVID-19: A report of four cases, J Med Virol. Published online
Furtado, Berwanger, Fonseca, Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial, The Lancet, doi:10.1016/S0140-6736(20)31862-6
Hood, Dahly, Wilkinson, Statistical review of Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial, Published online, doi:10.5281/zenodo.3779933
Hood, Goulao, Dahly, Yap, Statistical Review of Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial
Horby, Mafham, Linsell, Effect of Hydroxychloroquine in Hospitalized Patients with COVID-19: Preliminary results from a multi-centre, randomized, controlled trial. medRxiv, doi:10.1101/2020.07.15.20151852
Kalil, Patterson, Mehta, Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19, N Engl J Med. Published online, doi:10.1056/NEJMoa2031994
Li, Zhang, Hu, Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.10044
Lou, Liu, Qiu, Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: an Exploratory Randomized, Controlled Trial. medRxiv, doi:10.1101/2020.04.29.20085761
Lu, Hu, Fan, ACP risk grade: a simple mortality index for patients with confirmed or suspected severe acute respiratory syndrome coronavirus 2 disease (COVID-19) during the early stage of outbreak in Wuhan, China. medRxiv, doi:10.1101/2020.02.20.20025510
Maeda, Obata, Rizk, Kuno, The association of interleukin-6 value, interleukin inhibitors, and outcomes of patients with COVID-19 in New York City, J Med Virol. Published online
Mitja, Ubals, Corbacho, A Cluster-Randomized Trial of Hydroxychloroquine as Prevention of Covid-19 Transmission and Disease. medRxiv, doi:10.1101/2020.07.20.20157651
Pan, Peto, except HIV/AIDS, doi:10.1101/2020.10.15.20209817
Parackova, Zentsova, Bloomfield, Disharmonic Inflammatory Signatures in COVID-19: Augmented Neutrophils' but Impaired Monocytes' and Dendritic Cells' Responsiveness, Cells, doi:10.3390/cells9102206
Rajasingham, Bangdiwala, Nicol, Hydroxychloroquine as preexposure prophylaxis for COVID-19 in healthcare workers: a randomized trial. medRxiv, doi:10.1101/2020.09.18.20197327
Recovery, Dexamethasone in Hospitalized Patients with Covid-19 -Preliminary Report, New England Journal of Medicine, doi:10.1056/NEJMoa2021436
Robin, Turck, Hainard, pROC: an open-source package for R and S+ to analyze and compare ROC curves, BMC Bioinformatics, doi:10.1186/1471-2105-12-77
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review, JAMA, doi:10.1001/jama.2020.6019
Shi, Yu, Zhao, Wang, Zhao et al., Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan, Critical Care, doi:10.1186/s13054-020-2833-7
Shrestha, Budhathoki, Khadka, Shah, Pokharel et al., Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis, Virol J, doi:10.1186/s12985-020-01412-z
Siemieniuk, Bartoszko, Ge, Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ, doi:10.1136/bmj.m2980
Skipper, Pastick, Engen, Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19, Annals of Internal Medicine, doi:10.7326/M20-4207
Steegen, Tuerlinckx, Gelman, Vanpaemel, Increasing Transparency Through a Multiverse Analysis, Perspect Psychol Sci, doi:10.1177/1745691616658637
Sterne, Murthy, Diaz, Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis, JAMA. Published online, doi:10.1001/jama.2020.17023
Takahashi, Iwasaki, Watanabe, Case studies of SARS-CoV-2 treated with favipiravir among patients in critical or severe condition, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.08.047
Therneau, Grambsch, Modeling Survival Data: Extending the Cox Model
Tiwari, Khatib, Dixit, Anticoagulation in COVID -19: An Update, The Journal of Critical Care Medicine, doi:10.2478/jccm-2020-0033
Tobaiqy, Qashqary, Al-Dahery, Therapeutic management of patients with COVID-19: a systematic review, Infection Prevention in Practice, doi:10.1016/j.infpip.2020.100061
Valle, Kim-Schulze, Huang, An inflammatory cytokine signature predicts COVID-19 severity and survival, Nature Medicine, doi:10.1038/s41591-020-1051-9
Wickham, Averick, Bryan, Welcome to the Tidyverse, JOSS, doi:10.21105/joss.01686
Wilkinson, Dahly, Statistical Review of Favipiravir Versus Arbidol for COVID-19: A Randomized Clinical Trial
Williams, Storlie, Therneau, Jr, Hannig, A Bayesian Approach to Multistate Hidden Markov Models: Application to Dementia Progression, Journal of the American Statistical Association, doi:10.1080/01621459.2019.1594831
Wynants, Calster, Collins, Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal, BMJ, doi:10.1136/bmj.m1328
DOI record:
{
"DOI": "10.1101/2020.12.03.20239863",
"URL": "http://dx.doi.org/10.1101/2020.12.03.20239863",
"abstract": "<jats:title>Abstract</jats:title><jats:p>We collected a multi-centric retrospective dataset of patients (N = 213) who were admitted to ten hospitals in Czech Republic and tested positive for SARS-CoV-2. The dataset contains baseline patient characteristics, breathing support required, pharmacological treatment received and multiple markers on daily resolution. Patients in the dataset were treated with hydroxychloroquine (N = 108), azithromycin (N = 72), favipiravir (N = 9), convalescent plasma (N = 7), dexamethasone (N = 4) and remdesivir (N = 3), often in combination. Most patients were admitted during the first wave of the epidemic. To explore association between treatments and patient outcomes we performed multiverse analysis, observing how the conclusions change between defensible choices of statistical model, predictors included in the model and other analytical degrees of freedom. Weak evidence to constrain the potential efficacy of azithromycin and favipiravir can be extracted from the data. Additionally, we performed external validation of several proposed prognostic models for Covid-19 severity showing that they mostly perform unsatisfactorily on our dataset.</jats:p>",
"accepted": {
"date-parts": [
[
2020,
12,
22
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8886-7797",
"affiliation": [],
"authenticated-orcid": false,
"family": "Modrák",
"given": "Martin",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-5765-8995",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bürkner",
"given": "Paul-Christian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4960-1934",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sieger",
"given": "Tomáš",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slisz",
"given": "Tomáš",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vašáková",
"given": "Martina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mesežnikov",
"given": "Grigorij",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casas-Mendez",
"given": "Luis Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vajter",
"given": "Jaromír",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Táborský",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kubricht",
"given": "Viktor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suk",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Horejsek",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jedlička",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mifková",
"given": "Adriana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaroš",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kubiska",
"given": "Miroslav",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Váchalová",
"given": "Jana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Šín",
"given": "Robin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Veverková",
"given": "Markéta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pospíšil",
"given": "Zbyšek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vohryzková",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pokrievková",
"given": "Rebeka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hrušák",
"given": "Kristián",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Christozova",
"given": "Kristína",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8016-773X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Leos-Barajas",
"given": "Vianey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fišer",
"given": "Karel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hyánek",
"given": "Tomáš",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
12,
5
]
],
"date-time": "2020-12-05T05:18:27Z",
"timestamp": 1607145507000
},
"deposited": {
"date-parts": [
[
2020,
12,
26
]
],
"date-time": "2020-12-26T21:00:29Z",
"timestamp": 1609016429000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T20:29:43Z",
"timestamp": 1648585783666
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2020,
12,
4
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2020.12.03.20239863",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
12,
4
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2020,
12,
4
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1001/jama.2020.6019",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.1"
},
{
"DOI": "10.1016/j.infpip.2020.100061",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.2"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.3"
},
{
"DOI": "10.1001/jama.2020.17023",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.4"
},
{
"DOI": "10.1056/NEJMoa2031994",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.5"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.6"
},
{
"key": "2020122613000809000_2020.12.03.20239863v2.7",
"unstructured": "REMAP CAP. Tocilizumab announcement. Twitter. Accessed December 17, 2020. https://twitter.com/remap_cap/status/1329391734174826496"
},
{
"key": "2020122613000809000_2020.12.03.20239863v2.8",
"unstructured": "Regeneron Pharmaceuticals Inc. Regeneron’s COVID-19 Outpatient Trial Prospectively Demonstrates that REGN-COV2 Antibody Cocktail Significantly Reduced Virus Levels and Need for Further Medical Attention. Accessed December 17, 2020. https://investor.regeneron.com/news-releases/news-release-details/regenerons-covid-19-outpatient-trial-prospectively-demonstrates/"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.9"
},
{
"key": "2020122613000809000_2020.12.03.20239863v2.10",
"unstructured": "WHO Solidarity trial consortium, Pan H , Peto R , et al. Repurposed Antiviral Drugs for COVID-19 –Interim WHO SOLIDARITY Trial Results. Infectious Diseases (except HIV/AIDS); 2020. Accessed November 25, 2020. http://medrxiv.org/lookup/doi/10.1101/2020.10.15.20209817"
},
{
"DOI": "10.1101/2020.07.15.20151852",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.11"
},
{
"DOI": "10.7326/M20-4207",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.12"
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.13"
},
{
"DOI": "10.1101/2020.07.20.20157651",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.14"
},
{
"DOI": "10.1101/2020.09.18.20197327",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.15"
},
{
"DOI": "10.1016/S0140-6736(20)31862-6",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.16"
},
{
"key": "2020122613000809000_2020.12.03.20239863v2.17",
"unstructured": "Recovery CG . Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on Lopinavir-Ritonavir, 29 June 2020.; 2020. Accessed September 12, 2020. https://www.recoverytrial.net/files/lopinavir-ritonavir-recovery-statement-29062020_final.pdf"
},
{
"DOI": "10.1101/2020.09.03.20187252",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.18"
},
{
"DOI": "10.1001/jama.2020.10044",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.19"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106144",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.20"
},
{
"DOI": "10.1038/s41591-020-1051-9",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.21"
},
{
"DOI": "10.1016/j.ejim.2020.08.019",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.22"
},
{
"DOI": "10.1101/2020.03.17.20037432",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.23"
},
{
"key": "2020122613000809000_2020.12.03.20239863v2.24",
"unstructured": "Wilkinson J , Dahly D. Statistical Review of Favipiravir Versus Arbidol for COVID-19: A Randomized Clinical Trial. Zenodo; 2020. Accessed October 26, 2020. https://zenodo.org/record/3734198#.X5amU-0o-Uk"
},
{
"DOI": "10.1101/2020.04.29.20085761",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.25"
},
{
"key": "2020122613000809000_2020.12.03.20239863v2.26"
},
{
"DOI": "10.1016/j.ijid.2020.08.047",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.27"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.28"
},
{
"DOI": "10.1136/bmj.m2980",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.29"
},
{
"DOI": "10.2478/jccm-2020-0033",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.30"
},
{
"key": "2020122613000809000_2020.12.03.20239863v2.31"
},
{
"DOI": "10.1038/s41746-020-0256-0",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.32"
},
{
"DOI": "10.5281/zenodo.3779933",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.33"
},
{
"key": "2020122613000809000_2020.12.03.20239863v2.34",
"unstructured": "Hood K , Goulao B , Dahly D , Yap C. Statistical Review of Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Zenodo; 2020. Accessed September 12, 2020. https://zenodo.org/record/3819778#.X1yBlotS-Uk"
},
{
"DOI": "10.1136/bmj.m1328",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.35"
},
{
"DOI": "10.1177/1745691616658637",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.36"
},
{
"key": "2020122613000809000_2020.12.03.20239863v2.37",
"unstructured": "R Core Team. R: A Language and Environment for Statistical Computing.; 2020. https://www.R-project.org/"
},
{
"DOI": "10.21105/joss.01686",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.38"
},
{
"DOI": "10.1007/978-1-4757-3294-8",
"doi-asserted-by": "crossref",
"key": "2020122613000809000_2020.12.03.20239863v2.39",
"unstructured": "Therneau TM , Grambsch PM . Modeling Survival Data: Extending the Cox Model. Springer; 2000."
},
{
"DOI": "10.32614/RJ-2018-017",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.40"
},
{
"DOI": "10.1080/01621459.2019.1594831",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.41"
},
{
"DOI": "10.1186/1471-2105-12-77",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.42"
},
{
"DOI": "10.3390/cells9102206",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.43"
},
{
"DOI": "10.1101/2020.02.20.20025510",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.44"
},
{
"DOI": "10.1101/2020.04.13.20064329",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.45"
},
{
"DOI": "10.1186/s13054-020-2833-7",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.46"
},
{
"DOI": "10.1101/2020.02.24.20027268",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.47"
},
{
"DOI": "10.1210/clinem/dgaa346",
"doi-asserted-by": "publisher",
"key": "2020122613000809000_2020.12.03.20239863v2.48"
}
],
"reference-count": 48,
"references-count": 48,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2020.12.03.20239863"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Detailed disease progression of 213 patients hospitalized with Covid-19 in the Czech Republic: An exploratory analysis",
"type": "posted-content"
}
