Association of subcutaneous or intravenous route of administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in COVID-19
et al., medRxiv, doi:10.1101/2021.11.30.21266756, Dec 2021
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Prospective study comparing subcutaneous and intravenous casirivimab/imdevimab, and comparing to a PSM matched control set, showing significantly lower mortality and hospitalization with treatment. Controls were matched with EUA-eligible risk factors only, authors were unable to determine symptom severity.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
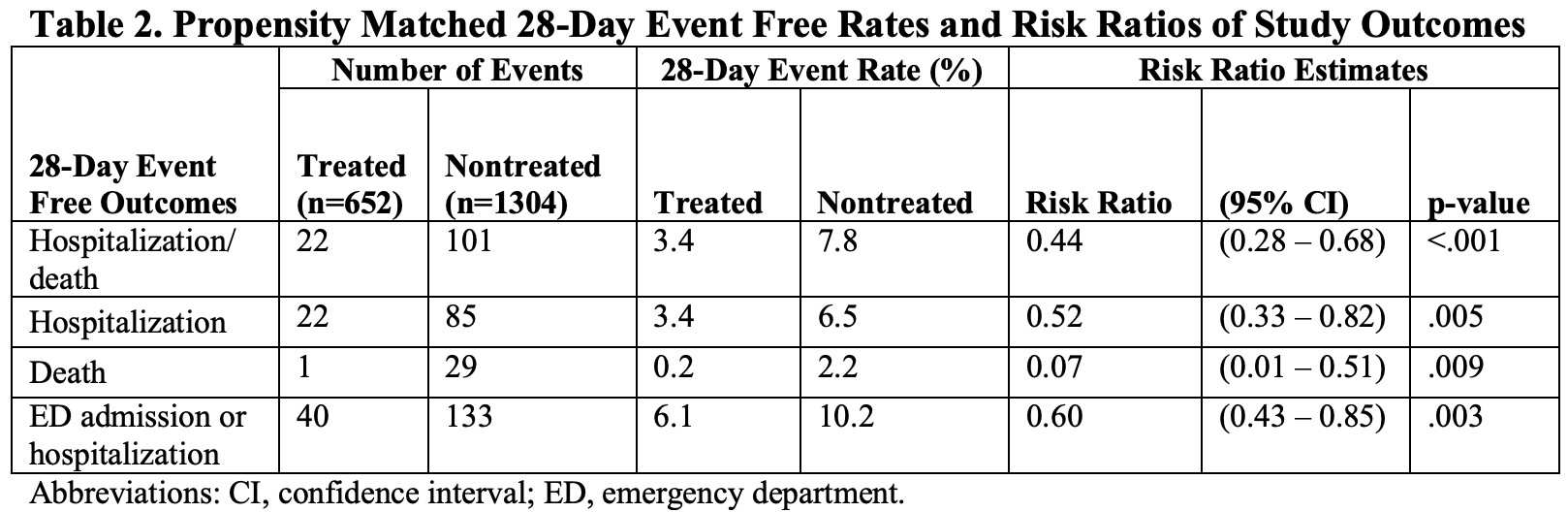
risk of death, 93.0% lower, RR 0.07, p = 0.009, treatment 1 of 652 (0.2%), control 29 of 1,304 (2.2%), NNT 48, propensity score matching.
|
|
risk of death/hospitalization, 56.0% lower, RR 0.44, p < 0.001, treatment 22 of 652 (3.4%), control 101 of 1,304 (7.7%), NNT 23, propensity score matching, primary outcome.
|
|
risk of hospitalization, 48.0% lower, RR 0.52, p = 0.005, treatment 22 of 652 (3.4%), control 85 of 1,304 (6.5%), NNT 32, propensity score matching.
|
|
risk of hospitalization/ER, 40.0% lower, RR 0.60, p = 0.003, treatment 40 of 652 (6.1%), control 133 of 1,304 (10.2%), NNT 25, propensity score matching.
|
|
SQ vs. IV death, 53.0% lower, RR 0.47, p = 0.52, treatment 1 of 969 (0.1%), control 3 of 1,216 (0.2%), NNT 697, adjusted per study.
|
|
SQ vs. IV death/hosp., 71.0% higher, RR 1.71, p = 0.06, treatment 27 of 969 (2.8%), control 21 of 1,216 (1.7%), adjusted per study.
|
|
SQ vs. IV hospitalization, 79.0% higher, RR 1.79, p = 0.046, treatment 27 of 969 (2.8%), control 20 of 1,216 (1.6%), adjusted per study.
|
|
SQ vs. IV ER/hosp., 15.0% lower, RR 0.85, p = 0.38, treatment 47 of 969 (4.9%), control 71 of 1,216 (5.8%), NNT 101, adjusted per study.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
McCreary et al., 1 Dec 2021, prospective, USA, preprint, 27 authors, study period 14 July, 2021 - 26 October, 2021, average treatment delay 6.0 days.
Association of subcutaneous or intravenous route of administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in COVID-19
doi:10.1101/2021.11.30.21266756
Findings: Among 1,956 propensity-matched adults, outpatients who received casirivimab and imdevimab subcutaneously had a 28-day rate of hospitalization or death of 3.4% (n=652) compared to 7.8% (n=1,304) in non-treated controls [risk ratio 0.44 (95% confidence interval: 0.28 to 0.68, p < .001)]. Among 2,185 outpatients who received subcutaneous (n=969) or intravenous (n=1,216) casirivimab and imdevimab, the 28-day rate of hospitalization/death was 2.8% vs. 1.7%, respectively, which resulted in an adjusted risk difference of 1.5% (95% confidence interval: -0.5% to 3.5%, p=.14). The 28-day adjusted risk differences comparing subcutaneous to intravenous route for death, ICU admission, and mechanical ventilation were 0.3% or less, although the 95% confidence intervals were wide. Meaning: Subcutaneously administered casirivimab and imdevimab is associated with reduced hospitalization or death amongst outpatients with mild to moderate COVID-19 compared to no treatment, and has a small, adjusted risk difference compared to patients treated intravenously.
Treated and Nontreated Analysis
References
Austin, An introduction to propensity score methods for reducing the effects of confounding in observational studies, Multivariate Behav Res, doi:10.1080/00273171.2011.568786
Bariola, Mccreary, Wadas, Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection, Open Forum Infect Dis, doi:10.1093/ofid/ofab254
Benchimol, Sl, Guttmann, Harron, The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement, PLoS Med
Gov, Uk, Patient information leaflet for Ronapreve
Huang, Mccreary, Bariola, The UPMC OPTIMISE-C19 (OPtimizing Treatment and Impact of Monoclonal antIbodieS through Evaluation for COVID-19) trial: a structured summary of a study protocol for an open-label, pragmatic, comparative effectiveness platform trial with response-adaptive randomization, Trials, doi:10.1186/s13063-021-05316-3
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19, N Engl J Med, doi:10.1056/NEJMoa2109682
Pfizer, A study of PF-07321332/ritonavir in non-hospitalized low-risk adult participants with COVID-19
Reitz, Seymour, Vates, Strategies to Promote ResiliencY (SPRY): a randomised embedded multifactorial adaptative platform (REMAP) clinical trial protocol to study interventions to improve recovery after surgery in high-risk patients, BMJ Open, doi:10.1136/bmjopen-2020-037690
Rosenbaum, Rubin, The Central Role of the Propensity Score in Observational Studies for Causal Effects, Biometrika, doi:10.2307/2335942
Sharp, Corporation, Efficacy and safety of molnupiravir (MK-4482) in nonhospitalized adult participants with COVID-19
Weinreich, Sivapalasingam, Norton, REGEN-COV Antibody Cocktail Clinical Outcomes Study in Covid-19 Outpatients, medRxiv, doi:10.1101/2021.05.19.21257469
DOI record:
{
"DOI": "10.1101/2021.11.30.21266756",
"URL": "http://dx.doi.org/10.1101/2021.11.30.21266756",
"abstract": "<jats:p>Importance: Monoclonal antibody (mAb) treatment decreases hospitalization and death in outpatients with mild to moderate COVID 19; however, only intravenous administration has been evaluated in randomized clinical trials of treatment. Subcutaneous administration may expand outpatient treatment capacity and qualified staff available to administer treatment, but association with patient outcomes is understudied. \nObjective: To evaluate whether or not, i.) subcutaneous casirivimab and imdevimab treatment is associated with reduced 28 days hospitalization/death than non-treatment among mAb-eligible patients, and ii.) subcutaneous casirivimab and imdevimab treatment is clinically and statistically similar to intravenous casirivimab and imdevimab treatment.\nDesign, Setting, and Participants: Prospective cohort study of outpatients in a learning health system in the United States with mild to moderate COVID 19 symptoms from July 14 to October 26, 2021 who were eligible for mAb treatment under emergency use authorization. A nontreated control group of eligible patients was also selected.\nIntervention: Subcutaneous injection or intravenous administration of the combined single dose of casirivimab 600mg and imdevimab 600mg.\nMain Outcomes and Measures: The primary outcome was the 28 day adjusted risk ratio or adjusted risk difference for hospitalization or death. Secondary outcomes included 28 day adjusted risk ratios/differences of hospitalization, death, composite endpoint of ED admission and hospitalization, and rates of adverse events.\nResults: Among 1,956 matched adults with mild to moderate COVID 19, patients who received casirivimab and imdevimab subcutaneously had a 28-day rate of hospitalization/death of 3.4% (n=652) compared to 7.8% (n=1,304) in nontreated controls [risk ratio 0.44 (95% confidence interval: 0.28 to 0.68, p < .001)]. Among 2,185 patients treated with subcutaneous (n=969) or intravenous (n=1,216) casirivimab and imdevimab, the 28 day rate of hospitalization/death was 2.8% vs. 1.7%, respectively which resulted in an adjusted risk difference of 1.5% (95% confidence interval: -0.5% to 3.5%, p=.14). The 28 day adjusted risk differences (subcutaneous and intravenous) for death, ICU admission, and mechanical ventilation were 0.3% or less, although the 95% confidence intervals were wide. \nConclusions and Relevance: Subcutaneously administered casirivimab and imdevimab is associated with reduced risk adjusted hospitalization or death amongst outpatients with mild to moderate COVID 19 compared to no treatment and indicates low adjusted risk difference compared to patients treated intravenously.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
12,
1
]
]
},
"author": [
{
"affiliation": [],
"family": "McCreary",
"given": "Erin K.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bariola",
"given": "J. Ryan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wadas",
"given": "Richard J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shovel",
"given": "Judith A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wisniewski",
"given": "Mary K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adam",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albin",
"given": "Debbie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Minnier",
"given": "Tami E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schmidhofer",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meyers",
"given": "Russell",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marroquin",
"given": "Oscar C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Collins",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garrard",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berrry",
"given": "Lindsay R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berry",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crawford",
"given": "Amy M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McGlothlin",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Linstrum",
"given": "Kelsey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nakayama",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montgomery",
"given": "Stephanie K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Snyder",
"given": "Graham M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yealy",
"given": "Donald M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Angus",
"given": "Derek C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kip",
"given": "Paula L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seymour",
"given": "Christopher W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "David T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kip",
"given": "Kevin E.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T23:25:15Z",
"timestamp": 1638401115000
},
"deposited": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T23:25:15Z",
"timestamp": 1638401115000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T23:50:08Z",
"timestamp": 1638402608075
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
12,
1
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.11.30.21266756",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
12,
1
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
12,
1
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Association of subcutaneous or intravenous route of administration of casirivimab and imdevimab monoclonal antibodies with clinical outcomes in COVID-19."
],
"type": "posted-content"
}
