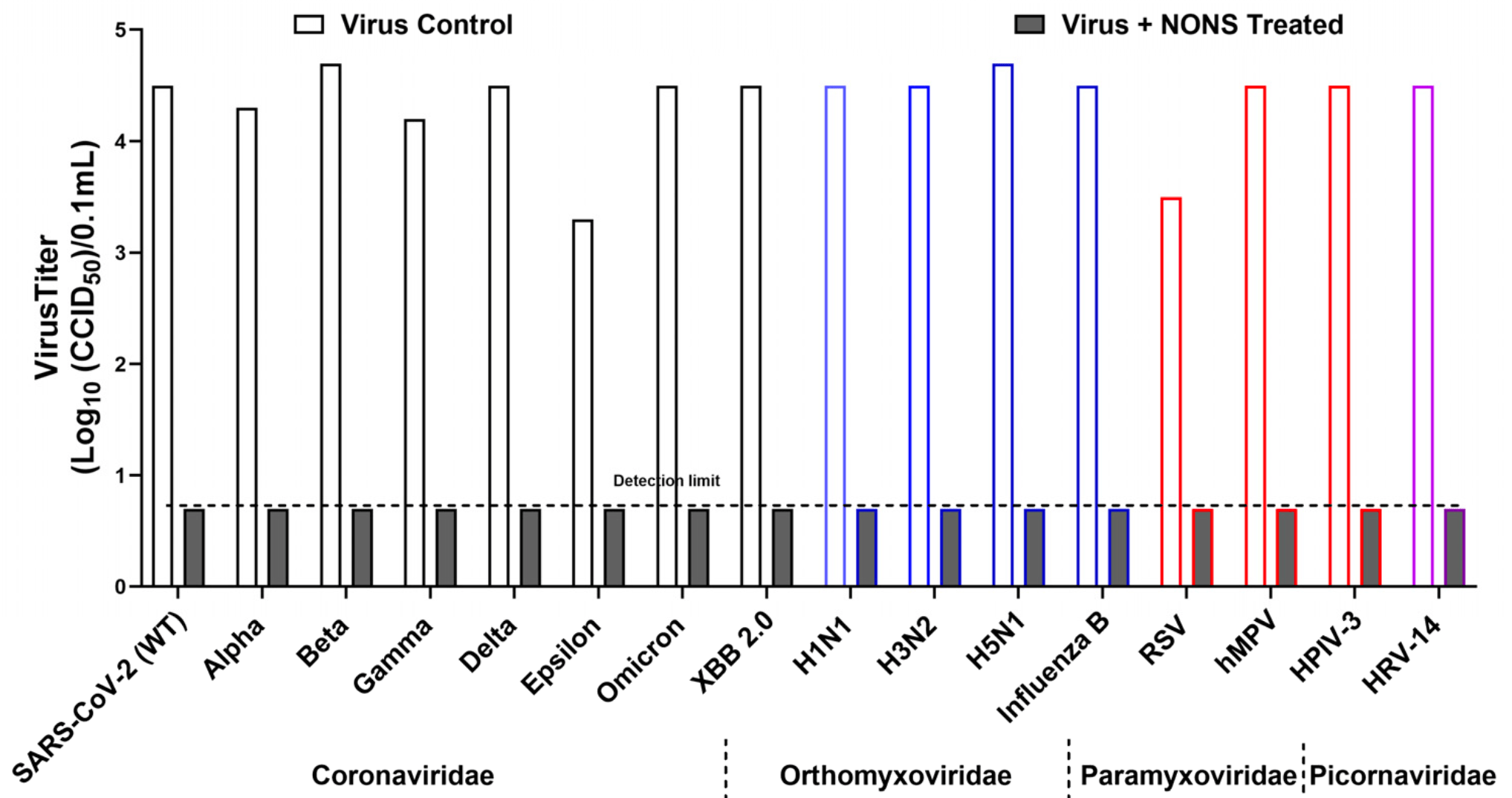
Broad-Spectrum Virucidal Activity of Nitric Oxide Nasal Spray (NONS) Against SARS-CoV-2 Variants and Major Respiratory Viruses
et al., Viruses, doi:10.3390/v18010091, Jan 2026
43rd treatment shown to reduce risk in
June 2022, now with p = 0.012 from 12 studies, recognized in 10 countries.
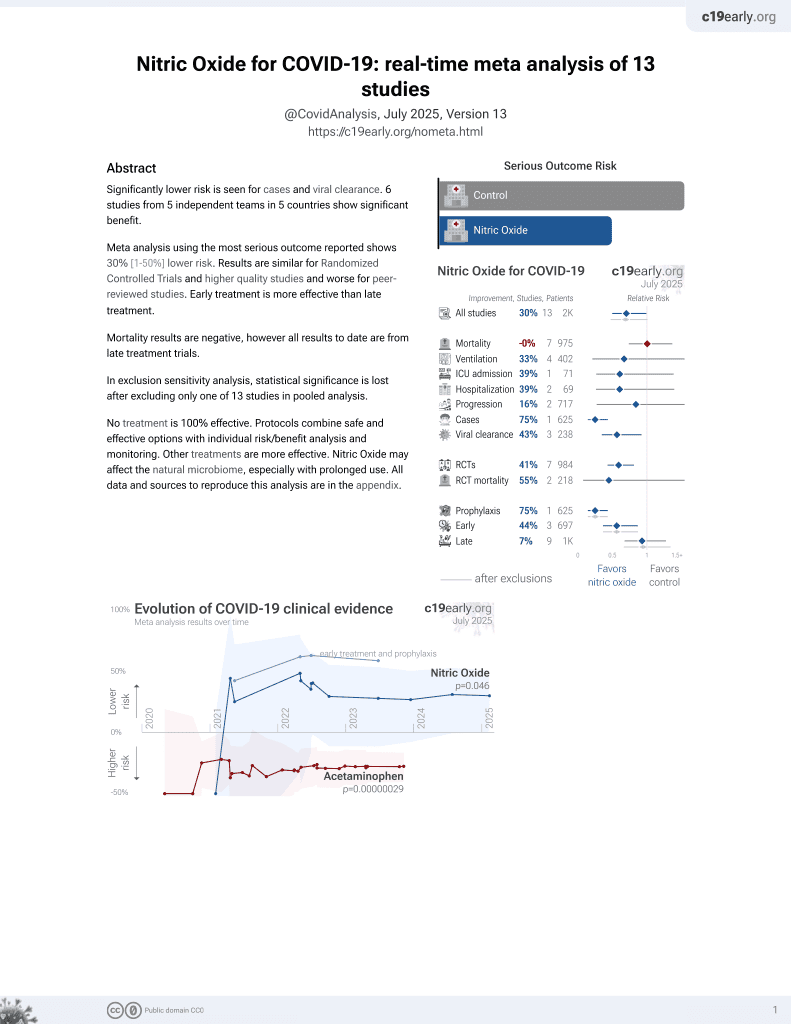
Lower risk for cases and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing broad-spectrum virucidal activity of nitric oxide nasal spray (NONS) against SARS-CoV-2 variants and major respiratory viruses. Authors found that NONS achieved >3 log10 reductions (>99.9% reduction) in viral infectivity within 15 seconds to 2 minutes across all tested viruses, including SARS-CoV-2 variants (Alpha, Beta, Gamma, Delta, Omicron BA.1, and XBB 2.0), influenza A subtypes (H1N1, H3N2, H5N1), influenza B, respiratory syncytial virus (RSV), human metapneumovirus (hMPV), human parainfluenza virus type 3 (HPIV-3), and human rhinovirus 14.
3 preclinical studies support the efficacy of nitric oxide for COVID-19:
1.
Martins et al., Broad-Spectrum Virucidal Activity of Nitric Oxide Nasal Spray (NONS) Against SARS-CoV-2 Variants and Major Respiratory Viruses, Viruses, doi:10.3390/v18010091.
Martins et al., 9 Jan 2026, USA, peer-reviewed, 4 authors.
Contact: selvarani.vimalanathan@ubc.ca (corresponding author), jmartins@sanotize.com, chris@sanotize.com, jeremy.road@vch.ca.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Broad-Spectrum Virucidal Activity of Nitric Oxide Nasal Spray (NONS) Against SARS-CoV-2 Variants and Major Respiratory Viruses
Viruses, doi:10.3390/v18010091
Respiratory viruses such as SARS-CoV-2, influenzas A and B, respiratory syncytial virus (RSV), human metapneumovirus (hMPV), human parainfluenza virus type 3 (HPIV-3), and rhinoviruses remain major causes of global morbidity. Their rapid evolution, high transmissibility, and limited therapeutic options, together with the absence of approved vaccines for several pathogens, highlight the need for broad-acting and pathogen-independent antiviral strategies. Nitric oxide exhibits antiviral activity through redox-dependent mechanisms, including S-nitrosylation of cysteine-containing viral proteins and disruption of redoxsensitive structural domains. Clinical studies conducted during the SARS-CoV-2 pandemic demonstrated that a nitric oxide nasal spray (NONS) rapidly reduced nasal viral load and transmission. In this study, we evaluated the in vitro virucidal activity of the NONS against a panel of clinically relevant respiratory viruses representing four major virus families. Virus suspensions of approximately 10 4 CCID 50 were exposed to a full-strength NONS for contact times ranging from 5 s to 2 min at room temperature, followed by neutralization and quantification of residual infectivity using endpoint dilution assays. The NONS rapidly reduced viral infectivity across all viruses tested, achieving >3 log 10 reductions within 2 min. SARS-CoV-2 variants including Alpha, Beta, Gamma, Delta, Omicron BA.1, and XBB 2.0 were reduced to levels at or below the assay detection limit within 30 s to 2 min. Influenza A and B viruses showed the fastest loss of infectivity, reaching detection limits within 10-15 s. RSV, hMPV, HPIV-3, and human rhinovirus 14 were similarly inactivated within 1-2 min. These findings demonstrate that the NONS exhibits rapid and broad-spectrum virucidal activity against diverse respiratory viruses and supports its potential role in pandemic preparedness but also seasonal use.
Author Contributions: Conceptualization, J.M., C.M. and S.V.; methodology, S.V.; validation, S.V., J.M., J.R. and C.M.; formal analysis, S.V.; investigation, S.V. and J.M.; resources, C.M.; data curation, S.V.; writing, original draft preparation, S.V.; writing, review and editing, S.V., J.M., J.R. and C.M.; visualization, S.V.; supervision, C.M.; project administration, C.M.; funding acquisition, J.M. and C.M. All authors have read and agreed to the published version of the manuscript. Conflicts of Interest: C.M. and J.M. are employees and shareholders of SaNOtize. S.V. and J.R. collaborate with SaNOtize on research activities and hold academic appointments at the University of British Columbia.
References
Akaike, Maeda, Nitric Oxide and Virus Infection, Immunology, doi:10.1046/j.1365-2567.2000.00142.x
Akerström, Mousavi-Jazi, Klingström, Leijon, Lundkvist et al., Nitric Oxide Inhibits the Replication Cycle of Severe Acute Respiratory Syndrome Coronavirus, J. Virol, doi:10.1128/JVI.79.3.1966-1969.2005
Altarawneh, Chemaitelly, Hasan, Ayoub, Qassim et al., Protection against the Omicron Variant from Previous SARS-CoV-2 Infection, N. Engl. J. Med, doi:10.1056/NEJMc2200133
Badorff, Fichtlscherer, Rhoads, Zeiher, Muelsch et al., Nitric Oxide Inhibits Dystrophin Proteolysis by Coxsackieviral Protease 2A through S-Nitrosylation: A Protective Mechanism against Enteroviral Cardiomyopathy, Circulation, doi:10.1161/01.CIR.102.18.2276
Bartlett, Uhart, Leveraging One Health as a Sentinel Approach for Pandemic Resilience, Virol. J, doi:10.1186/s12985-024-02545-1
Belongia, Simpson, King, Sundaram, Kelley et al., Variable Influenza Vaccine Effectiveness by Subtype: A Systematic Review and Meta-Analysis of Test-Negative Design Studies, Lancet Infect. Dis, doi:10.1016/S1473-3099(16)00129-8
Caliendo, Lewis, Pohlmann, Baillie, Banyard et al., Transatlantic Spread of Highly Pathogenic Avian Influenza H5N1 by Wild Birds from Europe to North America in 2021, Sci. Rep, doi:10.1038/s41598-022-13447-z
Carabelli, Peacock, Thorne, Harvey, Hughes, COVID-19
Cinelli, Do, Miley, Silverman, Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition, Med. Res. Rev, doi:10.1002/med.21599
Dawood, Jain, Finelli, Shaw, Lindstrom et al., Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans, N. Engl. J. Med, doi:10.1056/NEJMoa0903810
Harvey, Carabelli, Jackson, Gupta, Thomson et al., SARS-CoV-2 Variants, Spike Mutations and Immune Escape, Nat. Rev. Microbiol, doi:10.1038/s41579-021-00573-0
Keyaerts, Vijgen, Chen, Maes, Hedenstierna et al., Inhibition of SARS-Coronavirus Infection in Vitro by S-Nitroso-N-Acetylpenicillamine, a Nitric Oxide Donor Compound, Int. J. Infect. Dis, doi:10.1016/j.ijid.2004.04.012
Lane, Jeglinski, Avery-Gomm, Ballstaedt, Banyard et al., High Pathogenicity Avian Influenza (H5N1) in Northern Gannets (Morus bassanus): Global Spread, Clinical Signs and Demographic Consequences, IBIS, doi:10.1111/ibi.13275
Lipsitch, Dean, Understanding COVID-19 Vaccine Efficacy, Science, doi:10.1126/science.abe5938
Melero, Mas, Mclellan, Structural, Antigenic and Immunogenic Features of Respiratory Syncytial Virus Glycoproteins Relevant for Vaccine Development, Vaccine, doi:10.1016/j.vaccine.2016.09.045
Miller, Moore, Epidemiological Analysis of Nitric Oxide Nasal Spray (VirX) Use in Students Exposed to COVID-19 Infected Individuals, Respir. Ther
Más, Rodriguez, Olmedillas, Cano, Palomo et al., Structure and Immunogenicity of the Human Metapneumovirus F Protein in the Postfusion Conformation, PLoS Pathog, doi:10.1371/journal.ppat.1005859
Peacock, Barclay, De Silva, Towers, SARS-CoV-2 variant biology: Immune escape, transmission and fitness, Nat. Rev. Microbiol, doi:10.1038/s41579-022-00841-7
Plowright, Parrish, Mccallum, Hudson, Ko et al., Pathways to Zoonotic Spillover, Nat. Rev. Microbiol, doi:10.1038/nrmicro.2017.45
Puhach, Meyer, Eckerle, SARS-CoV-2 Viral Load and Shedding Kinetics, Nat. Rev. Microbiol, doi:10.1038/s41579-022-00822-w
Regev-Shoshani, Vimalanathan, Mcmullin, Road, Av-Gay et al., Gaseous Nitric Oxide Reduces Influenza Infectivity in Vitro, Nitric Oxide, doi:10.1016/j.niox.2013.03.007
Rimmelzwaan, Baars, De Lijster, Fouchier, Osterhaus, Inhibition of Influenza Virus Replication by Nitric Oxide, J. Virol, doi:10.1128/JVI.73.10.8880-8883.1999
Saura, Zaragoza, Mcmillan, Quick, Hohenadl et al., An Antiviral Mechanism of Nitric Oxide: Inhibition of a Viral Protease, Immunity, doi:10.1016/S1074-7613(00)80003-5
Sodano, Gazzano, Fruttero, Lazzarato, NO in Viral Infections: Role and Development of Antiviral Therapies, Molecules, doi:10.3390/molecules27072337
Sonvico, Colombo, Quarta, Guareschi, Banella et al., Nasal Delivery as a Strategy for the Prevention and Treatment of COVID-19, Expert Opin. Drug Deliv, doi:10.1080/17425247.2023.2263363
Tandon, Wu, Moore, Winchester, Tu et al., SARS-CoV-2 Accelerated Clearance Using a Novel Nitric Oxide Nasal Spray (NONS) Treatment: A Randomized Trial, Lancet Reg. Health-Southeast Asia, doi:10.1016/j.lansea.2022.100036
Taubenberger, Morens, Influenza: The Mother of All Pandemics, Emerg. Infect. Dis
Taubenberger, Morens, The Pathology of Influenza Virus Infections, Annu. Rev. Pathol, doi:10.1146/annurev.pathmechdis.3.121806.154316
Verberk, De Hoog, Westerhof, Van Goethem, Lammens et al., Transmission of SARS-CoV-2 within Households: A Remote Prospective Cohort Study in European Countries, Eur. J. Epidemiol, doi:10.1007/s10654-022-00870-9
Webster, Bean, Gorman, Chambers, Kawaoka, Evolution and Ecology of Influenza A Viruses, Microbiol. Rev, doi:10.1128/mr.56.1.152-179.1992
Winchester, John, Jabbar, John, Clinical Efficacy of Nitric Oxide Nasal Spray (NONS) for the Treatment of Mild COVID-19 Infection, J. Infect, doi:10.1016/j.jinf.2021.05.009
Yin, Paterson, Wen, Lamb, Jardetzky, Structure of the Uncleaved Ectodomain of the Paramyxovirus (HPIV3) Fusion Protein, Proc. Natl. Acad. Sci
Yin, Wen, Paterson, Lamb, Jardetzky, Structure of the Parainfluenza Virus 5 F Protein in Its Metastable, Prefusion Conformation, Nature, doi:10.1038/nature04322
Zaki, Van Boheemen, Bestebroer, Osterhaus, Fouchier, Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia, N. Engl. J. Med, doi:10.1056/NEJMoa1211721
Zamora, Vodovotz, Billiar, Inducible Nitric Oxide Synthase and Inflammatory Diseases, Mol. Med, doi:10.1007/BF03401781
Zell, Markgraf, Schmidtke, Görlach, Stelzner et al., Nitric Oxide Donors Inhibit the Coxsackievirus B3 Proteinases 2A and 3C in Vitro, Virus Production in Cells, and Signs of Myocarditis in Virus-Infected Mice, Med. Microbiol. Immunol, doi:10.1007/s00430-003-0198-6
Zhong, Zheng, Li, Poon; Xie, Chan et al., Epidemiology and Cause of Severe Acute Respiratory Syndrome (SARS) in Guangdong, People's Republic of China, Lancet, doi:10.1016/S0140-6736(03)14630-2
DOI record:
{
"DOI": "10.3390/v18010091",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v18010091",
"abstract": "<jats:p>Respiratory viruses such as SARS-CoV-2, influenzas A and B, respiratory syncytial virus (RSV), human metapneumovirus (hMPV), human parainfluenza virus type 3 (HPIV-3), and rhinoviruses remain major causes of global morbidity. Their rapid evolution, high transmissibility, and limited therapeutic options, together with the absence of approved vaccines for several pathogens, highlight the need for broad-acting and pathogen-independent antiviral strategies. Nitric oxide exhibits antiviral activity through redox-dependent mechanisms, including S-nitrosylation of cysteine-containing viral proteins and disruption of redox-sensitive structural domains. Clinical studies conducted during the SARS-CoV-2 pandemic demonstrated that a nitric oxide nasal spray (NONS) rapidly reduced nasal viral load and transmission. In this study, we evaluated the in vitro virucidal activity of the NONS against a panel of clinically relevant respiratory viruses representing four major virus families. Virus suspensions of approximately 104 CCID50 were exposed to a full-strength NONS for contact times ranging from 5 s to 2 min at room temperature, followed by neutralization and quantification of residual infectivity using endpoint dilution assays. The NONS rapidly reduced viral infectivity across all viruses tested, achieving >3 log10 reductions within 2 min. SARS-CoV-2 variants including Alpha, Beta, Gamma, Delta, Omicron BA.1, and XBB 2.0 were reduced to levels at or below the assay detection limit within 30 s to 2 min. Influenza A and B viruses showed the fastest loss of infectivity, reaching detection limits within 10–15 s. RSV, hMPV, HPIV-3, and human rhinovirus 14 were similarly inactivated within 1–2 min. These findings demonstrate that the NONS exhibits rapid and broad-spectrum virucidal activity against diverse respiratory viruses and supports its potential role in pandemic preparedness but also seasonal use.</jats:p>",
"alternative-id": [
"v18010091"
],
"author": [
{
"affiliation": [
{
"name": "SaNOtize Research & Development Corp., Vancouver, BC V6P 6T3, Canada"
}
],
"family": "Martins",
"given": "James",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC V6T 2B5, Canada"
}
],
"family": "Vimalanathan",
"given": "Selvarani",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Respiratory Medicine, University of British Columbia, Vancouver, BC V6T 1Z4, Canada"
}
],
"family": "Road",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "SaNOtize Research & Development Corp., Vancouver, BC V6P 6T3, Canada"
}
],
"family": "Miller",
"given": "Chris",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2026,
1,
9
]
],
"date-time": "2026-01-09T10:35:52Z",
"timestamp": 1767954952000
},
"deposited": {
"date-parts": [
[
2026,
1,
9
]
],
"date-time": "2026-01-09T10:39:24Z",
"timestamp": 1767955164000
},
"funder": [
{
"award": [
"NA"
],
"award-info": [
{
"award-number": [
"NA"
]
}
],
"name": "SaNOtize Research & Development Corp"
}
],
"indexed": {
"date-parts": [
[
2026,
1,
9
]
],
"date-time": "2026-01-09T12:03:10Z",
"timestamp": 1767960190611,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2026,
1,
9
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2026,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
9
]
],
"date-time": "2026-01-09T00:00:00Z",
"timestamp": 1767916800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/18/1/91/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "91",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2026,
1,
9
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
9
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1128/JVI.73.10.8880-8883.1999",
"article-title": "Inhibition of Influenza Virus Replication by Nitric Oxide",
"author": "Rimmelzwaan",
"doi-asserted-by": "crossref",
"first-page": "8880",
"journal-title": "J. Virol.",
"key": "ref_1",
"volume": "73",
"year": "1999"
},
{
"DOI": "10.1128/JVI.79.3.1966-1969.2005",
"article-title": "Nitric Oxide Inhibits the Replication Cycle of Severe Acute Respiratory Syndrome Coronavirus",
"author": "Leijon",
"doi-asserted-by": "crossref",
"first-page": "1966",
"journal-title": "J. Virol.",
"key": "ref_2",
"volume": "79",
"year": "2005"
},
{
"DOI": "10.3390/molecules27072337",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Sodano, F., Gazzano, E., Fruttero, R., and Lazzarato, L. (2022). NO in Viral Infections: Role and Development of Antiviral Therapies. Molecules, 27."
},
{
"DOI": "10.1056/NEJMoa1211721",
"article-title": "Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia",
"author": "Zaki",
"doi-asserted-by": "crossref",
"first-page": "1814",
"journal-title": "N. Engl. J. Med.",
"key": "ref_4",
"volume": "367",
"year": "2012"
},
{
"DOI": "10.1016/S0140-6736(03)14630-2",
"article-title": "Epidemiology and Cause of Severe Acute Respiratory Syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003",
"author": "Zhong",
"doi-asserted-by": "crossref",
"first-page": "1353",
"journal-title": "Lancet",
"key": "ref_5",
"volume": "362",
"year": "2003"
},
{
"DOI": "10.3201/eid1209.05-0979",
"article-title": "1918 Influenza: The Mother of All Pandemics",
"author": "Taubenberger",
"doi-asserted-by": "crossref",
"first-page": "15",
"journal-title": "Emerg. Infect. Dis.",
"key": "ref_6",
"volume": "12",
"year": "2006"
},
{
"DOI": "10.1056/NEJMoa0903810",
"article-title": "Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans",
"author": "Dawood",
"doi-asserted-by": "crossref",
"first-page": "2605",
"journal-title": "N. Engl. J. Med.",
"key": "ref_7",
"volume": "360",
"year": "2009"
},
{
"DOI": "10.1038/s41579-021-00573-0",
"article-title": "SARS-CoV-2 Variants, Spike Mutations and Immune Escape",
"author": "Harvey",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_8",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(16)00129-8",
"article-title": "Variable Influenza Vaccine Effectiveness by Subtype: A Systematic Review and Meta-Analysis of Test-Negative Design Studies",
"author": "Belongia",
"doi-asserted-by": "crossref",
"first-page": "942",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_9",
"volume": "16",
"year": "2016"
},
{
"DOI": "10.1056/NEJMc2200133",
"article-title": "Protection against the Omicron Variant from Previous SARS-CoV-2 Infection",
"author": "Altarawneh",
"doi-asserted-by": "crossref",
"first-page": "1288",
"journal-title": "N. Engl. J. Med.",
"key": "ref_10",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-13447-z",
"article-title": "Transatlantic Spread of Highly Pathogenic Avian Influenza H5N1 by Wild Birds from Europe to North America in 2021",
"author": "Caliendo",
"doi-asserted-by": "crossref",
"first-page": "11729",
"journal-title": "Sci. Rep.",
"key": "ref_11",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1186/s12985-024-02545-1",
"article-title": "Leveraging One Health as a Sentinel Approach for Pandemic Resilience",
"author": "Bartlett",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Virol. J.",
"key": "ref_12",
"volume": "21",
"year": "2024"
},
{
"DOI": "10.1111/ibi.13275",
"article-title": "High Pathogenicity Avian Influenza (H5N1) in Northern Gannets (Morus bassanus): Global Spread, Clinical Signs and Demographic Consequences",
"author": "Lane",
"doi-asserted-by": "crossref",
"first-page": "633",
"journal-title": "IBIS",
"key": "ref_13",
"volume": "166",
"year": "2024"
},
{
"DOI": "10.1128/mr.56.1.152-179.1992",
"article-title": "Evolution and Ecology of Influenza A Viruses",
"author": "Webster",
"doi-asserted-by": "crossref",
"first-page": "152",
"journal-title": "Microbiol. Rev.",
"key": "ref_14",
"volume": "56",
"year": "1992"
},
{
"DOI": "10.1038/nrmicro.2017.45",
"article-title": "Pathways to Zoonotic Spillover",
"author": "Plowright",
"doi-asserted-by": "crossref",
"first-page": "502",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_15",
"volume": "15",
"year": "2017"
},
{
"DOI": "10.1146/annurev.pathmechdis.3.121806.154316",
"article-title": "The Pathology of Influenza Virus Infections",
"author": "Taubenberger",
"doi-asserted-by": "crossref",
"first-page": "499",
"journal-title": "Annu. Rev. Pathol.",
"key": "ref_16",
"volume": "3",
"year": "2008"
},
{
"DOI": "10.1007/s10654-022-00870-9",
"article-title": "Transmission of SARS-CoV-2 within Households: A Remote Prospective Cohort Study in European Countries",
"author": "Verberk",
"doi-asserted-by": "crossref",
"first-page": "549",
"journal-title": "Eur. J. Epidemiol.",
"key": "ref_17",
"volume": "37",
"year": "2022"
},
{
"article-title": "SARS-CoV-2 Viral Load and Shedding Kinetics",
"author": "Puhach",
"first-page": "147",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_18",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1016/j.lansea.2022.100036",
"article-title": "SARS-CoV-2 Accelerated Clearance Using a Novel Nitric Oxide Nasal Spray (NONS) Treatment: A Randomized Trial",
"author": "Tandon",
"doi-asserted-by": "crossref",
"first-page": "100036",
"journal-title": "Lancet Reg. Health-Southeast Asia",
"key": "ref_19",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2021.05.009",
"article-title": "Clinical Efficacy of Nitric Oxide Nasal Spray (NONS) for the Treatment of Mild COVID-19 Infection",
"author": "Winchester",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "J. Infect.",
"key": "ref_20",
"volume": "83",
"year": "2021"
},
{
"article-title": "Epidemiological Analysis of Nitric Oxide Nasal Spray (VirX) Use in Students Exposed to COVID-19 Infected Individuals",
"author": "Miller",
"first-page": "38",
"journal-title": "Respir. Ther.",
"key": "ref_21",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.1016/S1074-7613(00)80003-5",
"article-title": "An Antiviral Mechanism of Nitric Oxide: Inhibition of a Viral Protease",
"author": "Saura",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Immunity",
"key": "ref_22",
"volume": "10",
"year": "1999"
},
{
"DOI": "10.1046/j.1365-2567.2000.00142.x",
"article-title": "Nitric Oxide and Virus Infection",
"author": "Akaike",
"doi-asserted-by": "crossref",
"first-page": "300",
"journal-title": "Immunology",
"key": "ref_23",
"volume": "101",
"year": "2000"
},
{
"DOI": "10.1002/med.21599",
"article-title": "Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition",
"author": "Cinelli",
"doi-asserted-by": "crossref",
"first-page": "158",
"journal-title": "Med. Res. Rev.",
"key": "ref_24",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1007/BF03401781",
"article-title": "Inducible Nitric Oxide Synthase and Inflammatory Diseases",
"author": "Zamora",
"doi-asserted-by": "crossref",
"first-page": "347",
"journal-title": "Mol. Med.",
"key": "ref_25",
"volume": "6",
"year": "2000"
},
{
"DOI": "10.1080/17425247.2023.2263363",
"article-title": "Nasal Delivery as a Strategy for the Prevention and Treatment of COVID-19",
"author": "Sonvico",
"doi-asserted-by": "crossref",
"first-page": "1115",
"journal-title": "Expert Opin. Drug Deliv.",
"key": "ref_26",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1016/j.ijid.2004.04.012",
"article-title": "Inhibition of SARS-Coronavirus Infection in Vitro by S-Nitroso-N-Acetylpenicillamine, a Nitric Oxide Donor Compound",
"author": "Keyaerts",
"doi-asserted-by": "crossref",
"first-page": "223",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_27",
"volume": "8",
"year": "2004"
},
{
"DOI": "10.1007/s00430-003-0198-6",
"article-title": "Nitric Oxide Donors Inhibit the Coxsackievirus B3 Proteinases 2A and 3C in Vitro, Virus Production in Cells, and Signs of Myocarditis in Virus-Infected Mice",
"author": "Zell",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "Med. Microbiol. Immunol.",
"key": "ref_28",
"volume": "193",
"year": "2004"
},
{
"DOI": "10.1161/01.CIR.102.18.2276",
"article-title": "Nitric Oxide Inhibits Dystrophin Proteolysis by Coxsackieviral Protease 2A through S-Nitrosylation: A Protective Mechanism against Enteroviral Cardiomyopathy",
"author": "Badorff",
"doi-asserted-by": "crossref",
"first-page": "2276",
"journal-title": "Circulation",
"key": "ref_29",
"volume": "102",
"year": "2000"
},
{
"DOI": "10.1016/j.niox.2013.03.007",
"article-title": "Gaseous Nitric Oxide Reduces Influenza Infectivity in Vitro",
"author": "Vimalanathan",
"doi-asserted-by": "crossref",
"first-page": "48",
"journal-title": "Nitric Oxide",
"key": "ref_30",
"volume": "31",
"year": "2013"
},
{
"DOI": "10.1073/pnas.0503989102",
"article-title": "Structure of the Uncleaved Ectodomain of the Paramyxovirus (HPIV3) Fusion Protein",
"author": "Yin",
"doi-asserted-by": "crossref",
"first-page": "9288",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_31",
"volume": "102",
"year": "2005"
},
{
"DOI": "10.1038/nature04322",
"article-title": "Structure of the Parainfluenza Virus 5 F Protein in Its Metastable, Prefusion Conformation",
"author": "Yin",
"doi-asserted-by": "crossref",
"first-page": "38",
"journal-title": "Nature",
"key": "ref_32",
"volume": "439",
"year": "2006"
},
{
"DOI": "10.1016/j.vaccine.2016.09.045",
"article-title": "Structural, Antigenic and Immunogenic Features of Respiratory Syncytial Virus Glycoproteins Relevant for Vaccine Development",
"author": "Melero",
"doi-asserted-by": "crossref",
"first-page": "461",
"journal-title": "Vaccine",
"key": "ref_33",
"volume": "35",
"year": "2017"
},
{
"DOI": "10.1371/journal.ppat.1005859",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Más, V., Rodriguez, L., Olmedillas, E., Cano, O., Palomo, C., Terrón, M.C., Luque, D., Melero, J.A., and McLellan, J.S. (2016). Engineering, Structure and Immunogenicity of the Human Metapneumovirus F Protein in the Postfusion Conformation. PLoS Pathog., 12."
},
{
"DOI": "10.1126/science.abe5938",
"article-title": "Understanding COVID-19 Vaccine Efficacy",
"author": "Lipsitch",
"doi-asserted-by": "crossref",
"first-page": "763",
"journal-title": "Science",
"key": "ref_35",
"volume": "370",
"year": "2020"
},
{
"article-title": "SARS-CoV-2 variant biology: Immune escape, transmission and fitness",
"author": "Carabelli",
"first-page": "162",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_36",
"volume": "21",
"year": "2023"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/18/1/91"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Broad-Spectrum Virucidal Activity of Nitric Oxide Nasal Spray (NONS) Against SARS-CoV-2 Variants and Major Respiratory Viruses",
"type": "journal-article",
"update-policy": "https://doi.org/10.3390/mdpi_crossmark_policy",
"volume": "18"
}