Convalescence plasma treatment of COVID-19: results from a prematurely terminated randomized controlled open-label study in Southern Sweden
et al., BMC Research Notes, doi:10.1186/s13104-021-05847-7, COP20, NCT04600440, Dec 2021
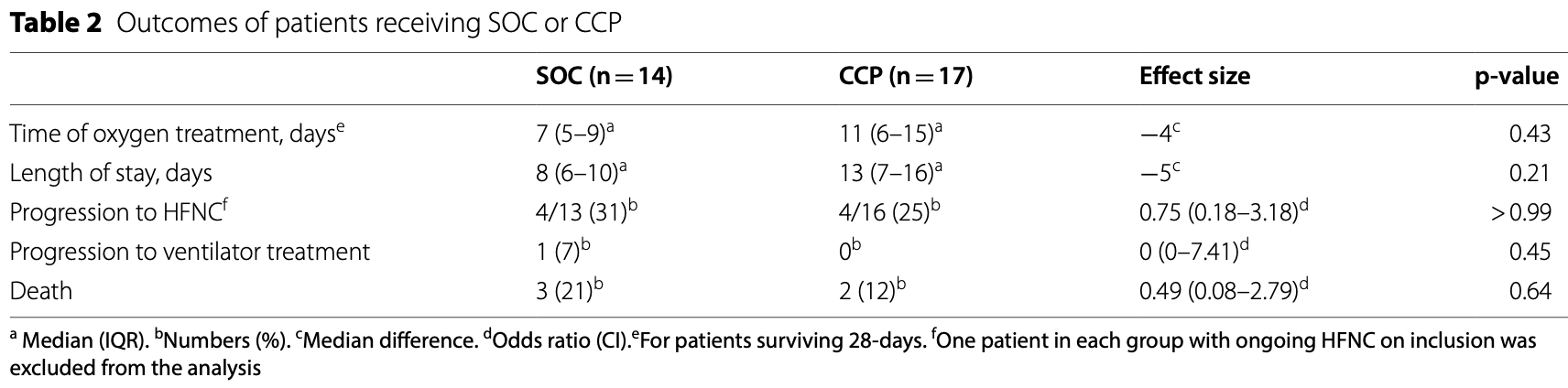
RCT 31 hospitalized patients requiring supplemental oxygen in Sweden, showing no significant difference in outcomes with convalescent plasma.
|
risk of death, 45.1% lower, RR 0.55, p = 0.64, treatment 2 of 17 (11.8%), control 3 of 14 (21.4%), NNT 10.
|
|
risk of mechanical ventilation, 68.9% lower, RR 0.31, p = 0.45, treatment 0 of 17 (0.0%), control 1 of 14 (7.1%), NNT 14, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of progression, 18.8% lower, RR 0.81, p = 1.00, treatment 4 of 16 (25.0%), control 4 of 13 (30.8%), NNT 17, progression to HFNC.
|
|
oxygen time, 57.1% higher, relative time 1.57, p = 0.43, treatment 17, control 14.
|
|
hospitalization time, 62.5% higher, relative time 1.62, p = 0.21, treatment 17, control 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Holm et al., 4 Dec 2021, Randomized Controlled Trial, Sweden, peer-reviewed, 14 authors, study period June 2020 - January 2021, average treatment delay 7.0 days, trial NCT04600440 (history) (COP20).
Contact: karin.holm@med.lu.se (corresponding author).
Convalescence plasma treatment of COVID-19: results from a prematurely terminated randomized controlled open-label study in Southern Sweden
BMC Research Notes, doi:10.1186/s13104-021-05847-7
Objective: Convalescent plasma has been tried as therapy for various viral infections. Early observational studies of convalescent plasma treatment for hospitalized COVID-19 patients were promising, but randomized controlled studies were lacking at the time. The objective of this study was to investigate if convalescent plasma is beneficial to hospitalized patients with COVID-19. Results: Hospitalized patients with confirmed COVID-19 and an oxygen saturation below 94% were randomized 1:1 to receive convalescent plasma in addition to standard of care or standard of care only. The primary outcome was number of days of oxygen treatment to keep saturation above 93% within 28 days from inclusion. The study was prematurely terminated when thirty-one of 100 intended patients had been included. The median time of oxygen treatment among survivors was 11 days (IQR 6-15) for the convalescent plasma group and 7 days (IQR 5-9) for the standard of care group (p = 0.4, median difference -4). Two patients in the convalescent plasma group and three patients in the standard of care group died (p = 0.64, OR 0.49, 95% CI 0.08-2.79). Thus no significant differences were observed between the groups.
Abbreviations
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s13104-021-05847-7. Additional file 1. SARS-CoV2 antibody detection and microneutralization assay. Authors' contributions KH: study design, inclusion of patients and donors, data collection and analysis, manuscript writing. MNL: study design, transfusion medicine expertise, donor screening and responsible for plasma collection and plasma analysis, data analysis and manuscript writing. JKK: study design, transfusion medicine expertise, data analysis and critical revision of the manuscript. OL, CW, JÖ: inclusion of patients, data collection and analysis, critical revision of the manuscript. NF: study design, inclusion of patients and donors, data collection. BB: study design, responsible for virology sampling and data analysis, critical revision of the manuscript. ER, AKÖ, JWB, MF: responsible for measuring
Declarations Ethics approval and consent to participate The Swedish Ethical Review Authority approved the study (Reference Number #2020-01744, 2020-03595). Written informed consent was required from donors and patients on inclusion.
Consent to publish Not applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ (Clin Res ed), doi:10.1136/bmj.m3939
Arabi, Hajeer, Luke, Raviprakash, Balkhy et al., Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia, Emerg Infect Dis, doi:10.3201/eid2209.151164
Betrains, Godinas, Woei, Rosseels, Van Herck et al., Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies, Br J Haematol, doi:10.1111/bjh.17266
Casadevall, Pirofski, The convalescent sera option for containing COVID-19, J Clin Invest, doi:10.1172/JCI138003
Cheng, Wong, Soo, Wong, Lee et al., Use of convalescent plasma therapy in SARS patients in Hong Kong, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-004-1271-9
Duan, Liu, Li, Zhang, Yu et al., Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci, doi:10.1073/pnas.2004168117
Hegerova, Gooley, Sweerus, Maree, Bailey et al., Use of convalescent plasma in hospitalized patients with COVID-19: case series, Blood, doi:10.1182/blood.2020006964
Horby, Estcourt, Peto, Emberson, Staplin et al., Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, doi:10.1101/2021.03.09.21252736
Hueso, Pouderoux, Péré, Beaumont, Raillon et al., Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19, Blood, doi:10.1182/blood.2020008423
Hung, To, Lee, Lee, Chan et al., Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection, Clin Infect Dis, doi:10.1093/cid/ciq106
Joyner, Wright, Fairweather, Senefeld, Bruno et al., Early safety indicators of COVID-19 convalescent plasma in 5000 patients, J Clin Investig, doi:10.1172/JCI140200
Libster, Marc, Wappner, Coviello, Bianchi et al., Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N Engl J Med, doi:10.1056/NEJMoa2033700
Mair-Jenkins, Saavedra-Campos, Baillie, Cleary, Khaw et al., The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis, J Infect Dis, doi:10.1093/infdis/jiu396
Rodionov, Biener, Spieth, Achleitner, Hölig et al., Potential benefit of convalescent plasma transfusions in immunocompromised patients with COVID-19, Lancet Microbe, doi:10.1016/S2666-5247(21)00030-6
Rojas, Rodríguez, Monsalve, Acosta-Ampudia, Camacho et al., Convalescent plasma in Covid-19: possible mechanisms of action, Autoimmun Rev, doi:10.1016/j.autrev.2020.102554
Sahr, Ansumana, Massaquoi, Idriss, Sesay et al., Evaluation of convalescent whole blood for treating Ebola Virus Disease in Freetown, Sierra Leone, J Infect, doi:10.1016/j.jinf.2016.11.009
Senefeld, Klassen, Ford, Wiggins, Bostrom et al., Therapeutic use of convalescent plasma in COVID-19 patients with immunodeficiency, medRxiv, doi:10.1101/2020.11.08.20224790
Simonovich, Pratx, Scibona, Beruto, Vallone et al., A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N Engl J Med, doi:10.1056/NEJMoa2031304
Zhou, Zhong, Guan, Treatment with convalescent plasma for influenza A (H5N1) Infection, N Engl J Med, doi:10.1056/NEJMc070359
DOI record:
{
"DOI": "10.1186/s13104-021-05847-7",
"ISSN": [
"1756-0500"
],
"URL": "http://dx.doi.org/10.1186/s13104-021-05847-7",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Objective</jats:title>\n <jats:p>Convalescent plasma has been tried as therapy for various viral infections. Early observational studies of convalescent plasma treatment for hospitalized COVID-19 patients were promising, but randomized controlled studies were lacking at the time. The objective of this study was to investigate if convalescent plasma is beneficial to hospitalized patients with COVID-19.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Hospitalized patients with confirmed COVID-19 and an oxygen saturation below 94% were randomized 1:1 to receive convalescent plasma in addition to standard of care or standard of care only. The primary outcome was number of days of oxygen treatment to keep saturation above 93% within 28 days from inclusion. The study was prematurely terminated when thirty-one of 100 intended patients had been included. The median time of oxygen treatment among survivors was 11 days (IQR 6–15) for the convalescent plasma group and 7 days (IQR 5–9) for the standard of care group (<jats:italic>p</jats:italic> = 0.4, median difference -4). Two patients in the convalescent plasma group and three patients in the standard of care group died (<jats:italic>p</jats:italic> = 0.64, OR 0.49, 95% CI 0.08–2.79). Thus no significant differences were observed between the groups.</jats:p>\n <jats:p><jats:italic>Trial registration</jats:italic> ClinicalTrials NCT04600440, retrospectively registered Oct 23, 2020.</jats:p>\n </jats:sec>",
"alternative-id": [
"5847"
],
"article-number": "440",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "2 July 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 November 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "4 December 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The Swedish Ethical Review Authority approved the study (Reference Number #2020-01744, 2020-03595). Written informed consent was required from donors and patients on inclusion."
},
{
"group": {
"label": "Consent to publish",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8289-5949",
"affiliation": [],
"authenticated-orcid": false,
"family": "Holm",
"given": "Karin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lundgren",
"given": "Maria N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kjeldsen-Kragh",
"given": "Jens",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ljungquist",
"given": "Oskar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Böttiger",
"given": "Blenda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wikén",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Öberg",
"given": "Jonas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernström",
"given": "Nils",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosendal",
"given": "Ebba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Överby",
"given": "Anna K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wigren Byström",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Forsell",
"given": "Mattias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landin-Olsson",
"given": "Mona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rasmussen",
"given": "Magnus",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04600440",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "BMC Research Notes",
"container-title-short": "BMC Res Notes",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
4
]
],
"date-time": "2021-12-04T09:02:48Z",
"timestamp": 1638608568000
},
"deposited": {
"date-parts": [
[
2021,
12,
4
]
],
"date-time": "2021-12-04T21:41:30Z",
"timestamp": 1638654090000
},
"funder": [
{
"DOI": "10.13039/501100009780",
"doi-asserted-by": "publisher",
"name": "region skåne"
}
],
"indexed": {
"date-parts": [
[
2023,
1,
14
]
],
"date-time": "2023-01-14T10:53:58Z",
"timestamp": 1673693638449
},
"is-referenced-by-count": 12,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 3,
"start": {
"date-parts": [
[
2021,
12,
4
]
],
"date-time": "2021-12-04T00:00:00Z",
"timestamp": 1638576000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13104-021-05847-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13104-021-05847-7/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13104-021-05847-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2021,
12
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
4
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.autrev.2020.102554",
"author": "M Rojas",
"doi-asserted-by": "publisher",
"issue": "7",
"journal-title": "Autoimmun Rev",
"key": "5847_CR1",
"unstructured": "Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020;19(7): 102554. https://doi.org/10.1016/j.autrev.2020.102554.",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciq106",
"author": "IF Hung",
"doi-asserted-by": "publisher",
"first-page": "447",
"issue": "4",
"journal-title": "Clin Infect Dis",
"key": "5847_CR2",
"unstructured": "Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–56. https://doi.org/10.1093/cid/ciq106.",
"volume": "52",
"year": "2011"
},
{
"DOI": "10.1093/infdis/jiu396",
"author": "J Mair-Jenkins",
"doi-asserted-by": "publisher",
"first-page": "80",
"issue": "1",
"journal-title": "J Infect Dis",
"key": "5847_CR3",
"unstructured": "Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F-M, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2014;211(1):80–90. https://doi.org/10.1093/infdis/jiu396.",
"volume": "211",
"year": "2014"
},
{
"DOI": "10.1056/NEJMc070359",
"author": "B Zhou",
"doi-asserted-by": "publisher",
"first-page": "1450",
"issue": "14",
"journal-title": "N Engl J Med",
"key": "5847_CR4",
"unstructured": "Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) Infection. N Engl J Med. 2007;357(14):1450–1. https://doi.org/10.1056/NEJMc070359.",
"volume": "357",
"year": "2007"
},
{
"DOI": "10.1007/s10096-004-1271-9",
"author": "Y Cheng",
"doi-asserted-by": "publisher",
"first-page": "44",
"issue": "1",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "5847_CR5",
"unstructured": "Cheng Y, Wong R, Soo YOY, Wong WS, Lee CK, Ng MHL, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–6. https://doi.org/10.1007/s10096-004-1271-9.",
"volume": "24",
"year": "2005"
},
{
"DOI": "10.3201/eid2209.151164",
"author": "YM Arabi",
"doi-asserted-by": "publisher",
"first-page": "1554",
"issue": "9",
"journal-title": "Emerg Infect Dis",
"key": "5847_CR6",
"unstructured": "Arabi YM, Hajeer AH, Luke T, Raviprakash K, Balkhy H, Johani S, et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22(9):1554–61. https://doi.org/10.3201/eid2209.151164.",
"volume": "22",
"year": "2016"
},
{
"DOI": "10.1016/j.jinf.2016.11.009",
"author": "F Sahr",
"doi-asserted-by": "publisher",
"first-page": "302",
"issue": "3",
"journal-title": "J Infect",
"key": "5847_CR7",
"unstructured": "Sahr F, Ansumana R, Massaquoi TA, Idriss BR, Sesay FR, Lamin JM, et al. Evaluation of convalescent whole blood for treating Ebola Virus Disease in Freetown, Sierra Leone. J Infect. 2017;74(3):302–9. https://doi.org/10.1016/j.jinf.2016.11.009.",
"volume": "74",
"year": "2017"
},
{
"DOI": "10.1172/JCI140200",
"author": "MJ Joyner",
"doi-asserted-by": "publisher",
"first-page": "4791",
"issue": "9",
"journal-title": "J Clin Investig",
"key": "5847_CR8",
"unstructured": "Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Investig. 2020;130(9):4791–7. https://doi.org/10.1172/JCI140200.",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1182/blood.2020006964",
"author": "L Hegerova",
"doi-asserted-by": "publisher",
"first-page": "759",
"issue": "6",
"journal-title": "Blood",
"key": "5847_CR9",
"unstructured": "Hegerova L, Gooley TA, Sweerus KA, Maree C, Bailey N, Bailey M, et al. Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood. 2020;136(6):759–62. https://doi.org/10.1182/blood.2020006964.",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1172/JCI138003",
"author": "A Casadevall",
"doi-asserted-by": "publisher",
"first-page": "1545",
"issue": "4",
"journal-title": "J Clin Invest",
"key": "5847_CR10",
"unstructured": "Casadevall A, Pirofski L-A. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–8. https://doi.org/10.1172/JCI138003.",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2004168117",
"author": "K Duan",
"doi-asserted-by": "publisher",
"first-page": "9490",
"issue": "17",
"journal-title": "Proc Natl Acad Sci",
"key": "5847_CR11",
"unstructured": "Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117(17):9490–6. https://doi.org/10.1073/pnas.2004168117.",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1101/2021.03.09.21252736",
"doi-asserted-by": "publisher",
"key": "5847_CR12",
"unstructured": "Horby PW, Estcourt L, Peto L, Emberson JR, Staplin N, Spata E, et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021:2021.03.09.21252736. https://doi.org/10.1101/2021.03.09.21252736"
},
{
"DOI": "10.1056/NEJMoa2031304",
"author": "VA Simonovich",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "5847_CR13",
"unstructured": "Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vazquez C, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020. https://doi.org/10.1056/NEJMoa2031304.",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2033700",
"author": "R Libster",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "5847_CR14",
"unstructured": "Libster R, Perez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2033700.",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m3939",
"author": "A Agarwal",
"doi-asserted-by": "publisher",
"journal-title": "BMJ (Clin Res ed)",
"key": "5847_CR15",
"unstructured": "Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ (Clin Res ed). 2020;371: m3939. https://doi.org/10.1136/bmj.m3939.",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1101/2020.11.08.20224790",
"doi-asserted-by": "publisher",
"key": "5847_CR16",
"unstructured": "Senefeld JW, Klassen SA, Ford SK, Wiggins CC, Bostrom BC, Thompson MA, et al. Therapeutic use of convalescent plasma in COVID-19 patients with immunodeficiency. medRxiv. 2020:2020.11.08.20224790. https://doi.org/10.1101/2020.11.08.20224790"
},
{
"DOI": "10.1016/S2666-5247(21)00030-6",
"author": "RN Rodionov",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "Lancet Microbe",
"key": "5847_CR17",
"unstructured": "Rodionov RN, Biener A, Spieth P, Achleitner M, Hölig K, Aringer M, et al. Potential benefit of convalescent plasma transfusions in immunocompromised patients with COVID-19. Lancet Microbe. 2021;2(4): e138. https://doi.org/10.1016/S2666-5247(21)00030-6.",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1111/bjh.17266",
"author": "A Betrains",
"doi-asserted-by": "publisher",
"journal-title": "Br J Haematol",
"key": "5847_CR18",
"unstructured": "Betrains A, Godinas L, Woei AJF, Rosseels W, Van Herck Y, Lorent N, et al. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol. 2020. https://doi.org/10.1111/bjh.17266.",
"year": "2020"
},
{
"DOI": "10.1182/blood.2020008423",
"author": "T Hueso",
"doi-asserted-by": "publisher",
"first-page": "2290",
"issue": "20",
"journal-title": "Blood",
"key": "5847_CR19",
"unstructured": "Hueso T, Pouderoux C, Péré H, Beaumont AL, Raillon LA, Ader F, et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136(20):2290–5. https://doi.org/10.1182/blood.2020008423.",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1016/s1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "5847_CR20",
"unstructured": "WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–7. https://doi.org/10.1016/s1473-3099(20)30483-7."
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcresnotes.biomedcentral.com/articles/10.1186/s13104-021-05847-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Biochemistry, Genetics and Molecular Biology",
"General Medicine"
],
"subtitle": [],
"title": "Convalescence plasma treatment of COVID-19: results from a prematurely terminated randomized controlled open-label study in Southern Sweden",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "14"
}