COVID-19 outcome is not affected by anti-CD20 or high-titer convalescent plasma in immunosuppressed patients
et al., Scientific Reports, doi:10.1038/s41598-023-48145-x, NCT04884477, Dec 2023
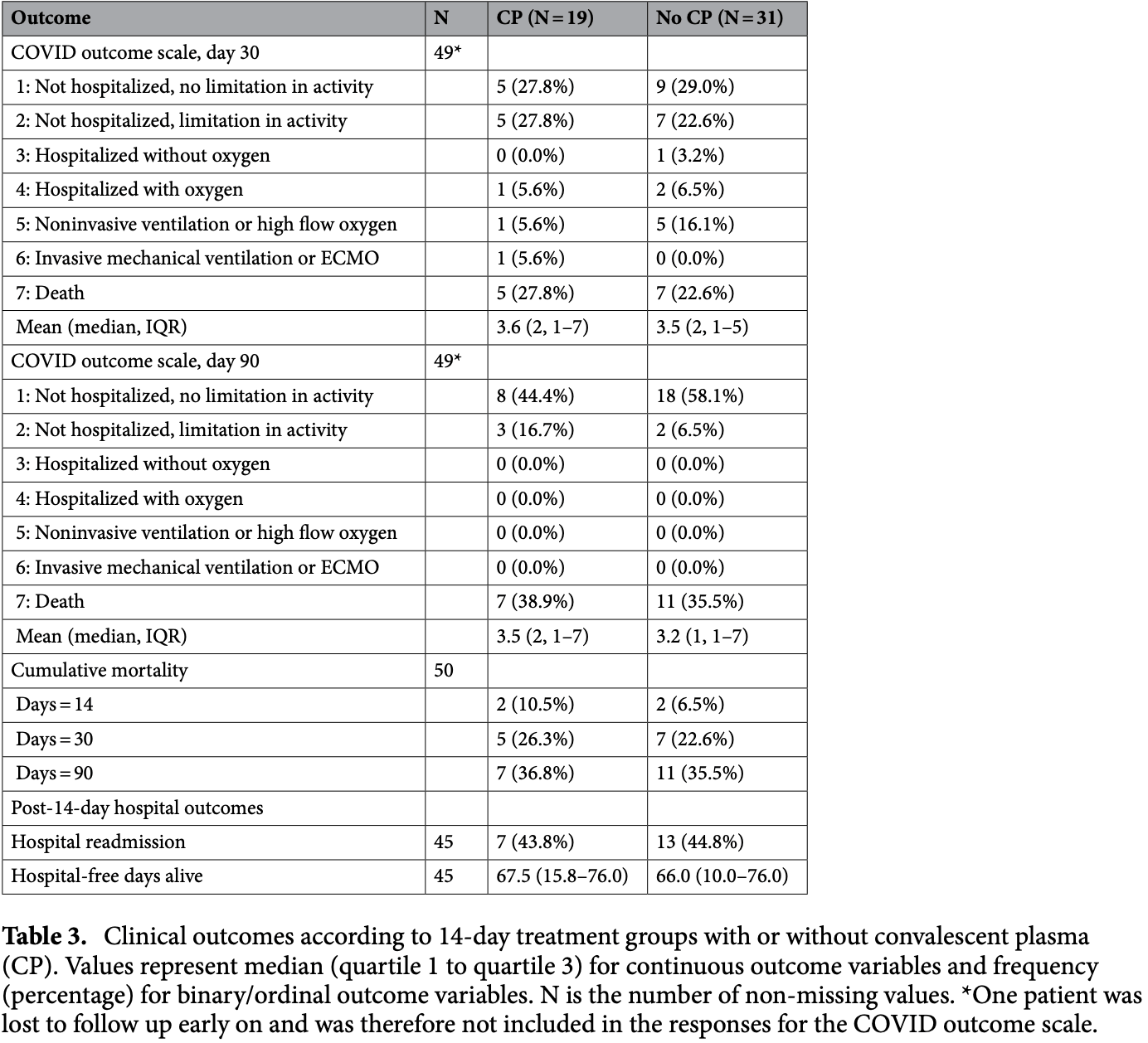
Retrospective 144 immunocompromised patients treated with anti-CD20 therapy prior to contracting COVID-19. Among 50 patients hospitalized within 14 days, administration of high-titer convalescent plasma in the first 14 days was not associated with improved outcomes.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 3.8% higher, RR 1.04, p = 1.00, treatment 7 of 19 (36.8%), control 11 of 31 (35.5%), day 90.
|
|
risk of death, 16.5% higher, RR 1.17, p = 1.00, treatment 5 of 19 (26.3%), control 7 of 31 (22.6%), day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kasten et al., 1 Dec 2023, retrospective, USA, peer-reviewed, median age 63.6, 13 authors, study period 1 September, 2020 - 28 February, 2021, trial NCT04884477 (history).
Contact: mkasten@mayo.edu, bauer.philippe@mayo.edu.
COVID-19 outcome is not affected by anti-CD20 or high-titer convalescent plasma in immunosuppressed patients
Scientific Reports, doi:10.1038/s41598-023-48145-x
The role of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescent plasma in the treatment of Coronavirus Disease 2019 (COVID-19) in immunosuppressed individuals remains controversial. We describe the course of COVID-19 in patients who had received anti-CD20 therapy within the 3 years prior to infection. We compared outcomes between those treated with and those not treated with high titer SARS-CoV2 convalescent plasma. We identified 144 adults treated at Mayo clinic sites who had received anti-CD20 therapies within a median of 5.9 months prior to the COVID-19 index date. About one-third (34.7%) were hospitalized within 14 days and nearly half (47.9%) within 90 days. COVID-19 directed therapy included anti-spike monoclonal antibodies (n = 30, 20.8%), and, among those hospitalized within 14 days (n = 50), remdesivir (n = 45, 90.0%), glucocorticoids (n = 36, 72.0%) and convalescent plasma (n = 24, 48.0%). The duration from receipt of last dose of anti-CD20 therapy did not correlate with outcomes. The overall 90-day mortality rate was 14.7%. Administration of convalescent plasma within 14 days of the COVID-19 diagnosis was not significantly associated with any study outcome. Further study of COVID-19 in CD20-depleted individuals is needed focusing on the early administration of new and potentially combination antiviral agents, associated or not with vaccine-boosted convalescent plasma.
Author contributions Authors M.J.K., B.D.L., Z.A.Y., J.C.O., M.J.E., D.J.I., R.R.R., A.S.S., P.V., P.R.B. were all involved with conception and design of the study.Authors M.J.K., A.P., Z.A.Y., J.C.O., M.J.E., P.M.F., R.O., R.R.R., A.S.S., P.V., P.R.B. were all involved in data acquisition.Authors M.J.K., B.D.L., A.P., Z.A.Y., J.C.O., M.J.E., D.J.I., P.M.F., R.O., R.R.R., A.S.S., P.V., P.R.B. were all involved in data interpetation.All Authors M.J.K., B.D.L., A.P., Z.A.Y., J.C.O., M.J.E., D.J.I., P.M.F., R.O., R.R.R., A.S.S., P.V., P.R.B. were involved with drafting and approving the manuscript.
Competing interests
Additional information
Supplementary Information The online version contains supplementary material available at https:// doi. org/
References
Avouac, COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: A cohort study, Lancet Rheumatol, doi:10.1016/S2665-9913(21)00059-X
Biernat, Early administration of convalescent plasma improves survival in patients with hematological malignancies and COVID-19, Viruses, doi:10.3390/v13030436
Booth, Key findings from the UKCCMP cohort of 877 patients with haematological malignancy and COVID-19: Disease control as an important factor relative to recent chemotherapy or anti-CD20 therapy, Br. J. Haematol
Cano, Impact of corticosteroids in coronavirus disease 2019 outcomes: Systematic review and meta-analysis, Chest, doi:10.1016/j.chest.2020.10.054
Chen, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2029849
Cook, Third primary SARS-CoV-2 mRNA vaccines enhance antibody responses in most patients with haematological malignancies, Nat Commun, doi:10.1038/s41467-022-34657-z
Core, R: A language and environment for statistical computing
Ferrari, Caprioli, Weber, Rambaldi, Lussana, Convalescent hyperimmune plasma for chemo-immunotherapy induced immunodeficiency in COVID-19 patients with hematological malignancies, Leuk. Lymphom, doi:10.1080/10428194.2021.1872070
Ford, Successful treatment of prolonged, severe coronavirus disease 2019 lower respiratory tract disease in a B cell acute lymphoblastic leukemia patient with an extended course of remdesivir and nirmatrelvir/ritonavir, Clin. Infect. Dis
Gerber, Protracted SARS-CoV-2 pneumonia with rituximab treatment: About two cases, J. Med. Virol, doi:10.1002/jmv.26921
Gottlieb, Early remdesivir to prevent progression to severe covid-19 in outpatients, N. Engl. J. Med, doi:10.1056/NEJMoa2116846
Hamilton, Lee, Arnold, Lilford, Hemming, Is convalescent plasma futile in COVID-19? A Bayesian reanalysis of the RECOVERY randomized controlled trial, Int. J. Infect. Dis, doi:10.1016/j.ijid.2021.06.034
Hammond, Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2118542
Harvey, Association of SARS-CoV-2 seropositive antibody test with risk of future infection, JAMA Intern. Med, doi:10.1001/jamainternmed.2021.0366
Hensley, Intractable coronavirus disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-cell therapy recipient: A case study, Clin. Infect. Dis, doi:10.1093/cid/ciab072
Herishanu, Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia, Blood, doi:10.1182/blood.2021011568
Hueso, Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19, Blood, doi:10.1182/blood.2020008423
Hughes, Clinical illness with viable severe acute respiratory coronavirus virus 2 (SARS-CoV-2) virus presenting 72 days after infection in an immunocompromised patient, Infect. Control Hosp. Epidemiol, doi:10.1017/ice.2021.120
Investigators, Interleukin-6 receptor antagonists in critically ill patients with covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2100433
Janiaud, Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: A systematic review and meta-analysis, JAMA, doi:10.1001/jama.2021.2747
Jeny, Correspondence on "glucocorticoid-induced relapse of COVID-19 in a patient with sarcoidosis, Ann. Rheum. Dis, doi:10.1136/annrheumdis-2020-218957
Joyner, Convalescent plasma antibody levels and the risk of death from covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2031893
Kalil, Baricitinib plus remdesivir for hospitalized adults with covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2031994
Kasten, Abbvie, Amgen, Lilly, Myers Squib et al., Grants from Nference, Inc and the MITRE corporation for COVID-19 research unrelated to the present work
Kenig, Ishay, Kharouf, Rubin, Treatment of B-cell depleted COVID-19 patients with convalescent plasma and plasmabased products, Clin. Immunol, doi:10.1016/j.clim.2021.108723
Kim, An, Kim, Hwang, Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis, PLoS Med, doi:10.1371/journal.pmed.1003501
Kim, Clinical characteristics and mortality of patients with hematologic malignancies and COVID-19: A systematic review, Eur. Rev. Med. Pharmacol. Sci, doi:10.26355/eurrev_202011_23852
Lancman, Mascarenhas, Bar-Natan, Severe COVID-19 virus reactivation following treatment for B cell acute lymphoblastic leukemia, J. Hematol. Oncol, doi:10.1186/s13045-020-00968-1
Levavi, Lancman, Gabrilove, Impact of rituximab on COVID-19 outcomes, Ann. Hematol
Levine, COVID-19 convalescent plasma outpatient therapy to prevent outpatient hospitalization: A meta-analysis of individual participant data from five randomized trials, Clin. Infect. Dis
Mckay, Rituximab infusion timing, cumulative dose, and hospitalization for COVID-19 in persons with multiple sclerosis in Sweden, JAMA. Netw. Open, doi:10.1001/jamanetworkopen
Misset, Convalescent plasma for Covid-19-induced ARDS in mechanically ventilated patients, NEJM
O'horo, Outcomes of COVID-19 with the Mayo Clinic model of care and research, Mayo Clin. Proc, doi:10.1016/j.mayocp.2020.12.006
O'shaughnessy, Revised Letter of Authorization
Razonable, research grants (funds to the institution) from Gilead, Regeneron and Roche; member of the Data and Safety Monitoring Board (Novartis) and Endpoint Adjudication Committee (Allovir); member of the Board of Directors
Ripoll, Vaccine-boosted convalescent plasma therapy for patients with immunosuppression and COVID-19, Blood Adv
Rubio-Rivas, WHO ordinal scale and inflammation risk categories in COVID-19. Comparative study of the severity scales, J. Gen. Intern. Med, doi:10.1007/s11606-022-07511-7
Rutherford, Risk factors for severe outcomes in patients with systemic vasculitis and COVID-19: A binational, registrybased cohort study, Arthrit. Rheumatol, doi:10.1002/art.41728
Senefeld, COVID-19 convalescent plasma for the treatment of immunocompromised patients: A systematic review and meta-analysis, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2022.50647
Senefeld, Use of convalescent plasma in COVID-19 patients with immunosuppression, Transfusion, doi:10.1111/trf.16525
Sharma, Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study, Lancet Haematol, doi:10.1016/S2352-3026(20)30429-4
Smith, Gonzales, Li, Langer-Gould, Analysis of rituximab use, time between rituximab and SARS-CoV-2 vaccination, and COVID-19 hospitalization or death in patients with multiple sclerosis, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2022.48664
Sun, Pleyer, Wiestner, COVID-19 vaccines for patients with haematological conditions, Lancet Haematol, doi:10.1016/S2352-3026(21)00073-9
Thompson, Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19, JAMA Oncol, doi:10.1001/jamaoncol.2021.1799
Tong, Core outcome measures for trials in people with coronavirus disease 2019: Respiratory failure, multiorgan failure, shortness of breath, and recovery, Crit. Care Med, doi:10.1097/CCM.0000000000004817
Trottier, Dual antiviral therapy for persistent coronavirus disease 2019 and associated organizing pneumonia in an immunocompromised host, Clin. Infect. Dis
Vardhana, Wolchok, The many faces of the anti-COVID immune response, J. Exp. Med, doi:10.1084/jem.20200678
Vijenthira, Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients, Blood, doi:10.1182/blood.2020008824
Von Elm, (STROBE) statement: Guidelines for reporting observational studies
Weinreich, REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2035002
DOI record:
{
"DOI": "10.1038/s41598-023-48145-x",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-023-48145-x",
"abstract": "<jats:title>Abstract</jats:title><jats:p>The role of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescent plasma in the treatment of Coronavirus Disease 2019 (COVID-19) in immunosuppressed individuals remains controversial. We describe the course of COVID-19 in patients who had received anti-CD20 therapy within the 3 years prior to infection. We compared outcomes between those treated with and those not treated with high titer SARS-CoV2 convalescent plasma. We identified 144 adults treated at Mayo clinic sites who had received anti-CD20 therapies within a median of 5.9 months prior to the COVID-19 index date. About one-third (34.7%) were hospitalized within 14 days and nearly half (47.9%) within 90 days. COVID-19 directed therapy included anti-spike monoclonal antibodies (n = 30, 20.8%), and, among those hospitalized within 14 days (n = 50), remdesivir (n = 45, 90.0%), glucocorticoids (n = 36, 72.0%) and convalescent plasma (n = 24, 48.0%). The duration from receipt of last dose of anti-CD20 therapy did not correlate with outcomes. The overall 90-day mortality rate was 14.7%. Administration of convalescent plasma within 14 days of the COVID-19 diagnosis was not significantly associated with any study outcome. Further study of COVID-19 in CD20-depleted individuals is needed focusing on the early administration of new and potentially combination antiviral agents, associated or not with vaccine-boosted convalescent plasma.</jats:p>",
"alternative-id": [
"48145"
],
"article-number": "21249",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "22 September 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "22 November 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "1 December 2023"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "Mary J. Kasten, M.D.: Stockholder: Abbott Laboratories, AbbVie, Amgen, Eli Lilly, Bristol Myers Squib, Amgen, Merck, GlaxoSmithKline, Baxter International, Pfizer, CVS Health Corporation, Viatris Inc, Medtronic, Zimmer Biomet, Masimo Corp, Takeda Pharmaceutical\nJohn C. O’Horo, M.D., M.P.H.: Grants from Nference, Inc and the MITRE corporation for COVID-19 research unrelated to the present work.\nRaymund R. Razonable, M.D.: research grants (funds to the institution) from Gilead, Regeneron and Roche; member of the Data and Safety Monitoring Board (Novartis) and Endpoint Adjudication Committee (Allovir); member of the Board of Directors, American Society of Transplantation\nPaschalis Vergidis, M.D.: research support from Ansun, Cidara, Scynexis and consultant AbbVie with all fees being paid to Mayo Clinic\nAll other authors declare no conflicts of interests."
}
],
"author": [
{
"affiliation": [],
"family": "Kasten",
"given": "Mary J.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lahr",
"given": "Brian D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parisapogu",
"given": "Anusha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yetmar",
"given": "Zachary A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O’Horo",
"given": "John C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Orenstein",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno Franco",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Razonable",
"given": "Raymund R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vergidis",
"given": "Paschalis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Aditya S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Enzler",
"given": "Mark J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Inwards",
"given": "David J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bauer",
"given": "Philippe R.",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T18:02:17Z",
"timestamp": 1701453737000
},
"deposited": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T18:11:18Z",
"timestamp": 1701454278000
},
"funder": [
{
"DOI": "10.13039/100000871",
"doi-asserted-by": "crossref",
"name": "Mayo Clinic"
}
],
"indexed": {
"date-parts": [
[
2023,
12,
2
]
],
"date-time": "2023-12-02T00:56:52Z",
"timestamp": 1701478612692
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
12,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T00:00:00Z",
"timestamp": 1701388800000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-023-48145-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-48145-x",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-48145-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2023,
12,
1
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
1
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "48145_CR1",
"unstructured": "Hammond, J. et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N. Engl. J. Med. 386, 1397–1408. https://doi.org/10.1056/NEJMoa2118542 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116846",
"author": "RL Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "305",
"journal-title": "N. Engl. J. Med.",
"key": "48145_CR2",
"unstructured": "Gottlieb, R. L. et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. N. Engl. J. Med. 386, 305–315. https://doi.org/10.1056/NEJMoa2116846 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2021436",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N. Engl. J. Med.",
"key": "48145_CR3",
"unstructured": "RECOVERY Collaborative Group et al. Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 384, 693–704. https://doi.org/10.1056/NEJMoa2021436 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17022",
"author": "The Writing Committee for the REMAP-CAP Investigators",
"doi-asserted-by": "publisher",
"first-page": "1317",
"journal-title": "JAMA",
"key": "48145_CR4",
"unstructured": "The Writing Committee for the REMAP-CAP Investigators. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: The remap-cap COVID-19 corticosteroid domain randomized clinical trial. JAMA 324, 1317–1329. https://doi.org/10.1001/jama.2020.17022 (2020).",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.10.054",
"author": "EJ Cano",
"doi-asserted-by": "publisher",
"first-page": "1019",
"journal-title": "Chest",
"key": "48145_CR5",
"unstructured": "Cano, E. J. et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: Systematic review and meta-analysis. Chest 159, 1019–1040. https://doi.org/10.1016/j.chest.2020.10.054 (2021).",
"volume": "159",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2100433",
"author": "REMAP-CAP Investigators",
"doi-asserted-by": "publisher",
"first-page": "1491",
"journal-title": "N. Engl. J. Med.",
"key": "48145_CR6",
"unstructured": "REMAP-CAP Investigators et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N. Engl. J. Med. 384, 1491–1502. https://doi.org/10.1056/NEJMoa2100433 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031994",
"author": "AC Kalil",
"doi-asserted-by": "publisher",
"first-page": "795",
"journal-title": "N. Engl. J. Med.",
"key": "48145_CR7",
"unstructured": "Kalil, A. C. et al. Baricitinib plus remdesivir for hospitalized adults with covid-19. N. Engl. J. Med. 384, 795–807. https://doi.org/10.1056/NEJMoa2031994 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2029849",
"author": "P Chen",
"doi-asserted-by": "publisher",
"first-page": "229",
"journal-title": "N. Engl. J. Med.",
"key": "48145_CR8",
"unstructured": "Chen, P. et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with covid-19. N. Engl. J. Med. 384, 229–237. https://doi.org/10.1056/NEJMoa2029849 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"author": "DM Weinreich",
"doi-asserted-by": "publisher",
"first-page": "238",
"journal-title": "N. Engl. J. Med.",
"key": "48145_CR9",
"unstructured": "Weinreich, D. M. et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N. Engl. J. Med. 384, 238–251. https://doi.org/10.1056/NEJMoa2035002 (2021).",
"volume": "384",
"year": "2021"
},
{
"key": "48145_CR10",
"unstructured": "COVID-19 Treatment Guidelines Panel. Statement on Tixagevimab Plus Cilgavimab (Evusheld) as Pre-Exposure Prophylaxis of COVID-19. Coronavirus Disease (COVID-19) Treatment Guidelines (2023, accessed 4 Apr 2023). https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-evusheld/."
},
{
"DOI": "10.1001/jama.2021.2747",
"author": "P Janiaud",
"doi-asserted-by": "publisher",
"first-page": "1185",
"journal-title": "JAMA",
"key": "48145_CR11",
"unstructured": "Janiaud, P. et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: A systematic review and meta-analysis. JAMA 325, 1185–1195. https://doi.org/10.1001/jama.2021.2747 (2021).",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031893",
"author": "MJ Joyner",
"doi-asserted-by": "publisher",
"first-page": "1015",
"journal-title": "N. Engl. J. Med.",
"key": "48145_CR12",
"unstructured": "Joyner, M. J. et al. Convalescent plasma antibody levels and the risk of death from covid-19. N. Engl. J. Med. 384, 1015–1027. https://doi.org/10.1056/NEJMoa2031893 (2021).",
"volume": "384",
"year": "2021"
},
{
"key": "48145_CR13",
"unstructured": "FDA News Release. FDA issues emergency use authorization for convalescent plasma as potential promising COVID–19 treatment, another achievement in administration’s fight against pandemic (2023, accessed 4 Apr 2023). https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment."
},
{
"key": "48145_CR14",
"unstructured": "FDA In Brief: FDA updates emergency use authorization for COVID-19 convalescent plasma to reflect new data (2023, accessed 4 Apr 2023). https://www.fda.gov/news-events/fda-brief/fda-brief-fda-updates-emergency-use-authorization-covid-19-convalescent-plasma-reflect-new-data."
},
{
"key": "48145_CR15",
"unstructured": "COVID-19 Treatment Guidelines Panel. COVID-19 Treatment Guidelines: COVID-19 Convalescent Plasma (2023, accessed 15 Sep 2023). https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/covid-19-convalescent-plasma/."
},
{
"key": "48145_CR16",
"unstructured": "Centers for Disease Control and Prevention. COVID Data Tracker, COVID-19 Vaccinations in the United States (2023, accessed 3 Mar 2023). https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-booster-percent-pop5."
},
{
"DOI": "10.1016/S2352-3026(21)00073-9",
"author": "C Sun",
"doi-asserted-by": "publisher",
"first-page": "e312",
"journal-title": "Lancet Haematol.",
"key": "48145_CR17",
"unstructured": "Sun, C., Pleyer, C. & Wiestner, A. COVID-19 vaccines for patients with haematological conditions. Lancet Haematol. 8, e312–e314. https://doi.org/10.1016/S2352-3026(21)00073-9 (2021).",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1182/blood.2021011568",
"author": "Y Herishanu",
"doi-asserted-by": "publisher",
"first-page": "3165",
"journal-title": "Blood",
"key": "48145_CR18",
"unstructured": "Herishanu, Y. et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 137, 3165–3173. https://doi.org/10.1182/blood.2021011568 (2021).",
"volume": "137",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2021.0366",
"author": "RA Harvey",
"doi-asserted-by": "publisher",
"first-page": "672",
"journal-title": "JAMA Intern. Med.",
"key": "48145_CR19",
"unstructured": "Harvey, R. A. et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern. Med. 181, 672–679. https://doi.org/10.1001/jamainternmed.2021.0366 (2021).",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.3390/v13030436",
"author": "MM Biernat",
"doi-asserted-by": "publisher",
"first-page": "436",
"journal-title": "Viruses",
"key": "48145_CR20",
"unstructured": "Biernat, M. M. et al. Early administration of convalescent plasma improves survival in patients with hematological malignancies and COVID-19. Viruses 13, 436. https://doi.org/10.3390/v13030436 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/S2665-9913(21)00059-X",
"author": "J Avouac",
"doi-asserted-by": "publisher",
"first-page": "e419",
"journal-title": "Lancet Rheumatol.",
"key": "48145_CR21",
"unstructured": "Avouac, J. et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: A cohort study. Lancet Rheumatol. 3, e419–e426. https://doi.org/10.1016/S2665-9913(21)00059-X (2021).",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab072",
"author": "MK Hensley",
"doi-asserted-by": "publisher",
"first-page": "e815",
"journal-title": "Clin. Infect. Dis.",
"key": "48145_CR22",
"unstructured": "Hensley, M. K. et al. Intractable coronavirus disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-cell therapy recipient: A case study. Clin. Infect. Dis. 73, e815–e821. https://doi.org/10.1093/cid/ciab072 (2021).",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1186/s13045-020-00968-1",
"author": "G Lancman",
"doi-asserted-by": "publisher",
"first-page": "131",
"journal-title": "J. Hematol. Oncol.",
"key": "48145_CR23",
"unstructured": "Lancman, G., Mascarenhas, J. & Bar-Natan, M. Severe COVID-19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. J. Hematol. Oncol. 13, 131. https://doi.org/10.1186/s13045-020-00968-1 (2020).",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1017/ice.2021.120",
"author": "CM Hughes",
"doi-asserted-by": "publisher",
"first-page": "820",
"journal-title": "Infect. Control Hosp. Epidemiol.",
"key": "48145_CR24",
"unstructured": "Hughes, C. M. et al. Clinical illness with viable severe acute respiratory coronavirus virus 2 (SARS-CoV-2) virus presenting 72 days after infection in an immunocompromised patient. Infect. Control Hosp. Epidemiol. 43, 820–822. https://doi.org/10.1017/ice.2021.120 (2022).",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.1084/jem.20200678",
"author": "SA Vardhana",
"doi-asserted-by": "publisher",
"first-page": "e20200678",
"journal-title": "J. Exp. Med.",
"key": "48145_CR25",
"unstructured": "Vardhana, S. A. & Wolchok, J. D. The many faces of the anti-COVID immune response. J. Exp. Med. 217, e20200678. https://doi.org/10.1084/jem.20200678 (2020).",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1002/art.41728",
"author": "MA Rutherford",
"doi-asserted-by": "publisher",
"first-page": "1713",
"journal-title": "Arthrit. Rheumatol.",
"key": "48145_CR26",
"unstructured": "Rutherford, M. A. et al. Risk factors for severe outcomes in patients with systemic vasculitis and COVID-19: A binational, registry-based cohort study. Arthrit. Rheumatol. 73, 1713–1719. https://doi.org/10.1002/art.41728 (2021).",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.26355/eurrev_202011_23852",
"author": "JS Kim",
"doi-asserted-by": "publisher",
"first-page": "11926",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "48145_CR27",
"unstructured": "Kim, J. S. et al. Clinical characteristics and mortality of patients with hematologic malignancies and COVID-19: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 24, 11926–11933. https://doi.org/10.26355/eurrev_202011_23852 (2020).",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1182/blood.2020008824",
"author": "A Vijenthira",
"doi-asserted-by": "publisher",
"first-page": "2881",
"journal-title": "Blood",
"key": "48145_CR28",
"unstructured": "Vijenthira, A. et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 136, 2881–2892. https://doi.org/10.1182/blood.2020008824 (2020).",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1016/S2352-3026(20)30429-4",
"author": "A Sharma",
"doi-asserted-by": "publisher",
"first-page": "e185",
"journal-title": "Lancet Haematol.",
"key": "48145_CR29",
"unstructured": "Sharma, A. et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol. 8, e185–e193. https://doi.org/10.1016/S2352-3026(20)30429-4 (2021).",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.clim.2021.108723",
"author": "A Kenig",
"doi-asserted-by": "publisher",
"first-page": "108723",
"journal-title": "Clin. Immunol.",
"key": "48145_CR30",
"unstructured": "Kenig, A., Ishay, Y., Kharouf, F. & Rubin, L. Treatment of B-cell depleted COVID-19 patients with convalescent plasma and plasma-based products. Clin. Immunol. 227, 108723. https://doi.org/10.1016/j.clim.2021.108723 (2021).",
"volume": "227",
"year": "2021"
},
{
"DOI": "10.1182/blood.2020008423",
"author": "T Hueso",
"doi-asserted-by": "publisher",
"first-page": "2290",
"journal-title": "Blood",
"key": "48145_CR31",
"unstructured": "Hueso, T. et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood 136, 2290–2295. https://doi.org/10.1182/blood.2020008423 (2020).",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1080/10428194.2021.1872070",
"author": "S Ferrari",
"doi-asserted-by": "publisher",
"first-page": "1490",
"journal-title": "Leuk. Lymphom.",
"key": "48145_CR32",
"unstructured": "Ferrari, S., Caprioli, C., Weber, A., Rambaldi, A. & Lussana, F. Convalescent hyperimmune plasma for chemo-immunotherapy induced immunodeficiency in COVID-19 patients with hematological malignancies. Leuk. Lymphom. 62, 1490–1496. https://doi.org/10.1080/10428194.2021.1872070 (2021).",
"volume": "62",
"year": "2021"
},
{
"DOI": "10.1001/jamaoncol.2021.1799",
"author": "MA Thompson",
"doi-asserted-by": "publisher",
"first-page": "1167",
"journal-title": "JAMA Oncol.",
"key": "48145_CR33",
"unstructured": "Thompson, M. A. et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol. 7, 1167–1175. https://doi.org/10.1001/jamaoncol.2021.1799 (2021).",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26921",
"author": "V Gerber",
"doi-asserted-by": "publisher",
"first-page": "4141",
"journal-title": "J. Med. Virol.",
"key": "48145_CR34",
"unstructured": "Gerber, V. et al. Protracted SARS-CoV-2 pneumonia with rituximab treatment: About two cases. J. Med. Virol. 93, 4141–4144. https://doi.org/10.1002/jmv.26921 (2021).",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.06.034",
"author": "FW Hamilton",
"doi-asserted-by": "publisher",
"first-page": "114",
"journal-title": "Int. J. Infect. Dis.",
"key": "48145_CR35",
"unstructured": "Hamilton, F. W., Lee, T., Arnold, D. T., Lilford, R. & Hemming, K. Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial. Int. J. Infect. Dis. 109, 114–117. https://doi.org/10.1016/j.ijid.2021.06.034 (2021).",
"volume": "109",
"year": "2021"
},
{
"DOI": "10.1111/trf.16525",
"author": "JW Senefeld",
"doi-asserted-by": "publisher",
"first-page": "2503",
"journal-title": "Transfusion",
"key": "48145_CR36",
"unstructured": "Senefeld, J. W. et al. Use of convalescent plasma in COVID-19 patients with immunosuppression. Transfusion 61, 2503–2511. https://doi.org/10.1111/trf.16525 (2021).",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2022.50647",
"author": "JW Senefeld",
"doi-asserted-by": "publisher",
"first-page": "e2250647",
"journal-title": "JAMA Netw. Open",
"key": "48145_CR37",
"unstructured": "Senefeld, J. W. et al. COVID-19 convalescent plasma for the treatment of immunocompromised patients: A systematic review and meta-analysis. JAMA Netw. Open 6, e2250647. https://doi.org/10.1001/jamanetworkopen.2022.50647 (2023).",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1097/EDE.0b013e3181577654",
"doi-asserted-by": "crossref",
"key": "48145_CR38",
"unstructured": "von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Equator Network (2023, accessed 18 Jan 2023). https://www.equator-network.org/reporting-guidelines/strobe/."
},
{
"DOI": "10.1097/CCM.0000000000004817",
"author": "A Tong",
"doi-asserted-by": "publisher",
"first-page": "503",
"journal-title": "Crit. Care Med.",
"key": "48145_CR39",
"unstructured": "Tong, A. et al. Core outcome measures for trials in people with coronavirus disease 2019: Respiratory failure, multiorgan failure, shortness of breath, and recovery. Crit. Care Med. 49, 503–516. https://doi.org/10.1097/CCM.0000000000004817 (2021).",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1016/j.mayocp.2020.12.006",
"author": "JC O'Horo",
"doi-asserted-by": "publisher",
"first-page": "601",
"journal-title": "Mayo Clin. Proc.",
"key": "48145_CR40",
"unstructured": "O’Horo, J. C. et al. Outcomes of COVID-19 with the Mayo Clinic model of care and research. Mayo Clin. Proc. 96, 601–618. https://doi.org/10.1016/j.mayocp.2020.12.006 (2021).",
"volume": "96",
"year": "2021"
},
{
"key": "48145_CR41",
"unstructured": "O’Shaughnessy, J. Revised Letter of Authorization (2021, accessed 9 Nov 2023). https://www.fda.gov/media/141477/download."
},
{
"DOI": "10.1136/annrheumdis-2020-218957",
"author": "F Jeny",
"doi-asserted-by": "publisher",
"first-page": "e241",
"journal-title": "Ann. Rheum. Dis.",
"key": "48145_CR42",
"unstructured": "Jeny, F. et al. Correspondence on “glucocorticoid-induced relapse of COVID-19 in a patient with sarcoidosis”. Ann. Rheum. Dis. 81, e241. https://doi.org/10.1136/annrheumdis-2020-218957 (2022).",
"volume": "81",
"year": "2022"
},
{
"DOI": "10.1371/journal.pmed.1003501",
"author": "MS Kim",
"doi-asserted-by": "publisher",
"first-page": "e1003501",
"journal-title": "PLoS Med.",
"key": "48145_CR43",
"unstructured": "Kim, M. S., An, M. H., Kim, W. J. & Hwang, T. H. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: A systematic review and network meta-analysis. PLoS Med. 17, e1003501. https://doi.org/10.1371/journal.pmed.1003501 (2020).",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1007/s11606-022-07511-7",
"author": "M Rubio-Rivas",
"doi-asserted-by": "publisher",
"first-page": "1980",
"journal-title": "J. Gen. Intern. Med.",
"key": "48145_CR44",
"unstructured": "Rubio-Rivas, M. et al. WHO ordinal scale and inflammation risk categories in COVID-19. Comparative study of the severity scales. J. Gen. Intern. Med. 37, 1980–1987. https://doi.org/10.1007/s11606-022-07511-7 (2022).",
"volume": "37",
"year": "2022"
},
{
"key": "48145_CR45",
"unstructured": "R Core Team. R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria (2020, accessed 18 Jan 2023). https://www.R-project.org/."
},
{
"DOI": "10.1001/jamanetworkopen.2022.48664",
"author": "JB Smith",
"doi-asserted-by": "publisher",
"first-page": "e2248664",
"journal-title": "JAMA Netw. Open",
"key": "48145_CR46",
"unstructured": "Smith, J. B., Gonzales, E. G., Li, B. H. & Langer-Gould, A. Analysis of rituximab use, time between rituximab and SARS-CoV-2 vaccination, and COVID-19 hospitalization or death in patients with multiple sclerosis. JAMA Netw. Open 5, e2248664. https://doi.org/10.1001/jamanetworkopen.2022.48664 (2022).",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1007/s00277-021-04662-1",
"author": "H Levavi",
"doi-asserted-by": "publisher",
"first-page": "2805",
"journal-title": "Ann. Hematol.",
"key": "48145_CR47",
"unstructured": "Levavi, H., Lancman, G. & Gabrilove, J. Impact of rituximab on COVID-19 outcomes. Ann. Hematol. 100, 2805–2812 (2021).",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.1111/bjh.17937",
"author": "S Booth",
"doi-asserted-by": "publisher",
"first-page": "892",
"journal-title": "Br. J. Haematol.",
"key": "48145_CR48",
"unstructured": "Booth, S. et al. Key findings from the UKCCMP cohort of 877 patients with haematological malignancy and COVID-19: Disease control as an important factor relative to recent chemotherapy or anti-CD20 therapy. Br. J. Haematol. 196, 892–901 (2021).",
"volume": "196",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen",
"author": "KA McKay",
"doi-asserted-by": "publisher",
"first-page": "e2136697",
"journal-title": "JAMA. Netw. Open.",
"key": "48145_CR49",
"unstructured": "McKay, K. A. et al. Rituximab infusion timing, cumulative dose, and hospitalization for COVID-19 in persons with multiple sclerosis in Sweden. JAMA. Netw. Open. 4, e2136697. https://doi.org/10.1001/jamanetworkopen (2021).",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2209502",
"author": "B Misset",
"doi-asserted-by": "publisher",
"first-page": "1590",
"journal-title": "NEJM",
"key": "48145_CR50",
"unstructured": "Misset, B. et al. Convalescent plasma for Covid-19-induced ARDS in mechanically ventilated patients. NEJM 389, 1590–1600 (2023).",
"volume": "389",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciad088",
"author": "AC Levine",
"doi-asserted-by": "publisher",
"first-page": "2077",
"journal-title": "Clin. Infect. Dis.",
"key": "48145_CR51",
"unstructured": "Levine, A. C. et al. COVID-19 convalescent plasma outpatient therapy to prevent outpatient hospitalization: A meta-analysis of individual participant data from five randomized trials. Clin. Infect. Dis. 76, 2077–2086 (2023).",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1182/bloodadvances.2022008932",
"author": "JG Ripoll",
"doi-asserted-by": "publisher",
"first-page": "5951",
"journal-title": "Blood Adv.",
"key": "48145_CR52",
"unstructured": "Ripoll, J. G. et al. Vaccine-boosted convalescent plasma therapy for patients with immunosuppression and COVID-19. Blood Adv. 6, 5951–5955 (2022).",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-34657-z",
"author": "LB Cook",
"doi-asserted-by": "publisher",
"first-page": "6922",
"journal-title": "Nat Commun.",
"key": "48145_CR53",
"unstructured": "Cook, L. B. et al. Third primary SARS-CoV-2 mRNA vaccines enhance antibody responses in most patients with haematological malignancies. Nat Commun. 13, 6922. https://doi.org/10.1038/s41467-022-34657-z (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac847",
"author": "CA Trottier",
"doi-asserted-by": "publisher",
"first-page": "923",
"journal-title": "Clin. Infect. Dis.",
"key": "48145_CR54",
"unstructured": "Trottier, C. A. et al. Dual antiviral therapy for persistent coronavirus disease 2019 and associated organizing pneumonia in an immunocompromised host. Clin. Infect. Dis. 76, 923–925 (2023).",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac868",
"author": "ES Ford",
"doi-asserted-by": "publisher",
"first-page": "926",
"journal-title": "Clin. Infect. Dis.",
"key": "48145_CR55",
"unstructured": "Ford, E. S. et al. Successful treatment of prolonged, severe coronavirus disease 2019 lower respiratory tract disease in a B cell acute lymphoblastic leukemia patient with an extended course of remdesivir and nirmatrelvir/ritonavir. Clin. Infect. Dis. 76, 926–929 (2023).",
"volume": "76",
"year": "2023"
}
],
"reference-count": 55,
"references-count": 55,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-023-48145-x"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "COVID-19 outcome is not affected by anti-CD20 or high-titer convalescent plasma in immunosuppressed patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}