Novel Tissue Factor Inhibition for Thromboprophylaxis in COVID-19: Primary Results of the ASPEN-COVID-19 Trial
et al., Arteriosclerosis, Thrombosis, and Vascular Biology, doi:10.1161/ATVBAHA.122.318748, ASPEN-COVID-19, NCT04655586, Aug 2023
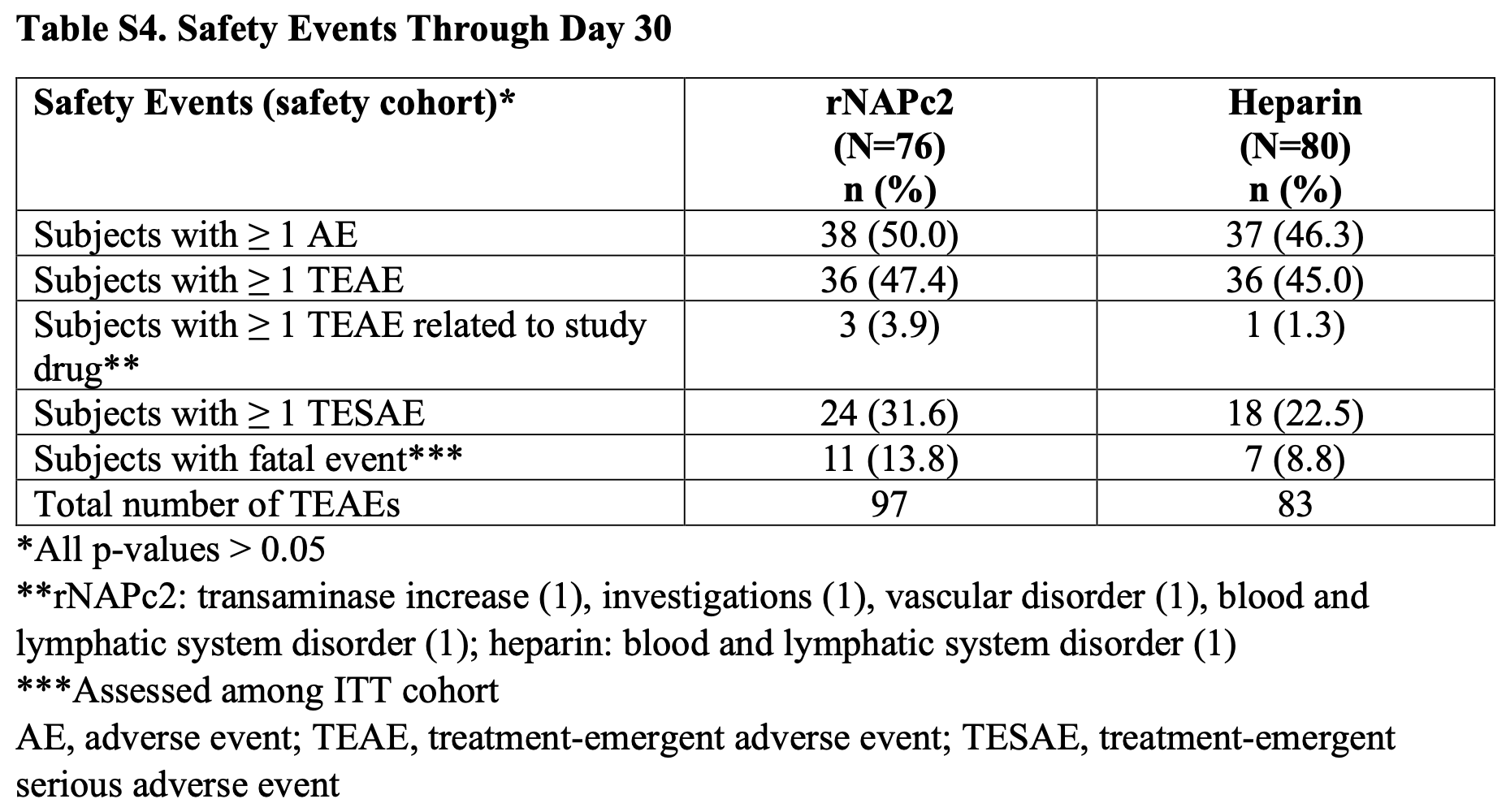
RCT 160 hospitalized COVID-19 patients showing no significant improvements with rNAPc2 (recombinant nematode anticoagulant protein c2) vs. heparin.
|
risk of death, 65.4% higher, RR 1.65, p = 0.32, treatment 11 of 76 (14.5%), control 7 of 80 (8.8%), Table S4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hess et al., 31 Aug 2023, Randomized Controlled Trial, multiple countries, peer-reviewed, median age 54.0, 22 authors, this trial compares with another treatment - results may be better when compared to placebo, trial NCT04655586 (history) (ASPEN-COVID-19).
Contact: connie.hess@cpcmed.org.
Novel Tissue Factor Inhibition for Thromboprophylaxis in COVID-19: Primary Results of the ASPEN-COVID-19 Trial
Arteriosclerosis, Thrombosis, and Vascular Biology, doi:10.1161/atvbaha.122.318748
BACKGROUND: Thrombo-inflammation is central to COVID-19-associated coagulopathy. TF (tissue factor), a driver of disordered coagulation and inflammation in viral infections, may be a therapeutic target in COVID-19. The safety and efficacy of the novel TF inhibitor rNAPc2 (recombinant nematode anticoagulation protein c2) in COVID-19 are unknown. METHODS: ASPEN-COVID-19 was an international, randomized, open-label, active comparator clinical trial with blinded end point adjudication. Hospitalized patients with COVID-19 and elevated D-dimer levels were randomized 1:1:2 to lower or higher dose rNAPc2 on days 1, 3, and 5 followed by heparin on day 8 or to heparin per local standard of care. In comparisons of the pooled rNAPc2 versus heparin groups, the primary safety end point was major or nonmajor clinically relevant International Society of Thrombosis and Haemostasis bleeding through day 8. The primary efficacy end point was proportional change in D-dimer concentration from baseline to day 8, or discharge if before day 8. Patients were followed for 30 days. RESULTS: Among 160 randomized patients, median age was 54 years, 43.1% were female, and 38.8% had severe baseline COVID-19. There were no significant differences between rNAPc2 and heparin in bleeding or other safety events. Overall, median change in D-dimer was -16.8% (interquartile range, -45.7 to 36.8; P=0.41) with rNAPc2 treatment and -11.2% (-36.0 to 34.4; P=0.91) with heparin (P intergroup =0.47). In prespecified analyses, in severely ill patients, D-dimer levels tended to increase more within the heparin (median, 29.0% [-14.9 to 145.2]; P=0.02) than the rNAPc2 group (median, 25.9% [-49.1 to 136.4]; P=0.14; P intergroup =0.96); in mildly ill patients, D-dimer levels were reduced within each group with a numerically greater reduction with rNAPc2 versus heparin (rNAPc2 median, -32.7% [-44.7 to 4.3]; P=0.007 and heparin median, -16.8% [-36.0 to 0.5]; P=0.008, P intergroup =0.34). CONCLUSIONS: rNAPc2 treatment in hospitalized patients with COVID-19 was well tolerated without excess bleeding or serious adverse events but did not significantly reduce D-dimer more than heparin at day 8.
ARTICLE INFORMATION Received
Sources of Funding Assessing Safety, Hospitalization, and Efficacy of rNAPc2 in COVID-19 (AS-PEN-COVID-19) was supported by a research grant from ARCA biopharma. Colorado Prevention Center Clinical Research held the database and performed all analyses, and the decision to submit this article for publication was made by the ASPEN-COVID-19 Executive Committee.
Supplemental Material Tables S1-S5 Figure S1
References
Ackermann, Verleden, Kuehnel, Haverich, Welte et al., Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19, N Engl J Med, doi:10.1056/NEJMoa2015432
Altman, Berning, Saxon, Adamek, Wagner et al., Myocardial injury and altered gene expression associated with SARS-CoV-2 infection or mRNA vaccination, JACC Basic Transl Sci, doi:10.1016/j.jacbts.2022.08.005
Antoniak, Mackman, Multiple roles of the coagulation protease cascade during virus infection, Blood, doi:10.1182/blood-2013-09-526277
Bachler, Bosch, Sturzel, Hell, Giebl et al., Impaired fibrinolysis in critically ill COVID-19 patients, Br J Anaesth, doi:10.1016/j.bja.2020.12.010
Becker, COVID-19 update: Covid-19-associated coagulopathy, J Thromb Thrombolysis, doi:10.1007/s11239-020-02134-3
Beigel, Tomashek, Dodd, Mehta, Zingman et al., ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 -final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bergum, Cruikshank, Maki, Kelly, Ruf et al., Role of zymogen and activated factor X as scaffolds for the inhibition of the blood coagulation factor VIIa-tissue factor complex by recombinant nematode anticoagulant protein c2, J Biol Chem, doi:10.1074/jbc.M009116200
Blasi, Meijenfeldt, Adelmeijer, Calvo, Ibañez et al., In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation, J Thromb Haemost, doi:10.1111/jth.15043
Bonaventura, Vecchie, Dagna, Martinod, Dixon et al., Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19, Nat Rev Immunol, doi:10.1038/s41577-021-00536-9
Burn, Duarte-Salles, Fernandez-Bertolin, Reyes, Kostka et al., Venous or arterial thrombosis and deaths among COVID-19 cases: a European network cohort study, Lancet Infect Dis
Campbell, Hisada, Denorme, Grover, Bouck et al., Comparison of the coagulopathies associated with COVID-19 and sepsis, Res Pract Thromb Haemost, doi:10.1002/rth2.12525
Canzano, Brambilla, Porro, Cosentino, Tortorici et al., Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients, JACC Basic Transl Sci, doi:10.1016/j.jacbts.2020.12.009
Dutch, Coalition, Dctc), Early effects of unfractionated heparin on clinical and radiological signs and D-dimer levels in patients with COVID-19 associated pulmonary embolism: an observational cohort study, Thromb Res, doi:10.1016/j.thromres.2021.01.023
Farkouh, Stone, Bagiella, Moreno, Nadkarni et al., Anticoagulation in patients with COVID-19: JACC review topic of the week, J Am Coll Cardiol, doi:10.1016/j.jacc.2021.12.023
Francischetti, Toomer, Zhang, Jani, Siddiqui et al., Upregulation of pulmonary tissue factor, loss of thrombomodulin and immunothrombosis in SARS-CoV-2 infection, EClini-calMedicine, doi:10.1016/j.eclinm.2021.101069
Garlapati, Molitor, Michna, Harms, Finger et al., Targeting myeloid cell coagulation signaling blocks MAP kinase/TGF-beta1 driven fibrotic remodeling in ischemic heart failure, J Clin Invest, doi:10.1172/JCI156436
Geisbert, Daddario-Dicaprio, Geisbert, Young, Formenty et al., Marburg virus Angola infection of rhesus macaques: pathogenesis and treatment with recombinant nematode anticoagulant protein c2, J Infect Dis, doi:10.1086/520608
Geisbert, Hensley, Jahrling, Larsen, Geisbert et al., Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys, Lancet, doi:10.1016/S0140-6736(03)15012-X
Giugliano, Wiviott, Stone, Simon, Schweiger et al., ANTHEM-TIMI-32 Investigators. Recombinant nematode anticoagulant protein c2 in patients with non-ST-segment elevation acute coronary syndrome: the ANTHEM-TIMI-32 trial, J Am Coll Cardiol, doi:10.1016/j.jacc.2007.02.065
Goligher, Bradbury, Mcverry, Lawler, Berger et al., Therapeutic anticoagulation with heparin in critically ill patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2103417
Gordon, Mouncey, Al-Beidh, Rowan, Nichol et al., REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2100433
Grosse, Grosse, Salzer, Dunser, Motz et al., Analysis of cardiopulmonary findings in COVID-19 fatalities: high incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia, Cardiovasc Pathol, doi:10.1016/j.carpath.2020.107263
Guervilly, Bonifay, Burtey, Sabatier, Cauchois et al., Dissemination of extreme levels of extracellular vesicles: tissue factor activity in patients with severe COVID-19, Blood Adv, doi:10.1182/bloodadvances.2020003308
Gungor, Atici, Baycan, Alici, Ozturk et al., Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: a systematic review and meta-analysis, Am J Emerg Med, doi:10.1016/j.ajem.2020.09.018
Hess, Capell, Bristow, Ruf, Szarek et al., Rationale and design of a study to assess the safety and efficacy of rNAPc2 in COVID-19: the Phase 2b ASPEN-COVID-19 trial, Am Heart J, doi:10.1016/j.ahj.2021.12.010
Hollerbach, Muller-Calleja, Pedrosa, Canisius, Sprinzl et al., Pathogenic lipidbinding antiphospholipid antibodies are associated with severity of COVID-19, J Thromb Haemost, doi:10.1111/jth.15455
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Joyner, Carter, Senefeld, Klassen, Mills et al., Convalescent plasma antibody levels and the risk of death from Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031893
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Lawler, Goligher, Berger, Neal, Mcverry et al., Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2105911
Lee, Vlasuk, Recombinant nematode anticoagulant protein c2 and other inhibitors targeting blood coagulation factor VIIa/tissue factor, J Intern Med, doi:10.1046/j.1365-2796.2003.01224.x
Liang, Kerschen, Hernandez, Basu, Zogg et al., EPCR-dependent PAR2 activation by the blood coagulation initiation complex regulates LPS-triggered interferon responses in mice, Blood, doi:10.1182/blood-2014-11-610717
Mackman, Grover, Antoniak, Tissue factor expression, extracellular vesicles, and thrombosis after infection with the respiratory viruses influenza A virus and coronavirus, J Thromb Haemost, doi:10.1111/jth.15509
Mast, Tissue factor pathway inhibitor: multiple anticoagulant activities for a single protein, Arterioscler Thromb Vasc Biol, doi:10.1161/ATVBAHA.115.305996
Moons, Peters, Bijsterveld, Piek, Prins et al., Recombinant nematode anticoagulant protein c2, an inhibitor of the tissue factor/factor VIIa complex, in patients undergoing elective coronary angioplasty, J Am Coll Cardiol, doi:10.1016/s0735-1097(03)00478-9
Muller-Calleja, Hollerbach, Royce, Ritter, Pedrosa et al., Lipid presentation by the protein C receptor links coagulation with autoimmunity, Science, doi:10.1126/science.abc0956
Nielsen, Rollins-Raval, Raval, Thachil, Is it hyperfibrinolysis or fibrinolytic shutdown in severe COVID-19?, Arterioscler Thromb Vasc Biol, doi:10.1016/j.thromres.2021.12.012
Rosell, Havervall, Meijenfeldt, Hisada, Aguilera et al., Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality-brief report, Arterioscler Thromb Vasc Biol, doi:10.1161/ATVBAHA.120.315547
Schulman, Kearon, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients, J Thromb Haemost, doi:10.1111/j.1538-7836.2005.01204.x
Spyropoulos, Goldin, Giannis, Diab, Wang et al., Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial, JAMA Intern Med, doi:10.1001/jamainternmed.2021.6203
Sutherland, Ruf, Pryzdial, Tissue factor and glycoprotein C on herpes simplex virus type 1 are protease-activated receptor 2 cofactors that enhance infection, Blood, doi:10.1182/blood-2011-08-376814
Sutherland, Simon, Shanina, Horwitz, Ruf et al., Virus envelope tissue factor promotes infection in mice, J Thromb Haemost, doi:10.1111/jth.14389
Talasaz, Sadeghipour, Kakavand, Aghakouchakzadeh, Kordzadeh-Kermani et al., Recent randomized trials of antithrombotic therapy for patients with COVID-19: JACC state-of-the-art review, J Am Coll Cardiol, doi:10.1016/j.jacc.2021.02.035
Uusitalo-Jarvinen, Kurokawa, Mueller, Andrade-Gordon, Friedlander et al., Role of protease activated receptor 1 and 2 signaling in hypoxia-induced angiogenesis, Arterioscler Thromb Vasc Biol, doi:10.1161/ATVBAHA.107.142539
Vasquez-Bonilla, Orozco, Argueta, Sierra, Zambrano et al., A review of the main histopathological findings in the Coronavirus Disease 2019 (COVID-19), Hum Pathol, doi:10.1016/j.humpath.2020.07.023
Wichmann, Sperhake, Lutgehetmann, Steurer, Edler et al., Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study, Ann Intern Med, doi:10.7326/M20-2003
Zelaya, Rothmeier, Ruf, Tissue factor at the crossroad of coagulation and cell signaling, J Thromb Haemost, doi:10.1111/jth.14246
Zuo, Estes, Ali, Gandhi, Yalavarthi et al., Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19, Sci Transl Med, doi:10.1126/scitranslmed.abd3876
DOI record:
{
"DOI": "10.1161/atvbaha.122.318748",
"ISSN": [
"1079-5642",
"1524-4636"
],
"URL": "http://dx.doi.org/10.1161/ATVBAHA.122.318748",
"abstract": "<jats:sec>\n <jats:title>BACKGROUND:</jats:title>\n <jats:p>Thrombo-inflammation is central to COVID-19-associated coagulopathy. TF (tissue factor), a driver of disordered coagulation and inflammation in viral infections, may be a therapeutic target in COVID-19. The safety and efficacy of the novel TF inhibitor rNAPc2 (recombinant nematode anticoagulation protein c2) in COVID-19 are unknown.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>METHODS:</jats:title>\n <jats:p>ASPEN-COVID-19 was an international, randomized, open-label, active comparator clinical trial with blinded end point adjudication. Hospitalized patients with COVID-19 and elevated D-dimer levels were randomized 1:1:2 to lower or higher dose rNAPc2 on days 1, 3, and 5 followed by heparin on day 8 or to heparin per local standard of care. In comparisons of the pooled rNAPc2 versus heparin groups, the primary safety end point was major or nonmajor clinically relevant International Society of Thrombosis and Haemostasis bleeding through day 8. The primary efficacy end point was proportional change in D-dimer concentration from baseline to day 8, or discharge if before day 8. Patients were followed for 30 days.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>RESULTS:</jats:title>\n <jats:p>\n Among 160 randomized patients, median age was 54 years, 43.1% were female, and 38.8% had severe baseline COVID-19. There were no significant differences between rNAPc2 and heparin in bleeding or other safety events. Overall, median change in D-dimer was −16.8% (interquartile range, −45.7 to 36.8;\n <jats:italic>P</jats:italic>\n =0.41) with rNAPc2 treatment and −11.2% (−36.0 to 34.4;\n <jats:italic>P</jats:italic>\n =0.91) with heparin (\n <jats:italic>P</jats:italic>\n <jats:sub>intergroup</jats:sub>\n =0.47). In prespecified analyses, in severely ill patients, D-dimer levels tended to increase more within the heparin (median, 29.0% [−14.9 to 145.2];\n <jats:italic>P</jats:italic>\n =0.02) than the rNAPc2 group (median, 25.9% [−49.1 to 136.4];\n <jats:italic>P</jats:italic>\n =0.14;\n <jats:italic>P</jats:italic>\n <jats:sub>intergroup</jats:sub>\n =0.96); in mildly ill patients, D-dimer levels were reduced within each group with a numerically greater reduction with rNAPc2 versus heparin (rNAPc2 median, −32.7% [−44.7 to 4.3];\n <jats:italic>P</jats:italic>\n =0.007 and heparin median, −16.8% [−36.0 to 0.5];\n <jats:italic>P</jats:italic>\n =0.008,\n <jats:italic>P</jats:italic>\n <jats:sub>intergroup</jats:sub>\n =0.34).\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>CONCLUSIONS:</jats:title>\n <jats:p>rNAPc2 treatment in hospitalized patients with COVID-19 was well tolerated without excess bleeding or serious adverse events but did not significantly reduce D-dimer more than heparin at day 8.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>REGISTRATION:</jats:title>\n <jats:p>\n URL:\n <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://www.clinicaltrials.gov\">https://www.clinicaltrials.gov</jats:ext-link>\n ; Unique identifier: NCT04655586.\n </jats:p>\n </jats:sec>",
"alternative-id": [
"10.1161/ATVBAHA.122.318748"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-11-09"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-05-26"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-06-29"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5830-0511",
"affiliation": [
{
"name": "Department of Medicine (C.N.H., J.H., M.S., W.H.C., M.R.B., M.P.B.), University of Colorado, Aurora."
},
{
"name": "CPC Clinical Research, Aurora, CO (C.N.H., J.H., M.R.N., M.S., W.H.C., R.G., M.P.B.)."
}
],
"authenticated-orcid": true,
"family": "Hess",
"given": "Connie N.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-3413-7836",
"affiliation": [
{
"name": "Department of Medicine (C.N.H., J.H., M.S., W.H.C., M.R.B., M.P.B.), University of Colorado, Aurora."
},
{
"name": "CPC Clinical Research, Aurora, CO (C.N.H., J.H., M.R.N., M.S., W.H.C., R.G., M.P.B.)."
}
],
"authenticated-orcid": true,
"family": "Hsia",
"given": "Judith",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2071-5479",
"affiliation": [
{
"name": "ARCA biopharma, Westminster, CO (I.A.C., D.M., C.A.G., S.P.H., T.K., M.R.B.)."
}
],
"authenticated-orcid": true,
"family": "Carroll",
"given": "Ian A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery (M.R.N., R.G.), University of Colorado, Aurora."
},
{
"name": "CPC Clinical Research, Aurora, CO (C.N.H., J.H., M.R.N., M.S., W.H.C., R.G., M.P.B.)."
}
],
"family": "Nehler",
"given": "Mark R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6064-2166",
"affiliation": [
{
"name": "Johannes Gutenberg University Medical Center, Mainz, Germany (W.R.)."
},
{
"name": "Scripps Research, La Jolla, CA (W.R.)."
}
],
"authenticated-orcid": true,
"family": "Ruf",
"given": "Wolfram",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9589-5382",
"affiliation": [
{
"name": "Brigham and Women’s Hospital, Harvard Medical School, Boston, MA (D.A.M.)."
}
],
"authenticated-orcid": true,
"family": "Morrow",
"given": "David A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9680-3689",
"affiliation": [
{
"name": "Instituto do Coracao (InCor), Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, SP, Brazil (J.C.N.)."
}
],
"authenticated-orcid": true,
"family": "Nicolau",
"given": "Jose C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Israelita Albert Einstein, Sao Paulo, Brazil (O.B.)."
}
],
"family": "Berwanger",
"given": "Otavio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0046-0264",
"affiliation": [
{
"name": "Department of Medicine (C.N.H., J.H., M.S., W.H.C., M.R.B., M.P.B.), University of Colorado, Aurora."
},
{
"name": "CPC Clinical Research, Aurora, CO (C.N.H., J.H., M.R.N., M.S., W.H.C., R.G., M.P.B.)."
},
{
"name": "The State University of New York Downstate Health Sciences University, Brooklyn (M.S.)."
}
],
"authenticated-orcid": true,
"family": "Szarek",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine (C.N.H., J.H., M.S., W.H.C., M.R.B., M.P.B.), University of Colorado, Aurora."
},
{
"name": "CPC Clinical Research, Aurora, CO (C.N.H., J.H., M.R.N., M.S., W.H.C., R.G., M.P.B.)."
}
],
"family": "Capell",
"given": "Warren H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pulmonary Associates of Richmond, VA (S.J.)."
}
],
"family": "Johri",
"given": "Shilpa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Eastern Shore Research Institute, Fairhope, AL (M.S.P.)."
}
],
"family": "Pursley",
"given": "Michael S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery (M.R.N., R.G.), University of Colorado, Aurora."
},
{
"name": "CPC Clinical Research, Aurora, CO (C.N.H., J.H., M.R.N., M.S., W.H.C., R.G., M.P.B.)."
}
],
"family": "Gupta",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "MultiCare Pulmonary Specialists, Tacoma, WA (P.S.M.)."
}
],
"family": "Meehan",
"given": "Patrick S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Florida College of Medicine, Jacksonville (F.F.)."
}
],
"family": "Franchi",
"given": "Francesco",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4050-5030",
"affiliation": [
{
"name": "Ochsner Medical Center, New Orleans, LA (M.B.E.)."
}
],
"authenticated-orcid": true,
"family": "Effron",
"given": "Mark B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ARCA biopharma, Westminster, CO (I.A.C., D.M., C.A.G., S.P.H., T.K., M.R.B.)."
}
],
"family": "Marshall",
"given": "Debra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ARCA biopharma, Westminster, CO (I.A.C., D.M., C.A.G., S.P.H., T.K., M.R.B.)."
}
],
"family": "Graybill",
"given": "Christopher A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9940-9604",
"affiliation": [
{
"name": "ARCA biopharma, Westminster, CO (I.A.C., D.M., C.A.G., S.P.H., T.K., M.R.B.)."
}
],
"authenticated-orcid": true,
"family": "Huebler",
"given": "Sophia P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ARCA biopharma, Westminster, CO (I.A.C., D.M., C.A.G., S.P.H., T.K., M.R.B.)."
}
],
"family": "Keuer",
"given": "Thomas",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8860-4698",
"affiliation": [
{
"name": "Department of Medicine (C.N.H., J.H., M.S., W.H.C., M.R.B., M.P.B.), University of Colorado, Aurora."
},
{
"name": "ARCA biopharma, Westminster, CO (I.A.C., D.M., C.A.G., S.P.H., T.K., M.R.B.)."
}
],
"authenticated-orcid": true,
"family": "Bristow",
"given": "Michael R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9860-3584",
"affiliation": [
{
"name": "Department of Medicine (C.N.H., J.H., M.S., W.H.C., M.R.B., M.P.B.), University of Colorado, Aurora."
},
{
"name": "CPC Clinical Research, Aurora, CO (C.N.H., J.H., M.R.N., M.S., W.H.C., R.G., M.P.B.)."
}
],
"authenticated-orcid": true,
"family": "Bonaca",
"given": "Marc P.",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04655586",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Arteriosclerosis, Thrombosis, and Vascular Biology",
"container-title-short": "ATVB",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.ahajournals.org"
]
},
"created": {
"date-parts": [
[
2023,
6,
29
]
],
"date-time": "2023-06-29T09:00:24Z",
"timestamp": 1688029224000
},
"deposited": {
"date-parts": [
[
2024,
5,
12
]
],
"date-time": "2024-05-12T23:29:18Z",
"timestamp": 1715556558000
},
"indexed": {
"date-parts": [
[
2024,
5,
13
]
],
"date-time": "2024-05-13T00:31:20Z",
"timestamp": 1715560280582
},
"is-referenced-by-count": 6,
"issue": "8",
"issued": {
"date-parts": [
[
2023,
8
]
]
},
"journal-issue": {
"issue": "8",
"published-print": {
"date-parts": [
[
2023,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
1
]
],
"date-time": "2023-08-01T00:00:00Z",
"timestamp": 1690848000000
}
}
],
"link": [
{
"URL": "https://www.ahajournals.org/doi/full/10.1161/ATVBAHA.122.318748",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "1572-1582",
"prefix": "10.1161",
"published": {
"date-parts": [
[
2023,
8
]
]
},
"published-print": {
"date-parts": [
[
2023,
8
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"key": "e_1_3_3_2_2",
"unstructured": "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed November 8 2022."
},
{
"DOI": "10.1056/NEJMoa2015432",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_3_2"
},
{
"DOI": "10.1016/j.humpath.2020.07.023",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_4_2"
},
{
"DOI": "10.7326/M20-2003",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_5_2"
},
{
"DOI": "10.1016/j.carpath.2020.107263",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_6_2"
},
{
"DOI": "10.1016/j.ajem.2020.09.018",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_7_2"
},
{
"DOI": "10.1016/j.jacc.2021.02.035",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_8_2"
},
{
"DOI": "10.1016/j.jacc.2021.12.023",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_9_2"
},
{
"DOI": "10.1007/s11239-020-02134-3",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_10_2"
},
{
"DOI": "10.1038/s41577-021-00536-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_11_2"
},
{
"DOI": "10.1111/jth.15043",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_12_2"
},
{
"DOI": "10.1111/jth.14246",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_13_2"
},
{
"DOI": "10.1111/jth.15509",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_14_2"
},
{
"DOI": "10.1182/blood-2013-09-526277",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_15_2"
},
{
"DOI": "10.1182/blood-2011-08-376814",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_16_2"
},
{
"DOI": "10.1111/jth.14389",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_17_2"
},
{
"DOI": "10.1161/ATVBAHA.120.315547",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_18_2"
},
{
"DOI": "10.1182/bloodadvances.2020003308",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_19_2"
},
{
"DOI": "10.1002/rth2.12525",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_20_2"
},
{
"DOI": "10.1016/j.eclinm.2021.101069",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_21_2"
},
{
"DOI": "10.1016/j.jacbts.2020.12.009",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_22_2"
},
{
"DOI": "10.1016/j.jacbts.2022.08.005",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_23_2"
},
{
"DOI": "10.1046/j.1365-2796.2003.01224.x",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_24_2"
},
{
"DOI": "10.1161/ATVBAHA.115.305996",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_25_2"
},
{
"DOI": "10.1074/jbc.M009116200",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_26_2"
},
{
"DOI": "10.1016/j.jacc.2007.02.065",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_27_2"
},
{
"DOI": "10.1016/s0735-1097(03)00478-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_28_2"
},
{
"DOI": "10.1016/S0140-6736(03)15012-X",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_29_2"
},
{
"DOI": "10.1086/520608",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_30_2"
},
{
"DOI": "10.1182/blood-2014-11-610717",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_31_2"
},
{
"DOI": "10.1126/science.abc0956",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_32_2"
},
{
"DOI": "10.1161/ATVBAHA.107.142539",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_33_2"
},
{
"DOI": "10.1172/JCI156436",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_34_2"
},
{
"DOI": "10.1016/j.ahj.2021.12.010",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_35_2"
},
{
"key": "e_1_3_3_36_2",
"unstructured": "WHO R&D Blueprint Novel Coronavirus COVID-19 Therapeutic Trial Synopsis. February 18 2020. https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis. Accessed August 4 2021."
},
{
"DOI": "10.1111/j.1538-7836.2005.01204.x",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_37_2"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_38_2"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_39_2"
},
{
"DOI": "10.1056/NEJMoa2031994",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_40_2"
},
{
"DOI": "10.1056/NEJMoa2100433",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_41_2"
},
{
"DOI": "10.1056/NEJMoa2031893",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_42_2"
},
{
"DOI": "10.1016/j.thromres.2021.01.023",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_43_2"
},
{
"DOI": "10.1016/j.bja.2020.12.010",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_44_2"
},
{
"DOI": "10.1016/j.thromres.2021.12.012",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_45_2"
},
{
"DOI": "10.1056/NEJMoa2105911",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_46_2"
},
{
"DOI": "10.1056/NEJMoa2103417",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_47_2"
},
{
"DOI": "10.1001/jamainternmed.2021.6203",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_48_2"
},
{
"DOI": "10.1016/S1473-3099(22)00223-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_49_2"
},
{
"DOI": "10.1126/scitranslmed.abd3876",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_50_2"
},
{
"DOI": "10.1111/jth.15455",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_51_2"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ahajournals.org/doi/10.1161/ATVBAHA.122.318748"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Novel Tissue Factor Inhibition for Thromboprophylaxis in COVID-19: Primary Results of the ASPEN-COVID-19 Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1161/crossmarkpolicy",
"volume": "43"
}