Real World Utilization of Bamlanivimab at a Rural Community Hospital
et al., Cureus, doi:10.7759/cureus.19747, Nov 2021
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 136 outpatients showing bamlanivimab reduced emergency department visits at 28 days, but not hospitalizations, compared to a control group prior to authoritzation in patients with mild to moderate COVID-19.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 29.9% lower, RR 0.70, p = 0.60, treatment 6 of 136 (4.4%), control 9 of 143 (6.3%), NNT 53, day 28.
|
|
risk of emergency care, 41.6% lower, RR 0.58, p = 0.04, treatment 20 of 136 (14.7%), control 36 of 143 (25.2%), NNT 9.6, day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Leavitt et al., 19 Nov 2021, retrospective, USA, peer-reviewed, median age 69.0, 9 authors, study period 2 December, 2020 - 8 January, 2021.
Contact: ashurst.john.32@gmail.com.
Real World Utilization of Bamlanivimab at a Rural Community Hospital
Cureus, doi:10.7759/cureus.19747
Introduction Although there were several proposed treatments for patients that were hospitalized with COVID-19, outpatient treatments for those with mild to moderate illness were limited prior to the emergency use authorization (EUA) of virus-neutralizing monoclonal antibodies. To assess the efficacy of outpatient monoclonal therapy, the investigators assessed the seven, 14, and 28-day emergency department and hospitalization rates of adult patients given bamlanivimab for the treatment of COVID-19 at a community hospital.
Methods A retrospective chart review was performed of all adult patients given bamlanivimab within the emergency department or an outpatient infusion center from December 2, 2020 through January 8, 2021 for the treatment of mild to moderate COVID-19. Patients were compared to a set of controls who would have qualified for bamlanivimab treatment prior to its authorization in reverse temporal order from November 30, 2020 through August 1, 2020. Abstracted data included patient demographics, allergic reactions, emergency department presentations, and hospitalizations at seven, 14, and 28 days post-infusion due to COVID-19 and any in-hospital mortality in those admitted with a COVID-19 complication.
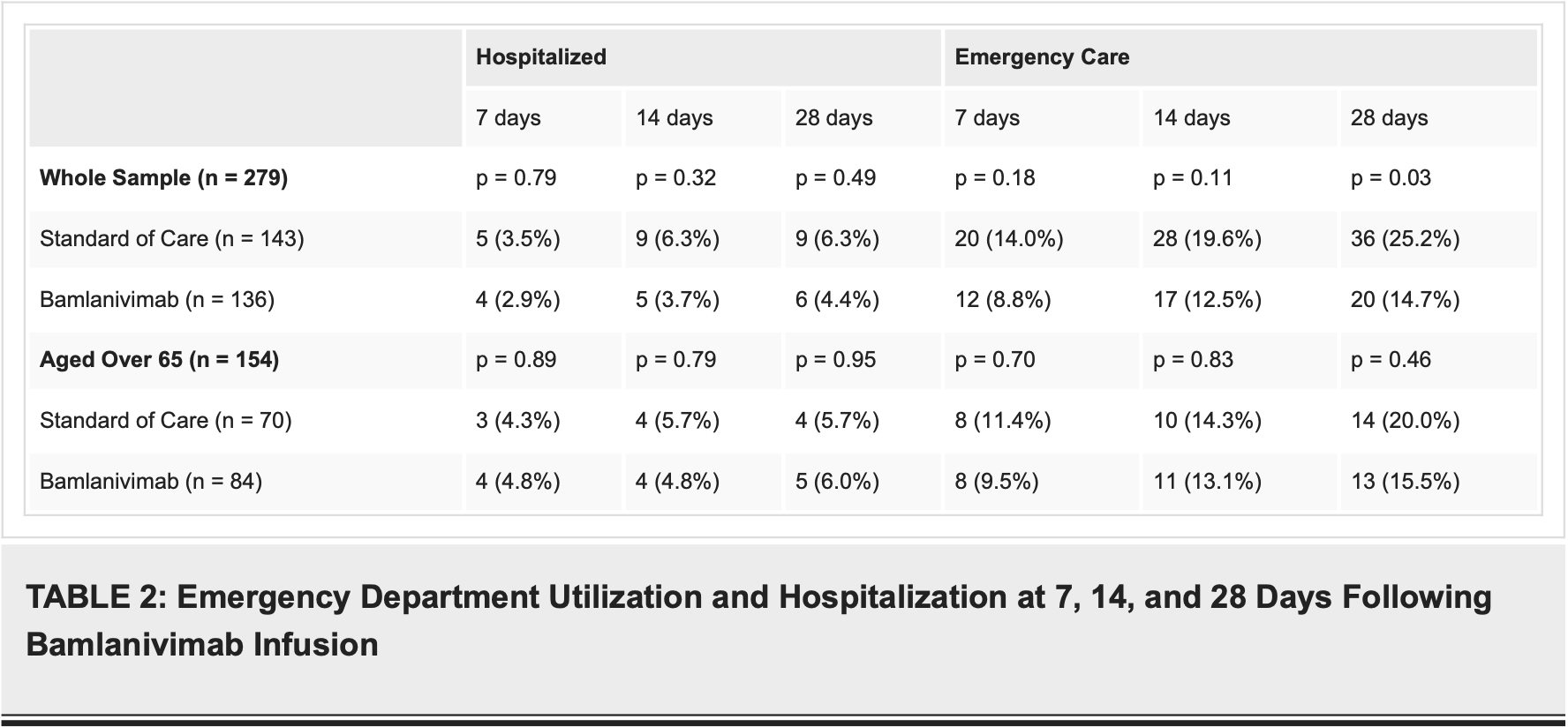
Results A total of 136 patients received bamlanivimab during the study period with none having an allergic reaction during infusion. In those who received bamlanivimab, 84 (61.8%) patients included were aged 65 years or older. At 28 days, there was a statistically significant reduction in emergency department visits in those who received bamlanivimab (20 vs 36 patients; p = 0.03) but not at seven days (12 vs 20 patients; p = 0.18) or 14 days (17 vs 28 patients; p = 0.11). No statistically significant difference in emergency department returns was noted in those aged 65 years or older at seven (eight vs eight patients; p = 0.70), 14 (11 vs 10 patients; p = 0.83), or 28 days (13 vs 14 patients, p = 0.46). A total of six (4.4%) patients were hospitalized at 28 days following the bamlanivimab infusion with five (83.3%) being aged 65 or older. No statistical difference was noted for decreased hospitalizations at seven (four vs five patients; p = 0.79), 14 (five vs nine patients; p = 0.32), or 28 days (six vs nine patients; p = 0.49) post-infusion. No patients suffered from in-hospital mortality after infusion with bamlanivimab.
Conclusion Outpatient infusion of bamlanivimab reduced the incidence of those with mild to moderate COVID-19 requiring subsequent care through the emergency department at 28 days but not hospitalizations within this time frame. No statistical difference was noted in either emergency department visits or hospitalizations in those aged 65 or greater who were treated as an outpatient with bamlanivimab for mild to moderate COVID-19.
Additional Information Disclosures Human subjects: Consent was obtained or waived by all participants in this study. Kingman Regional Medical Center issued approval 0277. The project was reviewed by the institutional review board at Kingman Regional Medical Center and found to be exempt. . Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
Ash, Leavitt, Dietrich, Schritter, Wells et al., Real world utilization of REGEN-COV2 at a community hospital, Am J Emerg Med, doi:10.1016/j.ajem.2021.07.050
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.0202
Jones, Brown-Augsburger, Corbett, The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates, Sci Transl Med, doi:10.1126/scitranslmed.abf1906
Kaji, Schriger, Green, Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies, Ann Emerg Med, doi:10.1016/j.annemergmed.2014.03.025
Leavitt, None, Cureus, doi:10.7759/cureus.197476of6
Lee, Santarelli, Caine, Schritter, Dietrich et al., Remdesivir for the treatment of severe COVID-19: a community hospital's experience, J Am Osteopath Assoc, doi:10.7556/jaoa.2020.156
Simonovich, Pratx, Scibona, A randomized trial of convalescent plasma in COVID-19 severe pneumonia, N Engl J Med, doi:10.1056/NEJMoa2031304
Worster, Bledsoe, Cleve, Fernandes, Upadhye et al., Reassessing the methods of medical record review studies in emergency medicine research, Ann Emerg Med, doi:10.1016/j.annemergmed.2004.11.021
Zhang, Penninger, Li, Zhong, Slutsky, Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target, Intensive Care Med, doi:10.1007/s00134-020-05985-9
DOI record:
{
"DOI": "10.7759/cureus.19747",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.19747",
"author": [
{
"affiliation": [],
"family": "Leavitt",
"given": "Rachael",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ash",
"given": "Jordan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hasenbalg",
"given": "Phillip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santarelli",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dietrich",
"given": "Tyson",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schritter",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wells",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dawson",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ashurst",
"given": "John",
"sequence": "additional"
}
],
"container-title": "Cureus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
11,
19
]
],
"date-time": "2021-11-19T16:26:00Z",
"timestamp": 1637339160000
},
"deposited": {
"date-parts": [
[
2024,
2,
9
]
],
"date-time": "2024-02-09T19:30:19Z",
"timestamp": 1707507019000
},
"indexed": {
"date-parts": [
[
2024,
2,
10
]
],
"date-time": "2024-02-10T00:19:49Z",
"timestamp": 1707524389808
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2021,
11,
19
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/74418-real-world-utilization-of-bamlanivimab-at-a-rural-community-hospital",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2021,
11,
19
]
]
},
"published-print": {
"date-parts": [
[
2021,
11,
19
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1007/s00134-020-05985-9",
"article-title": "Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target",
"author": "Zhang H",
"doi-asserted-by": "publisher",
"journal-title": "Intensive Care Med",
"key": "ref1",
"unstructured": "Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46:586-90. 10.1007/s00134-020-05985-9",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.7556/jaoa.2020.156",
"article-title": "Remdesivir for the treatment of severe COVID-19: a community hospital's experience",
"author": "Lee S",
"doi-asserted-by": "publisher",
"journal-title": "J Am Osteopath Assoc",
"key": "ref2",
"unstructured": "Lee S, Santarelli A, Caine K, Schritter S, Dietrich T, Ashurst J. Remdesivir for the treatment of severe COVID-19: a community hospital's experience. J Am Osteopath Assoc. 2020, 120:926-33. 10.7556/jaoa.2020.156",
"volume": "120",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031304",
"article-title": "A randomized trial of convalescent plasma in COVID-19 severe pneumonia",
"author": "Simonovich VA",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref3",
"unstructured": "Simonovich VA, Burgos Pratx LD, Scibona P, et al.. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2021, 384:619-29. 10.1056/NEJMoa2031304",
"volume": "384",
"year": "2021"
},
{
"key": "ref4",
"unstructured": "FDA News Release. Coronavirus (COVID-19) Update. FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. (2020). Accessed: November 13, 2020: http://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-tre...."
},
{
"DOI": "10.1126/scitranslmed.abf1906",
"article-title": "The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates",
"author": "Jones BE",
"doi-asserted-by": "publisher",
"journal-title": "Sci Transl Med",
"key": "ref5",
"unstructured": "Jones BE, Brown-Augsburger PL, Corbett KS, et al.. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci Transl Med. 2021, 13:eabf1906. 10.1126/scitranslmed.abf1906",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19",
"author": "Chen P",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref6",
"unstructured": "Chen P, Nirula A, Heller B, et al.. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021, 384:229-37. 10.1056/NEJMoa2029849",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb RL",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref7",
"unstructured": "Gottlieb RL, Nirula A, Chen P, et al.. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021, 325:632-44. 10.1001/jama.2021.0202",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/j.annemergmed.2004.11.021",
"article-title": "Reassessing the methods of medical record review studies in emergency medicine research",
"author": "Worster A",
"doi-asserted-by": "publisher",
"journal-title": "Ann Emerg Med",
"key": "ref8",
"unstructured": "Worster A, Bledsoe RD, Cleve P, Fernandes CM, Upadhye S, Eva K. Reassessing the methods of medical record review studies in emergency medicine research. Ann Emerg Med. 2005, 45:448-51. 10.1016/j.annemergmed.2004.11.021",
"volume": "45",
"year": "2005"
},
{
"DOI": "10.1016/j.annemergmed.2014.03.025",
"article-title": "Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies",
"author": "Kaji AH",
"doi-asserted-by": "publisher",
"journal-title": "Ann Emerg Med",
"key": "ref9",
"unstructured": "Kaji AH, Schriger D, Green S. Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies. Ann Emerg Med. 2014, 64:292-98. 10.1016/j.annemergmed.2014.03.025",
"volume": "64",
"year": "2014"
},
{
"DOI": "10.1016/j.ajem.2021.07.050",
"article-title": "Real world utilization of REGEN-COV2 at a community hospital",
"author": "Ash J",
"doi-asserted-by": "publisher",
"journal-title": "Am J Emerg Med",
"key": "ref10",
"unstructured": "Ash J, Leavitt R, Dietrich T, Schritter S, Wells J, Santarelli A, Ashurst J. Real world utilization of REGEN-COV2 at a community hospital. Am J Emerg Med. 2021, 50:129-31. 10.1016/j.ajem.2021.07.050",
"volume": "50",
"year": "2021"
}
],
"reference-count": 10,
"references-count": 10,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cureus.com/articles/74418-real-world-utilization-of-bamlanivimab-at-a-rural-community-hospital"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Aerospace Engineering"
],
"subtitle": [],
"title": "Real World Utilization of Bamlanivimab at a Rural Community Hospital",
"type": "journal-article"
}
