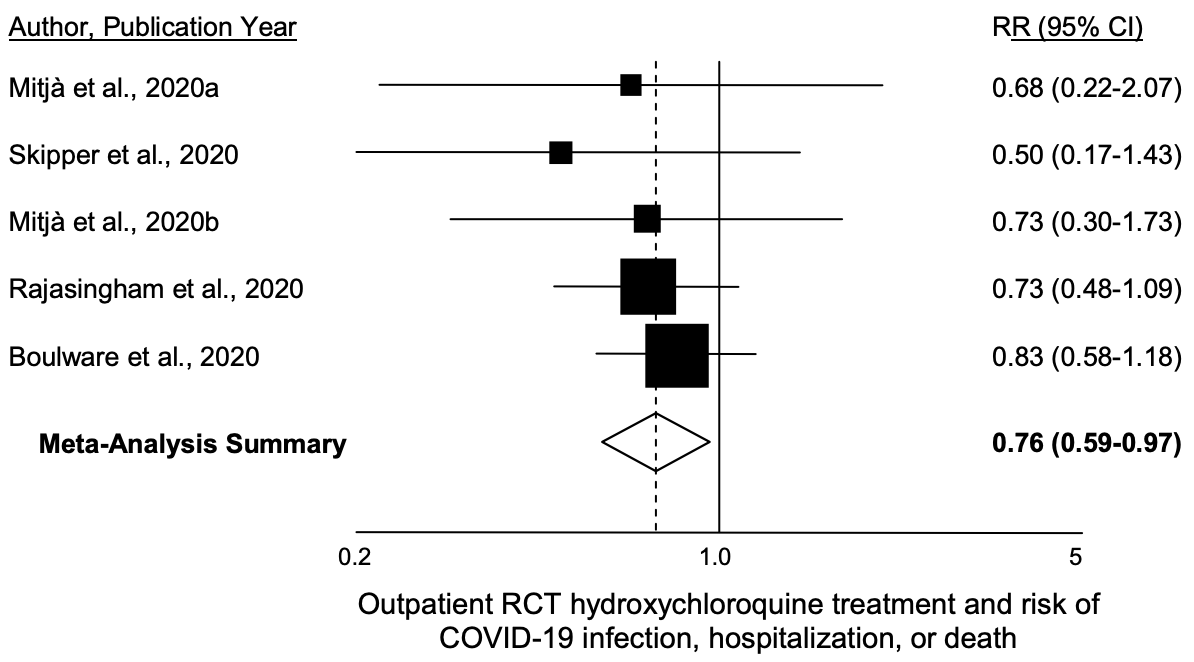
Randomized Controlled Trials of Early Ambulatory Hydroxychloroquine in the Prevention of COVID-19 Infection, Hospitalization, and Death: Meta-Analysis
et al., medRxiv, doi:10.1101/2020.09.30.20204693, Sep 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Meta analysis of prophylactic and early treatment RCTs, 24% reduction in cases, hospitalization or death with HCQ, RR 0.76, p=0.025. No serious adverse cardiac events were reported. 5,577 patients.
The Boulware study provides a breakdown for treatment delay. For the case of < ~4 days (2 days enrollment, ~46 hours shipping), the result of the meta analysis becomes RR 0.68, p=0.0097.
The actual effect may be larger due to treatment delays, followup loss, protocol deviation, active placebos, no severity analysis for cases, and suboptimal regimens.
10 meta-analyses show significant improvements with hydroxychloroquine for mortality6-9,
hospitalization6,
recovery10,
combined death/hospitalization/cases11,
cases12-14, and
viral clearance15.
Currently there are 38 HCQ for COVID-19 early treatment studies, showing 76% lower mortality [61‑85%], 67% lower ventilation [-710‑99%], 31% lower ICU admission [1‑53%], and 41% lower hospitalization [28‑51%].
|
risk of death/hospitalization/cases, 24.0% lower, RR 0.76, p = 0.03.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Boulware et al., A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, NEJM, June 3 2020, doi:10.1056/NEJMoa2016638.
2.
Mitjà et al., A Cluster-Randomized Trial of Hydroxychloroquine as Prevention of Covid-19 Transmission and Disease, NEJM, doi:10.1056/NEJMoa2021801.
3.
Rajasingham et al., Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial, Clinical Infectious Diseases, doi:10.1093/cid/ciaa1571.
4.
Skipper et al., Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial, Annals of Internal Medicine, doi:10.7326/M20-4207.
5.
Mitjà (B) et al., Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial, Clinical Infectious Diseases, ciaa1009, doi:10.1093/cid/ciaa1009.
6.
Landsteiner de Sampaio Amêndola et al., COVID-19 Infection in Rheumatic Patients on Chronic Antimalarial Drugs: A Systematic Review and Meta-Analysis, Journal of Clinical Medicine, doi:10.3390/jcm11226865.
7.
Risch, H., Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients that Should be Ramped-Up Immediately as Key to the Pandemic Crisis, American Journal of Epidemiology, kwaa093, 27 May 2020, doi:10.1093/aje/kwaa093.
8.
Risch (B), H., Response to: “Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients” and “Re: Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients that Should be Ramped-Up Immediately as Key to the Pandemic Crisis”, American Journal of Epidemiology, July 20, 2020, doi:10.1093/aje/kwaa152.
9.
Stricker et al., Hydroxychloroquine Pre-Exposure Prophylaxis for COVID-19 in Healthcare Workers from India: A Meta-Analysis, Journal of Infection and Public Health, doi:10.1016/j.jiph.2021.08.001.
10.
Prodromos et al., Hydroxychloroquine is effective, and consistently so used early, for Covid-19: A systematic review, New Microbes and New Infections, doi:10.1016/j.nmni.2020.100776.
11.
Ladapo et al., Randomized Controlled Trials of Early Ambulatory Hydroxychloroquine in the Prevention of COVID-19 Infection, Hospitalization, and Death: Meta-Analysis, medRxiv, doi:10.1101/2020.09.30.20204693.
12.
García-Albéniz et al., Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19, European Journal of Epidemiology, doi:10.1007/s10654-022-00891-4.
13.
Han et al., The efficacy and safety of hydroxychloroquine for COVID-19 prophylaxis and clinical assessment: an updated meta-analysis of randomized trials, Journal of Thoracic Disease, doi:10.21037/jtd-23-1043.
Ladapo et al., 30 Sep 2020, preprint, 4 authors.
Randomized Controlled Trials of Early Ambulatory Hydroxychloroquine in the Prevention of COVID-19 Infection, Hospitalization, and Death: Meta-Analysis
doi:10.1101/2020.09.30.20204693
Objective-To determine if hydroxychloroquine (HCQ) reduces the incidence of new illness, hospitalization or death among outpatients at risk for or infected with SARS-CoV-2 (COVID-19). Design-Systematic review and meta-analysis of randomized clinical trials. Data sources-Search of MEDLINE, EMBASE, PubMed, medRxiv, PROSPERO, and the Cochrane Central Register of Controlled Trials. Also review of reference lists from recent meta-analyses. Study selection-Randomized clinical trials in which participants were treated with HCQ or placebo/standard-of-care for pre-exposure prophylaxis, post-exposure prophylaxis, or outpatient therapy for COVID-19. Methods-Two investigators independently extracted data on trial design and outcomes. Medication side effects and adverse reactions were also assessed. The primary outcome was COVID-19 hospitalization or death. When unavailable, new COVID-19 infection was used. We calculated random effects meta-analysis according to the method of DerSimonian and Laird. Heterogeneity between the studies was evaluated by calculation of Cochran Q and I 2 parameters. An Egger funnel plot was drawn to investigate publication bias. We also calculated the fixed effects meta-analysis summary of the five studies. All calculations were done in Excel, and results were considered to be statistically significant at a two-sided threshold of P=.05. Results-Five randomized controlled clinical trials enrolling 5,577 patients were included. HCQ was associated with a 24% reduction in COVID-19 infection, hospitalization or death, P=.025
Conflicts of Interest Dr. Risch acknowledges past advisory consulting work with two of the more than 50 manufacturers of hydroxychloroquine, azithromycin and doxycycline. This past work was not related to any of these medications and was completed more than two years ago. He has no ongoing, planned or projected relationships with any of these companies, nor any other potential conflicts-of-interest to disclose. None of the other authors have any potential conflicts of interest to disclose.
Key Messages Box: What is already known on this topic • Various government, clinical and research entities have been trying to evaluate the degree of benefit of hydroxychloroquine in early COVID-19 outpatient treatment. • Seven nonrandomized but controlled clinical trials to date have shown significant reductions in hospitalization and mortality with early ambulatory hydroxychloroquine use, but individual randomized outpatient trials have not shown statistical significance of benefit with these or other outcomes.
What this study adds • The five outpatient randomized controlled studies to date examining new infection, hospitalization or mortality together show statistically significant evidence of reduced risk, RR=0.76 (95% CI 0.59 to 0.97). • No serious adverse cardiac events were reported in any of the studies. • The combined literature of seven nonrandomized controlled trials and five randomized controlled trials provides substantial and statistically significant evidence of benefit..
References
Arshad, Kilgore, Chaudhry, Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19, Int J Infect Dis, doi:10.1016/j.ijid.2020.06.099
Barbosa Esper, Da Silva, Costa Oikawa, Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed-up by telemedicine, Preprints, doi:10.21203/rs.3.rs-70219/v2
Benson, Hartz, A comparison of observational studies and randomized, controlled trials, N Engl J Med, doi:10.1056/NEJM200006223422506
Bernaola, Mena, Bernaola, Observational study of the efficiency of treatments in patients hospitalized with Covid-19 in Madrid, Preprints, doi:10.1101/2020.07.17.20155960
Brouqui, Gatineau, Raoult, Velthuis, Aj et al., Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture, New Microbes New Infect, doi:10.1016/j.nmni.2020
Catteau, Dauby, Montourcy, Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.10614422COVID-19RISK
Concato, Shah, Horwitz, Randomized, controlled trials, observational studies, and the hierarchy of research designs, N Engl J Med, doi:10.1056/NEJM200006223422507
Dersimonian, Laird, Meta-analysis in clinical trials revisited, Contemp Clin Trials, doi:10.1016/j.cct.2015.09.002
Dersimonian, Laird, Meta-analysis in clinical trials, Control Clin Trials, doi:10.1016/0197-2456(86
Horby, Lim, Dexamethasone in Hospitalized Patients with Covid-19 -Preliminary Report, N Engl J Med, doi:10.1056/NEJMoa2021436
Ip, Ahn, Zhou, Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: A multi-center observational study, Preprints, doi:10.1101/2020.08.20.20178772
Lagier, Million, Gautret, Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis, Travel Med Infect Dis
Lauriola, Pani, Ippoliti, Effect of combination therapy of hydroxychloroquine and azithromycin on mortality in COVID-19 patients, Clin Transl Sci, doi:10.1111/cts.12860
Ly, Zanini, Laforge, Pattern of SARS-CoV-2 infection among dependant elderly residents living in retirement homes in Marseille, France, Preprints
Mccullough, Kelly, Ruocco, Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection, Am J Med, doi:10.1016/j.amjmed.2020.07.003
Mikami, Miyashita, Yamada, Risk factors for mortality in patients with COVID-19 in New York City, J Gen Intern Med, doi:10.1007/s11606-020-05983-z
Mitjà, Corbacho-Monné, Ubals, Hydroxychloroquine for early treatment of adults with mild COVID-19: A randomized-controlled trial, Clin Infect Dis, doi:10.1093/cid/ciaa1009
Park, Decloedt, Rayner, Cotton, Mills, Clinical trials of disease stages in COVID 19: complicated and often misinterpreted, Lancet Glob Health, doi:10.1016/S2214-109X(20)30365-X
Rajasingham, Bangdiwala, Nicol, Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial, N Engl J Med, doi:10.1101/2020.09.18.20197327v1
Skipper, Pastick, Engen, Hydroxychloroquine in nonhospitalized adults with early COVID-19: A randomized trial, Ann Intern Med, doi:10.7326/M20-4207
Sulaiman, Mohana, Alawdah, The effect of early hydroxychloroquine-based therapy in COVID-19 patients in ambulatory care settings: A nationwide prospective cohort study, Preprints, doi:10.1101/2020.09.09.20184143
Watanabe, Efficacy of hydroxychloroquine as prophylaxis for Covid-19, Preprints
DOI record:
{
"DOI": "10.1101/2020.09.30.20204693",
"URL": "http://dx.doi.org/10.1101/2020.09.30.20204693",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Objective</jats:title><jats:p>To determine if hydroxychloroquine (HCQ) reduces the incidence of new illness, hospitalization or death among outpatients at risk for or infected with SARS-CoV-2 (COVID-19).</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>Systematic review and meta-analysis of randomized clinical trials.</jats:p></jats:sec><jats:sec><jats:title>Data sources</jats:title><jats:p>Search of MEDLINE, EMBASE, PubMed, medRxiv, PROSPERO, and the Cochrane Central Register of Controlled Trials. Also review of reference lists from recent meta-analyses.</jats:p></jats:sec><jats:sec><jats:title>Study selection</jats:title><jats:p>Randomized clinical trials in which participants were treated with HCQ or placebo/standard-of-care for pre-exposure prophylaxis, post-exposure prophylaxis, or outpatient therapy for COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Two investigators independently extracted data on trial design and outcomes. Medication side effects and adverse reactions were also assessed. The primary outcome was COVID-19 hospitalization or death. When unavailable, new COVID-19 infection was used. We calculated random effects meta-analysis according to the method of DerSimonian and Laird. Heterogeneity between the studies was evaluated by calculation of Cochran Q and I<jats:sup>2</jats:sup>parameters. An Egger funnel plot was drawn to investigate publication bias. We also calculated the fixed effects meta-analysis summary of the five studies. All calculations were done in Excel, and results were considered to be statistically significant at a two-sided threshold of P=.05.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Five randomized controlled clinical trials enrolling 5,577 patients were included. HCQ was associated with a 24% reduction in COVID-19 infection, hospitalization or death, P=.025 (RR, 0.76 [95% CI, 0.59 to 0.97]). No serious adverse cardiac events were reported. The most common side effects were gastrointestinal.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Hydroxychloroquine use in outpatients reduces the incidence of the composite outcome of COVID-19 infection, hospitalization, and death. Serious adverse events were not reported and cardiac arrhythmia was rare.</jats:p></jats:sec><jats:sec><jats:title>Systematic review registration</jats:title><jats:p>This review was not registered.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2020,
9,
30
]
]
},
"author": [
{
"affiliation": [],
"family": "Ladapo",
"given": "Joseph A.",
"sequence": "first"
},
{
"affiliation": [],
"family": "McKinnon",
"given": "John E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCullough",
"given": "Peter A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5337-3941",
"affiliation": [],
"authenticated-orcid": false,
"family": "Risch",
"given": "Harvey A.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
10,
1
]
],
"date-time": "2020-10-01T03:55:35Z",
"timestamp": 1601524535000
},
"deposited": {
"date-parts": [
[
2022,
11,
21
]
],
"date-time": "2022-11-21T13:28:40Z",
"timestamp": 1669037320000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
11,
29
]
],
"date-time": "2023-11-29T13:39:45Z",
"timestamp": 1701265185482
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 15,
"issued": {
"date-parts": [
[
2020,
9,
30
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2020.09.30.20204693",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
9,
30
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2020,
9,
30
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2021021707251007000_2020.09.30.20204693v1.1",
"unstructured": "RECOVERY Collaborative Group, Horby P , Lim WS , et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med 2020 Jul 17:EJMoa2021436. https://www.nejm.org/doi/10.1056/NEJMoa2021436"
},
{
"DOI": "10.1016/j.nmni.2020.100707",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.2",
"unstructured": "02 Brouqui P , Giraud-Gatineau A , Raoult D. Remdesivir investigational trials in COVID-19: a critical reappraisal. New Microbes New Infect 2020 Jun 7;100707. https://doi.org/10.1016/j.nmni.2020.100707"
},
{
"DOI": "10.1371/journal.ppat.1001176",
"doi-asserted-by": "publisher",
"key": "2021021707251007000_2020.09.30.20204693v1.3"
},
{
"key": "2021021707251007000_2020.09.30.20204693v1.4",
"unstructured": "Barbosa Esper R , Souza da Silva R , Teiichi Costa Oikawa F , et al. Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed-up by telemedicine. April 15, 2020. Accessed April 30, 2020. https://pgibertie.files.wordpress.com/2020/04/2020.04.15-journal-manuscript-final.pdf"
},
{
"DOI": "10.21203/rs.3.rs-70219/v2",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.5",
"unstructured": "Heras E , Garibaldi P , Boix M , et al. COVID-19 mortality risk factors in older people in a long-term care center. Preprints September 9, 2020. https://doi.org/10.21203/rs.3.rs-70219/v2"
},
{
"DOI": "10.1101/2020.08.20.20178772",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.6",
"unstructured": "Ip A , Ahn J , Zhou Y , et al. Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: A multi-center observational study. Preprints August 25, 2020. https://doi.org/10.1101/2020.08.20.20178772"
},
{
"key": "2021021707251007000_2020.09.30.20204693v1.7",
"unstructured": "Lagier JC , Million M , Gautret P , et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med Infect Dis 2020 Jun 25:101791. https://www.sciencedirect.com/science/article/pii/S1477893920302817"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106219",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.8",
"unstructured": "Ly TDA , Zanini D , Laforge V , et al. Pattern of SARS-CoV-2 infection among dependant elderly residents living in retirement homes in Marseille, France, March-June 2020. Preprints August 20,2020. https://www.mediterranee-infection.com/wp-content/uploads/2020/08/Abstract-COVID-EHPAD.pdf"
},
{
"DOI": "10.1101/2020.09.09.20184143",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.9",
"unstructured": "Sulaiman T , Mohana A , Alawdah L , et al. The effect of early hydroxychloroquine-based therapy in COVID-19 patients in ambulatory care settings: A nationwide prospective cohort study. Preprints September 13, 2020. https://doi.org/10.1101/2020.09.09.20184143"
},
{
"DOI": "10.1101/2020.07.20.20157651",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.10",
"unstructured": "Mitjà O , Ubals M , Corbacho-Monné M , et al. A cluster-randomized trial of hydroxychloroquine as prevention of COVID-19 transmission and disease. Preprints July 26, 2020. https://doi.org/10.1101/2020.07.20.20157651"
},
{
"DOI": "10.1093/cid/ciaa1009",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.11",
"unstructured": "Mitjà O , Corbacho-Monné M , Ubals M , et al. Hydroxychloroquine for early treatment of adults with mild COVID-19: A randomized-controlled trial. Clin Infect Dis 2020 Jul 16:ciaa1009. https://doi.org/10.1093/cid/ciaa1009"
},
{
"key": "2021021707251007000_2020.09.30.20204693v1.12",
"unstructured": "Skipper CP , Pastick KA , Engen NW , et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: A randomized trial. Ann Intern Med 2020 Jul 16:M20–4207. https://www.acpjournals.org/doi/10.7326/M20-4207"
},
{
"DOI": "10.1101/2020.09.18.20197327",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.13",
"unstructured": "Rajasingham R , Bangdiwala AS , Nicol MR , et al. Hydroxychloroquine as pre-exposure prophylaxis for COVID-19 in healthcare workers: a randomized trial. Preprints September 21, 2020. https://www.medrxiv.org/content/10.1101/2020.09.18.20197327v1"
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "2021021707251007000_2020.09.30.20204693v1.14"
},
{
"DOI": "10.1016/0197-2456(86)90046-2",
"doi-asserted-by": "publisher",
"key": "2021021707251007000_2020.09.30.20204693v1.15"
},
{
"DOI": "10.1016/j.cct.2015.09.002",
"doi-asserted-by": "publisher",
"key": "2021021707251007000_2020.09.30.20204693v1.16"
},
{
"key": "2021021707251007000_2020.09.30.20204693v1.17",
"unstructured": "Watanabe M. Efficacy of hydroxychloroquine as prophylaxis for Covid-19. Preprints July 21, 2020. https://arxiv.org/abs/2007.09477"
},
{
"DOI": "10.1016/S2214-109X(20)30365-X",
"article-title": "Clinical trials of disease stages in COVID 19: complicated and often misinterpreted",
"doi-asserted-by": "crossref",
"first-page": "e1249",
"issue": "10",
"journal-title": "Lancet Glob Health",
"key": "2021021707251007000_2020.09.30.20204693v1.18",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.06.099",
"doi-asserted-by": "publisher",
"key": "2021021707251007000_2020.09.30.20204693v1.19"
},
{
"DOI": "10.1101/2020.07.17.20155960",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.20",
"unstructured": "Bernaola N , Mena R , Bernaola A , et al. Observational study of the efficiency of treatments in patients hospitalized with Covid-19 in Madrid. Preprints July 21, 2020. https://doi.org/10.1101/2020.07.17.20155960"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106144",
"doi-asserted-by": "publisher",
"key": "2021021707251007000_2020.09.30.20204693v1.21"
},
{
"DOI": "10.1016/j.ejim.2020.08.019",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.22",
"unstructured": "COVID-19 RISK and Treatments (CORIST) Collaboration. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study. Eur J Intern Med 2020;S0953-6205(20)30335-6. https://doi.org/10.1016/j.ejim.2020.08.019"
},
{
"DOI": "10.1111/cts.12860",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.23",
"unstructured": "Lauriola M , Pani A , Ippoliti G , et al. Effect of combination therapy of hydroxychloroquine and azithromycin on mortality in COVID-19 patients. Clin Transl Sci. 2020. https://doi.org/10.1111/cts.12860"
},
{
"DOI": "10.2139/ssrn.3588532",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.24",
"unstructured": "Mikami T , Miyashita H , Yamada T , et al. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med 2020:1–10. https://link.springer.com/article/10.1007/s11606-020-05983-z"
},
{
"DOI": "10.1056/NEJM200006223422506",
"doi-asserted-by": "publisher",
"key": "2021021707251007000_2020.09.30.20204693v1.25"
},
{
"DOI": "10.1056/NEJM200006223422507",
"doi-asserted-by": "publisher",
"key": "2021021707251007000_2020.09.30.20204693v1.26"
},
{
"DOI": "10.1016/j.amjmed.2020.07.003",
"doi-asserted-by": "crossref",
"key": "2021021707251007000_2020.09.30.20204693v1.27",
"unstructured": "McCullough PA , Kelly RJ , Ruocco G , et al. Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. Am J Med. 2020 Aug 7:S0002-9343(20)30673-2. Advance online publication. https://doi.org/10.1016/j.amjmed.2020.07.003"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2020.09.30.20204693"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Randomized Controlled Trials of Early Ambulatory Hydroxychloroquine in the Prevention of COVID-19 Infection, Hospitalization, and Death: Meta-Analysis",
"type": "posted-content"
}
