Efficacy of convalescent plasma for treatment of COVID-19 in Uganda
et al., BMJ Open Respiratory Research, doi:10.1136/bmjresp-2021-001017, COVIDIT, NCT04542941, Aug 2021
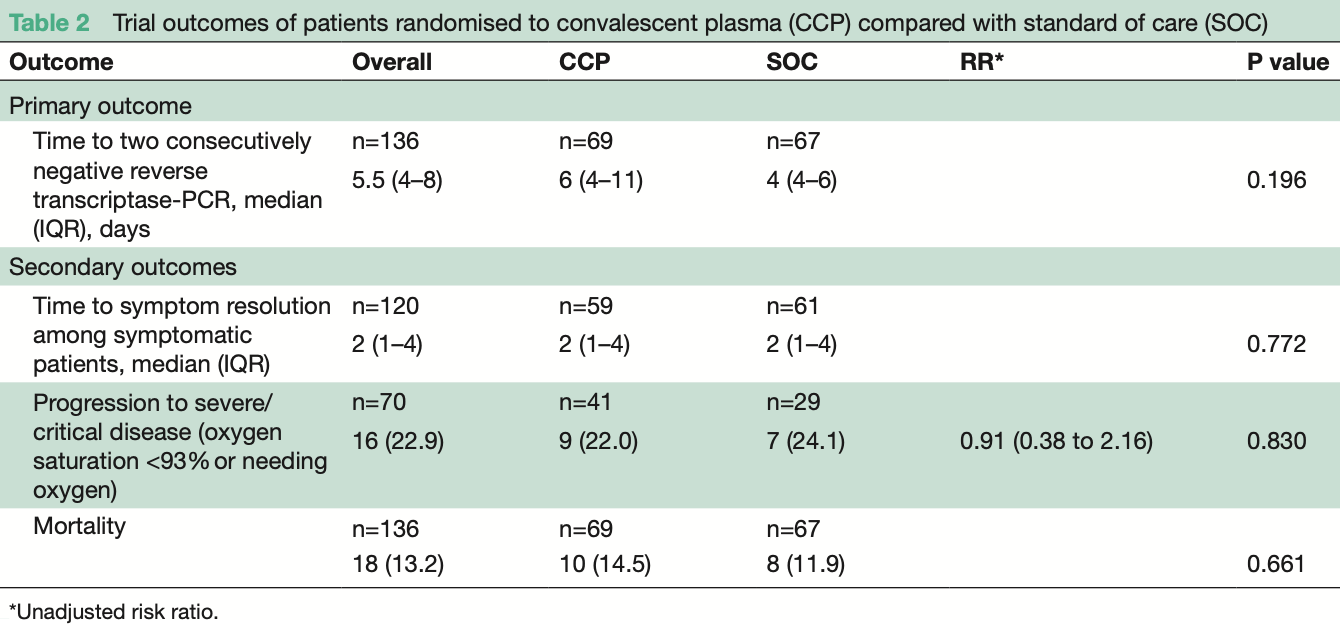
RCT 136 hospitalized COVID-19 patients in Uganda, showing no significant benefit with convalescent plasma treatment.
|
risk of death, 21.4% higher, RR 1.21, p = 0.80, treatment 10 of 69 (14.5%), control 8 of 67 (11.9%).
|
|
risk of progression, 9.1% lower, RR 0.91, p = 1.00, treatment 9 of 41 (22.0%), control 7 of 29 (24.1%), NNT 46.
|
|
recovery time, no change, relative time 1.00, p = 0.77, treatment 59, control 61.
|
|
time to viral-, 50.0% higher, relative time 1.50, p = 0.20, treatment 67, control 67.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kirenga et al., 9 Aug 2021, Randomized Controlled Trial, Uganda, peer-reviewed, median age 50.0, 30 authors, study period 16 June, 2020 - 31 December, 2020, average treatment delay 7.0 days, trial NCT04542941 (history) (COVIDIT).
Contact: brucekirenga@yahoo.co.uk.
Efficacy of convalescent plasma for treatment of COVID-19 in Uganda
BMJ Open Respiratory Research, doi:10.1136/bmjresp-2021-001017
Rationale Convalescent plasma (CCP) has been studied as a potential therapy for COVID-19, but data on its efficacy in Africa are limited. Objective In this trial we set out to determine the efficacy of CCP for treatment of COVID-19 in Uganda. Measurements Patients with a positive SARS-CoV-2 reverse transcriptase (RT)-PCR test irrespective of disease severity were hospitalised and randomised to receive either COVID-19 CCP plus standard of care (SOC) or SOC alone. The primary outcome was time to viral clearance, defined as having two consecutive RT-PCR-negative tests by day 28. Secondary outcomes included time to symptom resolution, clinical status on the modified WHO Ordinal Clinical Scale (≥1-point increase), progression to severe/ critical condition (defined as oxygen saturation <93% or needing oxygen), mortality and safety. Main results A total of 136 patients were randomised, 69 to CCP+SOC and 67 to SOC only. The median age was 50 years (IQR: 38.5-62.0), 71.3% were male and the median duration of symptom was 7 days (IQR=4-8). Time to viral clearance was not different between the CCP+SOC and SOC arms (median of 6 days (IQR=4-11) vs 4 (IQR=4-6), p=0.196). There were no statistically significant differences in secondary outcomes in CCP+SOC versus SOC: time to symptom resolution (median=7 (IQR=5-7) vs 7 (IQR=5-10) days, p=0.450), disease progression (9 (22.0%) vs 7 (24.0%) patients, p=0.830) and mortality (10 (14.5%) vs 8 (11.9%) deaths, p=0.476). Conclusion In this African trial, CCP therapy did not result in beneficial virological or clinical improvements. Further trials are needed to determine subgroups of patients who may benefit from CCP in Africa. Trial registration number NCT04542941.
Competing interests None declared. Patient consent for publication Not required. Ethics approval Trial ethical and regulatory approvals were obtained from the Mulago Hospital Research and Ethics Committee (MHREC) under reference number MHREC 1902, the National Drug Authority, and the Uganda National Council for Science and Technology under reference number HS816 ES. This study was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice. Provenance and peer review Not commissioned; externally peer reviewed. Data availability statement Data are available upon reasonable request. Data collected for this trial, including de-identified individual participant data and a data dictionary defining each field in the set, will be made available to others upon reasonable request. Additional, related documents including study protocol, statistical analysis plan and informed consent forms will be made available upon reasonable request. A formal request should be sent via email to the clinical trial principal investigator Dr Bruce Kirenga at brucekirenga@ yahoo. com. After approval of a proposal by an institutional research board with a signed data access agreement, data will be made available.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not..
References
Abolghasemi, Eshghi, Cheraghali, Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study, Transfus Apher Sci, doi:10.1016/j.transci.2020.102875
Agarwal, Mukherjee, Kumar, Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial), BMJ, doi:10.1136/bmj.m3939
Borghesi, Maroldi, Rjlrm, COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression, Radiol Med, doi:10.1007/s11547-020-01200-3
Budhiraja, Dewan, Aggarwal, Effectiveness of convalescent plasma in Indian patients with COVID-19, Blood Cells Mol Dis, doi:10.1016/j.bcmd.2021.102548
Callaway, Ledford, How to redesign COVID vaccines so they protect against variants, Nature, doi:10.1038/d41586-021-00241-6
Cheng, Wong, Soo, Use of convalescent plasma therapy in SARS patients in Hong Kong, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-004-1271-9
Dai, Gu, Hao, Potential benefits, mechanisms, and uncertainties of convalescent plasma therapy for COVID-19, Blood Sci, doi:10.1097/BS9.0000000000000047
Flyak, Ilinykh, Murin, Mechanism of human antibodymediated neutralization of Marburg virus, Cell, doi:10.1016/j.cell.2015.01.031
Garraud, Heshmati, Pozzetto, Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow, Transfus Clin Biol, doi:10.1016/j.tracli.2015.12.003
Gharbharan, Jordans, Geurtsvankessel, Convalescent plasma for COVID-19. A randomized clinical trial, Medrxiv, doi:10.1101/2020.07.01.20139857
Gharbharan, Jordans, Geurtsvankessel, Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection, Nat Commun, doi:10.1038/s41467-021-23469-2
Horby, Pessoa-Amorim, Peto, Tocilizumab in patients admitted to hospital with COVID-19 (recovery): preliminary results of a randomised, controlled, open-label, platform trial, doi:10.1101/2021.02.11.21249258
Joyner, Senefeld, Klassen, Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience, medRxiv, doi:10.1101/2020.08.12.20169359
Ko, Joo, Park, Neutralizing antibody production in asymptomatic and mild COVID-19 patients, in comparison with pneumonic COVID-19 patients, J Clin Med, doi:10.3390/jcm9072268
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.10044
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and lifethreatening COVID-19, JAMA, doi:10.1001/jama.2020.10044
Libster, Marc, Wappner, Early high-titer plasma therapy to prevent severe Covid-19 in older adults
Liu, Fan, Li, Antibody-dependent-cellular-cytotoxicityinducing antibodies significantly affect the post-exposure treatment of Ebola virus infection, Sci Rep, doi:10.1038/srep45552
Liu, Morgenstern, Kelly, The impact of nonpharmaceutical interventions on SARS-CoV-2 transmission across 130 countries and territories, BMC Med, doi:10.1186/s12916-020-01872-8
Lu, Murakowski, Bournazos, Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo, Science, doi:10.1126/science.aaf1279
Mahase, Covid-19: what new variants are emerging and how are they being investigated?, BMJ, doi:10.1136/bmj.n158
Medicine, Interleukin-6 receptor antagonists in critically ill patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2100433
Muttamba, Lusiba, Namakula, Feasibility of collecting and processing of COVID-19 convalescent plasma for treatment of COVID-19 in Uganda
Muttamba, Lusiba, Namakula, Feasibility of collecting and processing of COVID-19 convalescent plasma for treatment of COVID-19 in Uganda, PLoS One, doi:10.1371/journal.pone.0252306
Nio, The COVID-19 treatment guidelines panel's statement on the emergency use authorization of convalescent plasma for the treatment of COVID-19
Olivares-Gazca, Priesca-Marín, Ojeda-Laguna, Infusion of convalescent plasma is associated with clinical improvement in critically ill patients with COVID-19: a pilot study, RIC, doi:10.24875/RIC.20000237
Rajendran, Krishnasamy, Rangarajan, Convalescent plasma transfusion for the treatment of COVID-19: systematic review, J Med Virol, doi:10.1002/jmv.25961
Rambar, Convalescent serum and pooled plasma in communicable diseases, U S Nav Med Bull
Rcgjnejo, Dexamethasone in hospitalized patients with Covid-19-preliminary report
Simonovich, Pratx, Scibona, A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N Engl J Med, doi:10.1056/NEJMoa2031304
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
Winkler, Koepsell, Sajcoih, The use of convalescent plasma to treat emerging infectious diseases, Curr Opin Hematol, doi:10.1097/MOH.0000000000000191
Xu, Han, Li, Effective treatment of severe COVID-19 patients with tocilizumab, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2005615117
DOI record:
{
"DOI": "10.1136/bmjresp-2021-001017",
"ISSN": [
"2052-4439"
],
"URL": "http://dx.doi.org/10.1136/bmjresp-2021-001017",
"abstract": "<jats:sec><jats:title>Rationale</jats:title><jats:p>Convalescent plasma (CCP) has been studied as a potential therapy for COVID-19, but data on its efficacy in Africa are limited.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>In this trial we set out to determine the efficacy of CCP for treatment of COVID-19 in Uganda.</jats:p></jats:sec><jats:sec><jats:title>Measurements</jats:title><jats:p>Patients with a positive SARS-CoV-2 reverse transcriptase (RT)-PCR test irrespective of disease severity were hospitalised and randomised to receive either COVID-19 CCP plus standard of care (SOC) or SOC alone. The primary outcome was time to viral clearance, defined as having two consecutive RT-PCR-negative tests by day 28. Secondary outcomes included time to symptom resolution, clinical status on the modified WHO Ordinal Clinical Scale (≥1-point increase), progression to severe/critical condition (defined as oxygen saturation <93% or needing oxygen), mortality and safety.</jats:p></jats:sec><jats:sec><jats:title>Main results</jats:title><jats:p>A total of 136 patients were randomised, 69 to CCP+SOC and 67 to SOC only. The median age was 50 years (IQR: 38.5–62.0), 71.3% were male and the median duration of symptom was 7 days (IQR=4–8). Time to viral clearance was not different between the CCP+SOC and SOC arms (median of 6 days (IQR=4–11) vs 4 (IQR=4–6), p=0.196). There were no statistically significant differences in secondary outcomes in CCP+SOC versus SOC: time to symptom resolution (median=7 (IQR=5–7) vs 7 (IQR=5–10) days, p=0.450), disease progression (9 (22.0%) vs 7 (24.0%) patients, p=0.830) and mortality (10 (14.5%) vs 8 (11.9%) deaths, p=0.476).</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>In this African trial, CCP therapy did not result in beneficial virological or clinical improvements. Further trials are needed to determine subgroups of patients who may benefit from CCP in Africa.</jats:p><jats:p><jats:bold>Trial registration number</jats:bold><jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04542941\">NCT04542941</jats:ext-link>.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmjresp-2021-001017"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2023-2840",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kirenga",
"given": "Bruce",
"sequence": "first"
},
{
"affiliation": [],
"family": "Byakika-Kibwika",
"given": "Pauline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muttamba",
"given": "Winters",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kayongo",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loryndah",
"given": "Namakula Olive",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mugenyi",
"given": "Levicatus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kiwanuka",
"given": "Noah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lusiba",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Atukunda",
"given": "Angella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mugume",
"given": "Raymond",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ssali",
"given": "Francis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ddungu",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Katagira",
"given": "Winceslaus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sekibira",
"given": "Rogers",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kityo",
"given": "Cissy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kyeyune",
"given": "Dorothy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Acana",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aanyu-Tukamuhebwa",
"given": "Hellen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kabweru",
"given": "Wilberforce",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nakwagala",
"given": "Fred",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bagaya",
"given": "Bernard Sentalo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kimuli",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nantanda",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buregyeya",
"given": "Esther",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Byarugaba",
"given": "Baterana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olaro",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mwebesa",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joloba",
"given": "Moses Lutaakome",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siddharthan",
"given": "Trishul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bazeyo",
"given": "William",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04542941",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "BMJ Open Respiratory Research",
"container-title-short": "BMJ Open Resp Res",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2021,
8,
10
]
],
"date-time": "2021-08-10T03:11:10Z",
"timestamp": 1628565070000
},
"deposited": {
"date-parts": [
[
2022,
6,
30
]
],
"date-time": "2022-06-30T07:10:34Z",
"timestamp": 1656573034000
},
"funder": [
{
"award": [
"MakRIF"
],
"name": "Government of Uganda"
}
],
"indexed": {
"date-parts": [
[
2023,
4,
8
]
],
"date-time": "2023-04-08T22:47:07Z",
"timestamp": 1680994027187
},
"is-referenced-by-count": 22,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
8
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2021,
8,
9
]
]
},
"published-print": {
"date-parts": [
[
2021,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 7,
"start": {
"date-parts": [
[
2021,
8,
8
]
],
"date-time": "2021-08-08T00:00:00Z",
"timestamp": 1628380800000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjresp-2021-001017",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e001017",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2021,
8
]
]
},
"published-online": {
"date-parts": [
[
2021,
8,
9
]
]
},
"published-print": {
"date-parts": [
[
2021,
8
]
]
},
"publisher": "BMJ",
"reference": [
{
"key": "2022063000100525000_8.1.e001017.1",
"unstructured": "Uganda Ministry of health Covid 19 pandemic. Available: https://www.health.go.ug/covid/about-corona-virus/ [Accessed 20 Mar 2021]."
},
{
"DOI": "10.1186/s12916-021-01924-7",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.2"
},
{
"DOI": "10.1038/d41586-021-00241-6",
"article-title": "How to redesign COVID vaccines so they protect against variants",
"author": "Callaway",
"doi-asserted-by": "crossref",
"first-page": "15",
"journal-title": "Nature",
"key": "2022063000100525000_8.1.e001017.3",
"volume": "590",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n158",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.4"
},
{
"key": "2022063000100525000_8.1.e001017.5",
"unstructured": "Government of Uganda . National Guidelines for Management of Covid-19 - Ministry of Health. Available: https://www.health.go.ug/cause/national-guidelines-for-management-of-covid-19/ [Accessed 10 Mar 2021]."
},
{
"DOI": "10.1073/pnas.2005615117",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.6"
},
{
"DOI": "10.1056/NEJMoa2100433",
"doi-asserted-by": "crossref",
"key": "2022063000100525000_8.1.e001017.7",
"unstructured": "Medicine R-CIJNEJo . Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med 2021.doi:10.1056/NEJMoa2100433"
},
{
"DOI": "10.1101/2021.02.11.21249258",
"doi-asserted-by": "crossref",
"key": "2022063000100525000_8.1.e001017.8",
"unstructured": "Horby PW , Pessoa-Amorim G , Peto L . Tocilizumab in patients admitted to hospital with COVID-19 (recovery): preliminary results of a randomised, controlled, open-label, platform trial. Medrxiv 2021.doi:10.1101/2021.02.11.21249258"
},
{
"key": "2022063000100525000_8.1.e001017.9",
"unstructured": "NIo H . The COVID-19 treatment guidelines panel’s statement on the emergency use authorization of convalescent plasma for the treatment of COVID-19, 2020."
},
{
"key": "2022063000100525000_8.1.e001017.10",
"unstructured": "Medicine RCGJNEJo . Dexamethasone in hospitalized patients with Covid-19—preliminary report, 2020."
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.11"
},
{
"article-title": "Convalescent serum and pooled plasma in communicable diseases",
"author": "RAMBAR",
"first-page": "93",
"journal-title": "U S Nav Med Bull",
"key": "2022063000100525000_8.1.e001017.12",
"volume": "46",
"year": "1946"
},
{
"DOI": "10.1016/j.tracli.2015.12.003",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.13"
},
{
"DOI": "10.1097/moh.0000000000000191",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.14"
},
{
"DOI": "10.1016/j.bcmd.2021.102548",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.15"
},
{
"DOI": "10.24875/RIC.20000237",
"article-title": "Infusion of convalescent plasma is associated with clinical improvement in critically ill patients with COVID-19: a pilot study",
"author": "Olivares-Gazca",
"doi-asserted-by": "crossref",
"first-page": "159",
"journal-title": "RIC",
"key": "2022063000100525000_8.1.e001017.16",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031304",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.17"
},
{
"key": "2022063000100525000_8.1.e001017.18",
"unstructured": "Libster R , Pérez Marc G , Wappner D . Early high-titer plasma therapy to prevent severe Covid-19 in older adults 2021."
},
{
"DOI": "10.1001/jama.2020.1004",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.19"
},
{
"article-title": "Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience",
"author": "Joyner",
"journal-title": "medRxiv",
"key": "2022063000100525000_8.1.e001017.20",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-20241-w",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.21"
},
{
"DOI": "10.1136/bmj.m3939",
"article-title": "Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial)",
"author": "Agarwal",
"doi-asserted-by": "crossref",
"first-page": "m3939",
"journal-title": "BMJ",
"key": "2022063000100525000_8.1.e001017.22",
"volume": "151",
"year": "2020"
},
{
"DOI": "10.1097/BS9.0000000000000047",
"article-title": "Potential benefits, mechanisms, and uncertainties of convalescent plasma therapy for COVID-19",
"author": "Dai",
"doi-asserted-by": "crossref",
"first-page": "71",
"journal-title": "Blood Sci",
"key": "2022063000100525000_8.1.e001017.23",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2015.01.031",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.24"
},
{
"DOI": "10.1126/science.aaf1279",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.25"
},
{
"DOI": "10.1038/srep41926",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.26"
},
{
"DOI": "10.1371/journal.pone.0252306",
"article-title": "Feasibility of collecting and processing of COVID-19 convalescent plasma for treatment of COVID-19 in Uganda",
"author": "Muttamba",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "2022063000100525000_8.1.e001017.27",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1101/2020.10.29.20222067",
"doi-asserted-by": "crossref",
"key": "2022063000100525000_8.1.e001017.28",
"unstructured": "Muttamba W , Lusiba J , Namakula LO . Feasibility of collecting and processing of COVID-19 convalescent plasma for treatment of COVID-19 in Uganda 2020."
},
{
"key": "2022063000100525000_8.1.e001017.29",
"unstructured": "SARS-CoV-2 (COVID-19) antibody titer assay kit (Spike protein RBD) - ACROBiosystems. Available: https://www.acrobiosystems.com/P3184-SARS-CoV-2-%28COVID-19%29-antibody-titer-assay-kit-%28Spike-protein-RBD%29.html [Accessed 05 Sep 2020]."
},
{
"DOI": "10.1007/s11547-020-01200-3",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.30"
},
{
"DOI": "10.1002/jmv.25961",
"article-title": "Convalescent plasma transfusion for the treatment of COVID-19: systematic review",
"author": "Rajendran",
"doi-asserted-by": "crossref",
"first-page": "1475",
"journal-title": "J Med Virol",
"key": "2022063000100525000_8.1.e001017.31",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1004",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.32"
},
{
"DOI": "10.1007/s10096-004-1271-9",
"doi-asserted-by": "publisher",
"key": "2022063000100525000_8.1.e001017.33"
},
{
"DOI": "10.1016/j.transci.2020.102875",
"article-title": "Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study",
"author": "Abolghasemi",
"doi-asserted-by": "crossref",
"first-page": "102875",
"journal-title": "Transfus Apher Sci",
"key": "2022063000100525000_8.1.e001017.34",
"volume": "59",
"year": "2020"
},
{
"DOI": "10.3390/jcm9072268",
"article-title": "Neutralizing antibody production in asymptomatic and mild COVID-19 patients, in comparison with pneumonic COVID-19 patients",
"author": "Ko",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Med",
"key": "2022063000100525000_8.1.e001017.35",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1101/2020.07.01.20139857",
"doi-asserted-by": "crossref",
"key": "2022063000100525000_8.1.e001017.36",
"unstructured": "Gharbharan A , Jordans CC , GeurtsvanKessel C . Convalescent plasma for COVID-19. A randomized clinical trial. Medrxiv 2020.doi:10.1101/2020.07.01.20139857"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmjopenrespres.bmj.com/lookup/doi/10.1136/bmjresp-2021-001017"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Efficacy of convalescent plasma for treatment of COVID-19 in Uganda",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "8"
}