COVID-19, Infection Inhibitors and Medicines
, T., MDPI AG, doi:10.20944/preprints202501.1042.v1, Jan 2025
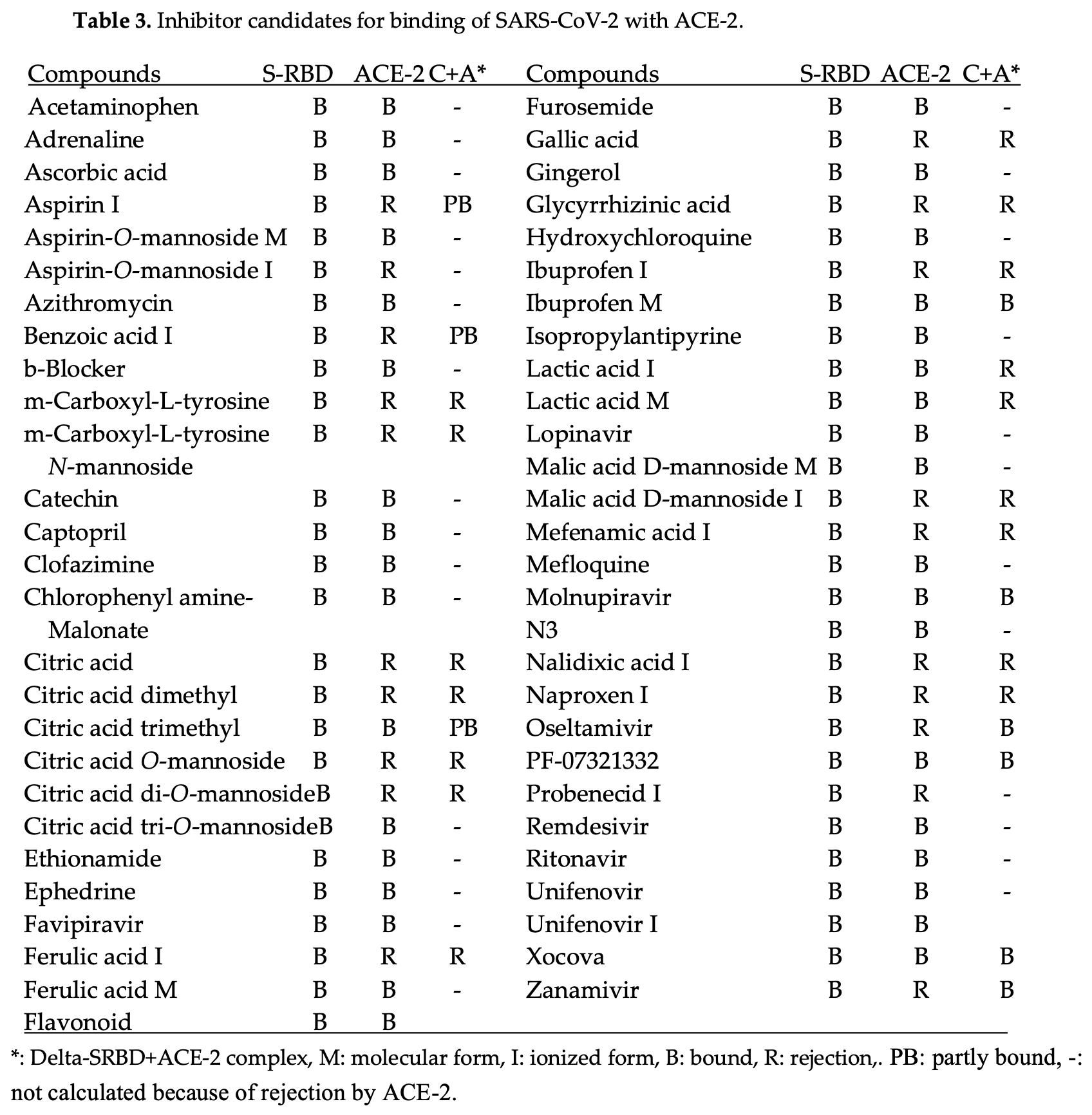
In Silico analysis of potential inhibitors and treatments for COVID-19 by analyzing the molecular interactions between the SARS-CoV-2 spike protein (S-RBD) and the human ACE-2 receptor. Author identifies tricarboxylic acids (citric acid, malic acid) and natural polyphenols (gallic acid, ferulic acid, glycyrrhizinic acid) as effective binding inhibitors, preventing viral entry. Common medications such as aspirin, ibuprofen, ritonavir, oseltamivir, and zanamivir also exhibited inhibitory effects. Modified versions of existing drugs that incorporate acidic groups may improve efficacy. Author emphasizes the rapid mutation of SARS-CoV-2 variants, reducing the efficacy of monoclonal antibodies, and underscores the potential of existing compounds to counteract COVID-19.
Hanai et al., 14 Jan 2025, preprint, 1 author.
Contact: hanai104@kf7.so-net.ne.jp.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
COVID-19, Infection Inhibitors and Medicines
doi:10.20944/preprints202501.1042.v1
The fast mutation of COVID-19 viruses still confuses us, and the mRNA vaccines do not inhibit the infection and may protect against the heavy disease. The infection mechanism is described with the protein-protein binding stereo structure; therefore, the infection strength of variants has been estimated from the protein-protein (S-RBD binding with ACE-2) interaction energy values calculated using a molecular mechanics program. The binding strength order was Alfa < Lambda < WT < FE.1 < XBB1.5 < EG.5 ≈ BQ.1 ≈ Alpha+E484K ≈ Omicron XBB.1.16 ≈ Epsilon, Iota < EG.5 < Delta plus ≈ Beta, Kappa B.1.621 ≈ KP.3 ≈ Kappa B.1.617.1 ≈ Delta B.1.517.2 < KP.2 < BA.2.86 ≈ JN.1 ≈ HV.1 ≈ BA.1 < BA.2. The mutation from acidic amino acid to basic amino acid strength the binding. The substitute size of amino acids causes the steric hindrance for the binding. The affinity level supports the infection strength. Various proposed infection inhibitors are quantitatively analyzed. TCA acids and natural polyphenols inhibit the binding of S-RBD to ACE-2. The cocktail dose of known medicines may enhance their performance. The inhibiting multiplication may be achieved using glycated compounds that bind glycoproteins and reduce glycoprotein activities.
References
Abbasi, None, Miki Matsui-Masai
Ahmad A Toubasi, Allon, Bagnato, Disseminated histoplasmosis mimicking postvaccination side effects in an immunocompromised person with multiple sclerosis, Multiple Sclerosis Journal-Experimental, doi:10.1177/20552173241271790
Anthony M Kyriakopoulos 1, Nigh2, Peter A Mccullough3, Seneff, Clinical rationale for dietary lutein supplementation in long COVID and mRNA vaccine injury syndromes [version 3; peer, review: 2 approved, Research, doi:10.12688/f1000research.143517.1
Barmada, Klein, Ramaswamy, Brodsky, Joycox et al., Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine-associated myocarditis, Additional introduction, Sci. Immunol, doi:10.1126/sciimmunol.adh3455
Barmada, Klein, Ramaswamy, Brodsky, Joycox et al., Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine-associated myocarditis, Additional introduction, Sci. Immunol, doi:10.1126/sciimmunol.adh3455
Belinky, Ramos-Benitez, Boritz, Lach, Herr et al., SARS-CoV-2 infection and persistence in the human body and brain at autopsy, Nature, doi:10.1038/s41586-022-65542-y
Bettinger, Irvine, Hennady, Shulha, Valiquette et al., Adverse Events Following Immunization With mRNA and Viral Vector Vaccines in Individuals With Previous Severe Acute Respiratory Syndrome Coronavirus 2 Infection From the Canadian National Vaccine Safety Network, Research Network CID
Bianchini, Crivelli, Abernathy, Guerra, Palus et al., Human neutralizing antibodies to cold linear epitopes and subdomain 1 of the SARS-CoV-2 spike glycoprotein, Sci. Immunol, doi:10.1126/sciimunol.ade0958.2023
Bjorkman, Gisslen, Gullberg, Ludvigsson, The Swedish COVID-19 approach: a scientific dialogue on mitigation policies, Frontiers in Public Health, doi:10.3389/fpubh.2023.1206732
Bladh, Aguilera, Marking, Kihlgren, Greilert Norin et al., Comparison of SARS-CoV-2 spike-specific IgA and IgG in nasal secretions, saliva and serum, Frontiers in Immunology, doi:10.3389/fimmu.2024.1346749
Bodie, Hashimoto, Connolly, Chu, Takayama et al., Design of a chimeric ACE-2/Tc-silent fusion protein with ultrahigh affinity and neutralizing capacity for SARS-CoV-2 variants, Antib. Ther
Boretti, mRNA vaccine boosters and impaired immune system response in immune compromised individuals: a narrative review, Clinical and Experimental Medicine, doi:10.1007/s10238-023-01264-1
Brevini, Maes, Webb, John, Fuchs et al., FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2, Nature, doi:10.1038/s41586-022-05594-0
Briere, Favier, Gimenez-Roqueple, Rustin, Tricarboxylic acid cycle dysfunction as a cause of human diseases and tumor formation, Am. J. Physiol. Cell Physiol
Cheriyedath, Understanding immune responses to Sars-Cov-2 infections in children, News Med. Sci
Christopher D Richardson, Heterologous ChAdOx1-nCoV19-BNT162b2 vaccination provides superior immunogenicity against COVID-19, Lancet, doi:10.1016/S2213-2600(21)00366-0,www.thelancet.com/respiratory
Chuang, Lin, Chang, Tsai, Tsau, Pediatric glomerulopathy after COVID-19 vaccination: A case series and review of the literature, Journal of the Formosan Medical Association, doi:10.1016/j.jfma.2023.04.014
Clementi, Scagnolari, 'amore, Palombi, Criscuolo et al., Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro, Pharmacological Research, doi:10.1016/j.phrs.2020.105255
Cohen, Missing immune cells may explain why COVID-19 vaccine protection quickly wanes, New insights on what stimulates long-lived antibody production could spur better vaccines, SCIENCE INSIDER
Cordero, Research study to evaluate the clinical effectiveness of the nutritional supplement VITAMIC BIOSEN®, Combination of Curcumin, Vitamin C, and Boswellia Serrata, on individuals with symptoms consistent with LONG COVID who have been vaccinated against the SARS-CoV2, Biomed J Sci & Tech Res, doi:10.26717/BISTR.2022.47.007512
Debnath, Mitra, Dewake, Prabhakar, Tadala et al., N-acetyl cysteine: A tool to perturb SARS-CoV-2 spike protein conformation, doi:10.26434/chemrxiv.12687923.v2
Diblasi, Monteverde, Nonis, Phd, Sangorrín, At Least 55Undeclared Chemical Elements Found in COVID-19 Vaccines from AstraZeneca, CanSino, Moderna, Pfizer, Sinopharm and Sputnik V, with Precise ICP-MS, International Journal of Vaccine Theory, Practice, and Research, doi:10.56098/mt1njj52
Doctrow, How COVID-19 variants evade the immune response, NIH Research Matter
Domingo, A review of the scientifc literature on experimental toxicity studies of COVID-19 vaccines, with special attention to publications in toxicology journals, Archives of Toxicology, doi:10.1007/s00204-024-03854-8
Esmat, Jamil, Kaml Kheder, John Kombe Kombe, Zeng et al., Immunoglobulin A response to SARS-CoV-2 infection and immunity, REVIEW ARTICLE HELYON, doi:10.1016/j.heliyon.2024.e24031
Frank, Ball, Hopkins, Kelley, Kuzma et al., SARS-CoV-2 S1 subunit produces a protracted priming of the neuroinflammatory, physiological, and behavioral responses to a remote immune challenge: A role for corticosteroids, Brain, Behavior and Immunity, doi:10.1016/j.bbi.2024.07.034121
Fulop, Larbi, Pawelec, Cohen, Provost et al., Immunosenescence and Altered Vaccine Efficiency in Older Subjects: A Myth Difficult to Change, Vaccines, doi:10.3390/vaccines10040607
Gao, Xing, Hao, Zhang, Zhong et al., Diverse immune responses in vaccinated individuals with and without symptoms after omicron exposure during the recent outbreak in Guangzhou, China, Heliyon, doi:10.1016/j.heliyon.2024.e24030
Ghosh, Chakraborty, Biswas, Chowdhuri, Evaluation of green tea polyphenols, as novel coronavirus (SARS CoV-2) main protease (Mpro) inhibitors -an in silico docking and molecular dynamics simulation study, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1779818
Ghosh, Sialic acid and biology of life: An introduction, Sialic Acids and Sialoglycoconjugates in the Biology of Life, Health and Disease, doi:10.1016/B978-0-12-816126-5.00001-9.110
Gibo, Kojima, Fujisawa, Kikuchi, Fukushima, Increased Age-Adjusted Cancer Mortality After the Third mRNA-Lipid Nanoparticle Vaccine Dose During the COVID-19 Pandemic in Japan, Cureus, doi:10.7759/cureus.57860
Grunst, Qin, Dodero-Rojas, Ding, Prévost et al., Preprints.org
Guan, Tang, Li, Shu, Zhao et al., Herbal medicine and gut microbiota: exploring untapped therapeutic potential in neurodegenerative disease management, Archives of Pharmacal Research, doi:10.1007/s12272-023-01484-9.118
Hammond, Leister-Tebbe, Gardner, Abreu, Bao et al., Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Han, Li, Liu, Wang, Zhang et al., Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2, Cell, doi:10.1016/j.cell.2022.01.001
Hanai, Further quantitative in silico analysis of SARS-CoV-2 S-RBD Omicron BA, Talanta, doi:10.1016/j.talanta.2022.124127
Hanai, Kato, Furukoshi, Hennmi, Imoto et al., Evaluation of measuring methods of human serum albumin-drug binding affinity, Current Pharmaceutical Analysis
Hanai, Quantitative in Silico Analytical Chemistry, Quantitative analysis of molecular interactions from chromatography retention to enzyme selectivity
Hanai, Quantitative in silico Chromatography
Hanai, Quantitative in silico analysis of SARS-CoV-2 S-RBD omicron mutant transmissibility, Talanta, doi:10.1016/j.talanta.2022.123206
Hanai, Quantitative in silico analytical chemistry/ COVID-19 transmissibility and designing the binding inhibitors
Hanai, Quantitative structure-retention relationship of phenolic compounds without Hammet's equations, J. Chromatogr. A
Hanai, Shimada, Koyama, Nohara, Takayanagi et al., Quantitative analysis of selective glycosylation of saccharides with aromatic amines, Carbohydrate Research, doi:10.1016/j.carres.2020.108171
Hanai, and designing the binding inhibitors
Heil, Self-DNA driven inflammation in COVID-19 and after mRNAbased vaccination: lessons for non-COVID-19 pathologies, Immunol, doi:10.3389/fimmu.2023.1259879
Henss, Auste, Schürmann, Schmidt, Von Rhein et al., The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection, J Gen Virol, doi:10.1099/jgv.0.001574
Hentzien, Autran, Pitoh, Yazdanpanah, Calmy, A monoclonal antibody stands out against omicron subvariants: a call to action for wider access to Bebtelovimab, Lancet, www.thelancet, doi:10.1016/S1473-3099(22)00495-9
Hentzien, Autran, Pitoh, Yazdanpanah, Calmy, A monoclonal antibody stands out against omicron subvariants: a call to action for wider access to Bebtelovimab, Lancet, www.thelancet, doi:10.1016/S1473-3099(22)00495-9
Heo, Jae, Jeon, Min, Ha, Long-Term Risk of Autoimmune and Autoinflammatory Connective Tissue Disorders Following COVID-19, JAMA Dermatol, doi:10.1001/jamadermatol.2024.4233
Horton, Offline: Covid-19 is not a pandemic, Lancet
Hossein, Mehrabadi, Hajimoradi, Es-Haghi, Kalantari et al., Safety and Immunogenicity of Intranasal Razi Cov Pars as a COVID-19 Booster Vaccine in Adults: Promising Results from a Groundbreaking Clinical Trial, Vaccines, doi:10.3390/vaccines12111255
Hu, Lewandowski, Tan, Zhang, Morgan et al., Naturally Occurring Mutations of SARS-CoV-2 Main Protease Confer Drug Resistance to Nirmatrelvir, ACS Cent. Sci, doi:10.1021/acscentsci.3c00538
Irrgang, Gerling, Kocher, Lapuente, Steininger et al., Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination, Sci. Immunol, doi:10.1126/sciimunol.ade2798
Jose, Domingo, A review of the scientifc literature on experimental toxicity studies of COVID-19 vaccines, with special attention to publications in toxicology journals, Archives of Toxicology, doi:10.1007/s00204-024-03854-8
Kaiser, Kaiser, Reis, Marschalek, Quantification of objective concentrations of DNA impurities in mRNA vaccines
Kaku, Okumura, Padilla-Blanco, Kosugi, Uriu et al., Virological characteristics of the SARS-CoV-2 JN.1 variant, Lancet Infect Dis, doi:10.1016/51473-3099(23)00813-7
Kayano, Sasanami, Nishiura, Science-based exit from stringent countermeasures against COVID-19: Mortality prediction using immune landscape between 2021 and 2022 in Japan, Vaccine: X, doi:10.1016/j.jvacx.2024.100547
Kissel, René, Toes, Thomas, Huizinga1 et al., Glycobiology of rheumatic, diseases, Nat Rev Rheumatol, doi:10.1038/s41584-022-00867-4
Kozlov, Omicron overpowers key CONID antibody treatments in early tests, Nature, doi:10.1038/d41586-021-03829-0
Kusunoki, Ohkusa, Iida, Saito, Kawahara et al., Increase in antibody titer and change over time associated with severe acute respiratory syndrome coronavirus 2 infection after mRNA vaccination: Consideration of the significance of additional vaccination, Clin Case Rep, doi:10.1002/ccr3.8953
Lee, Leong, Rustagi, Beck, Zeng et al., SARS-CoV-2 escapes direct NK cell killing through Nsp1-mediated downregulation of ligands for NKG2D, Cell Reports, doi:10.1016/j.celrep.2022.111892
Lewis, Toukach, Bolton, Chen, Frank et al., Cataloging natural sialic and other nonulosonic acids (NulOs), and their representation using the Symbol Nomenclature for Glycans, Glycobiology, doi:10.1093/glycob/cwac072
Ling, Chang, Babji, Latip, Koketsu et al., Review of sialic acid's biochemistry, sources, extraction and functions with special reference to edible bird's nest Affiliations expand, Food Chem, doi:10.1016/j.foodchem.2021.130755
Liu, Zhong, Wu, Su, Wang et al., Potential Beneficial Effects of Naringin and Naringenin on Long COVID-A Review of the Literature, Microorganisms, doi:10.3390/microorganisms12020332103
Lu, Yang, Ran, Zhan, Li et al., Discovery of orally bioavailable SARS-CoV-2 papain-like protease inhibitor as a potential treatment for COVID-19, Nature Communications, doi:10.1038/s41467-024-54462-0124
Mallapaty, Kids Covid: Why young immune systems are still on top, Nature, doi:10.1038/d41586-02502423-8
Mallapaty, Kids show mysteriously low levels of COVID-19 antibodies, Nature
Marco Alessandria 1, Malatesta, Berrino, Donzelli, A Critical Analysis of All-Cause Deaths during COVID-19 Vaccination in an Italian Province, Microorganisms
Mortezavi, Sloan, Shankar, Singh, Chen et al., Virologic Response and Safety of Ibuzatrelvir, a Novel SARS-cov-2 Antiviral, in Adults With COVID-19, Clinical Infectious Diseases, doi:10.1093/cid/ciae529123
Muhammad, Khalid, Frischmeyer-Guerrerio, The conundrum of COVID-19 mRNA vaccine-induced anaphylaxi, J ALLERGY CLIN IMMUNOL GLOBAL FEBRUARY, doi:10.1016/j.jacig.2022.10.00
Muik, Lui, Diao, Fu, Bacher et al., Progressive loss of conserved spike protein neutralizing antibody sites in Omicron sublineages is balanced by preserved T-cell recognition epitopes, Cell Reports, doi:10.1016/j.celrep.2023.112888
Muik, Lui, Quandt, Diao, Fu et al., Progressive loss of conserved spike protein neutralizing antibody sites in Omicron sublineages is balanced by preserved T cell immunity, Cell Reports, doi:10.1016/j.celrep.2023.112888
Nishimura, Okamoto, Dapat, Katsumi, Oshitani, Inactivation of SARS-CoV-2 by Catechins from Green Tea, doi:10.7883/yoken.JJID.2020.902
Offit, Bivalent Covid-19 Vaccines -A Cautionary Tale, N. Engl. J. Med, doi:10.1056/NEJMp2215780
Palacios, Cancer Mortality Surges Post COVID ModRNA Vaccination Ronald Palacios Castrillo, European Journal of Clinical and Biomedical Sciences, doi:10.11648/j.ejcbs.20241002.11
Park, Park, Jang, Park, Therapeutic Potential of EGCG, a Green Tea Polyphenol, for Treatment of Coronavirus Diseases, Life, doi:10.3390/life11030197
Pavelic, Pavelic, Open questions over the COVID-19 pandemic, Science, Art and Religion, doi:10.5005/jp-hournals-11005-0027
Pflumm, Seidel, Klein, Groß, Krutzke et al., Heterologous DNA-prime/ protein-boost immunization with a monomeric SARS-CoV-2 spike antigen redundantizes the trimeric receptor-binding domain structure to induce neutralizing antibodies in old mice, Frontiers in Immunology, doi:10.3389/fimmu.2023.123127441./
Planas, Staropoli, Planchais, Yab, Jeyarajah et al., Escape of SARS-CoV-2 variants KP1.1, LB.1 and KP3.3 from approved monoclonal antibodies, doi:10.1101/2024.08.20.608835
Pramanik, Healthy lifestyle habits dramatically cut long-term COVID-19 risks
Prillaman, Prior omicron infection protects against BA.4 and BA.5 variants, Nature News, doi:10.1038/d41586-022-01950-2
Ruggiero, Balzano, Di Napoli, Mascolo, Berrino et al., Capillary leak syndrome following COVID-19 vaccination: Front, Immunol, doi:10.3389/fimmu.2022.956825
Said, Al-Rubkhi, Jaju, Koh, Al-Balushi et al., Association of the Magnitude of Preprints
Schwab, Domke, Hartmann, Stenzinger, Longerich et al., Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination, Clin. Res. Cardiol, doi:10.1007/s00392-022-02129-5
Scudellari, How SARS-CoV-2 infects cells -and why Delta is so dangerous, Nature, doi:10.1038/d41586-021-02039-y
Seneff, Nigh, Kyriakopoulos, Mccullough, Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs, Food and Chemical Toxicology, doi:10.1016/j.fct.2022.113008
Sheward, Kim, Fischbach, Muschiol, Ehling et al., Evasion of neutralizing antibodies by omicron sublineage BA.2.75, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00524-2
Shi1, Shi1, Wang, You, Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications, Signal Transduction and Targeted Therapy, doi:10.1038/s41392-024-02002-z
Shmerling, Is the Covid-19 pandemic over, or not?
Smith, Willett, Food in the Anthropocene: the EAT-Lancet commission on healthy diets from sustainable food systems, doi:10.1016/s170-6736(18)31788-4
Staufer, Gupta, Bucher, Kohler, Sigl et al., Synthetic virions reveal fatty acid-coupled adaptive immunogenicity of SARS-CoV-2 spike glycoprotein, Nature Communications, doi:10.1038/s41467-022-28446-x
Stein, Ramelli, Grazioli, Chung, Singh et al., Preprints.org
Stencel-Baerenwalg, Reiss, Reiter, Stehle, Dermody, The sweet spot: defining virussialic acid interactions, Nature Reviews Microbiology
Sunil, WimalawansaUnlocking insights: Navigating COVID-19 challenges and Emulating future pandemic Resilience strategies with strengthening natural immunity, Heliyon, doi:10.1016/j.heliyon.2024.e34691
Takahashi, Barbosa, Lima, Cardoso, Consigli et al., Antiviral fungal metabolites and some insights into their contribution to the current COVID-19 pandemic, Bioorg. Med. Chem, doi:10.1016/j.bmc.2021.116366
Tallei, Fatimawali, Niode, Idroes, Zidan et al., A Comprehensive Review of the Potential Use of Green Tea Polyphenols in the Management of COVID-19, Evid Based Complement Alternat Med, doi:10.1155/2021/7170736
Thabet, COVID-19 mortality paradox (United States vs Africa): Mass vaccination vs early treatment, World J Exp Med, doi:10.5493/wjem.v14.i1.88674
Topol, An optimistic outlook, Ground Truths
Topol, Long-term Long Covid, Ground Truths
Topol, The BA.5 story, The takeover by this omicron sub-variant is not pretty, Ground Truths
Topol, The BA2.86 variant and the new tooster, Ground Truths
Topol, The virus takes a detour in its evolution arc, Ground Truths
Tozser, Benko, Natural compounds as regulators of NLRP3 inflammasome-mediated IK-1b production, Mediator of Inflammation, doi:10.1155/2016/5460302
Tozser, Benko, Natural compounds as regulators of NLRP3 inflammasome-mediated IK-1b production, Mediator of Inflammation, doi:10.1155/2016/5460302.s
Tuekprakhon, Nutalai, Dijokaite-Guraliu, Zhou, Ginn et al., Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum, Cell, doi:10.1016/j.cell.2022.06.005
Tutunchi, Naeini, Ostadrahimi, Javad, Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19, Phytotherapy Research, doi:10.1002/ptr.6781104
Tye, Jinks, Haigh, Kaul, Patek et al., Mutations in SARS-CoV-2 spike protein impair epitope-specific CD4+ T cell recognition, Nat. Immunol, doi:10.1038/s41590-022-01351-7
Valdes Angues, Bustos, SARS-CoV-2 Vaccination and the Multi-Hit Hypothesis of Oncogenesis, Cureus, doi:10.7759/cureus.50703
Van Breemen, Muchiri, Bates, Weinstein, Leier et al., Cannabinoids block cellular entry of SARS-CoV-2 and the emerging variants, J. Nat. Prod
Van Breemen, Muchiri, Bates, Weistein, Leier et al., Cannabinoids block cellular entry of SARS-CoV-2 and the emerging variants, J. Nat. Rod, doi:10.1021/acs.jbaprof.1c00946
Villadiego, García-Arriaza, Ramírez-Lorca, García-Swinburn, Cabello-Rivera et al., Full protection from SARS-CoV-2 brain infection and damage in susceptible transgenic mice conferred by MVA-CoV2-S vaccine candidate, Nature Neuroscience
Villadiego, García-Arriaza, Ramírez-Lorca, García-Swinburn, Cabello-Rivera et al., Full protection from SARS-CoV-2 brain infection and damage in susceptible transgenic mice conferred by MVA-CoV2-S vaccine candidate, Nature Neuroscience, doi:10.1038/s41593-022-01242-y
Wesley Alberca1, Mouradian, Teixeira, Beserra, Araujo De Oliveira et al., The Potential Effects Preprints, doi:10.3389/fimmu.2020.570919
Whitford, Mothes, Li, Structure and inhibition of SARS-CoV-2 spike refolding in membranes, SCIENCE, doi:10.1126/science.adn5658
Williamson, Effects of polyphenols on glucose-induced metabolic changes in healthy human subjects and on glucose transporters, Mol. Nutr. Food Res, doi:10.1002/mnfr.202101113
Yagisawa, Foster, Hanaki, Omura, Global trends in clinical trials of Ivermectin for Covid-19, Part 2, The Japanese Journal of Antibiotics
Yamasoba, Kosugi, Kimura, Fujita, Uriu et al., Neutralisation sensitivity of SARS-CoV-2 omicron subvariants to therapeutic monoclonal antibodies, Lancet, www.thelancet.com/infection, doi:10.1016/S1473-3099(22)00365-6
Yang, Xiao, Lidsky, Wu, Bonser et al., Fluorogenic reporter enables identification of compounds that inhibit SARS-CoV-2, Nature Microbiology, doi:10.1038/s41564-022-01288-5
Yang, Yu-Hsiang, Shih, Lung, The association between COVID-19 vaccine/infection and new-onset asthma in children -based on the global TriNetX database, Infection Open access, doi:10.1007/s15010-024-02329-3
Yasui, Hayashi, Tockary, Kohara, Kazunori, Carrier-free mRNA vaccine induces robust immunity against SARS-CoV-2 in mice and non-human primates without systemic reactogenicity, Molecular Therapy, doi:10.1016/j.ymthe.2024.03.022
Yonker, Swank, Bartsch, Burns, Kane et al., Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis, Circulation, doi:10.1161/CIRCULATIONAHA.122.061025
Yoshimura, Sakamoto, Ozuru, Kurihara, Itoh et al., Insufficient anti-spike RBD IgA responses after triple vaccination with intramuscular mRNA BNT162b2 vaccine against SARS-CoV-2, Received, doi:10.1016/j.heliyon.2023.e23595
Yue, Song, Wang, Jian, Chen et al., ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5, The Lancet Infectious Diseases, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00010-5
Zaidi, Dehgani-Mobaraki, The mechanisms of action of Ivermectin against SARS-CoV-2-an extensive review, J. Antibiotics
Zha, Fu, Vascular Endothelial Glycocalyx Damage and Potential Targeted Therapy in COVID-19, Cells, doi:10.3390/cells11121972
Zhang, Ivermectin could be a powerful drug for fighting cancer, The Epoch Times
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduction and Targeted Therapy, doi:10.1038/s41392-021-00835-6
DOI record:
{
"DOI": "10.20944/preprints202501.1042.v1",
"URL": "http://dx.doi.org/10.20944/preprints202501.1042.v1",
"abstract": "<jats:p>The fast mutation of COVID-19 viruses still confuses us, and the mRNA vaccines do not inhibit the infection and may protect against the heavy disease. The infection mechanism is described with the protein-protein binding stereo structure; therefore, the infection strength of variants has been estimated from the protein-protein (S-RBD binding with ACE-2) interaction energy values calculated using a molecular mechanics program. The binding strength order was Alfa &lt; Lambda &lt; WT &lt; FE.1 &lt; XBB1.5 &lt; EG.5 ≈ BQ.1 ≈ Alpha+E484K ≈ Omicron XBB.1.16 ≈ Epsilon, Iota &lt; EG.5 &lt; Delta plus ≈ Beta, Kappa B.1.621 ≈ KP.3 ≈ Kappa B.1.617.1 ≈ Delta B.1.517.2 &lt; KP.2 &lt; BA.2.86 ≈ JN.1 ≈ HV.1 ≈ BA.1 &lt; BA.2. The mutation from acidic amino acid to basic amino acid strength the binding. The substitute size of amino acids causes the steric hindrance for the binding. The affinity level supports the infection strength. Various proposed infection inhibitors are quantitatively analyzed. TCA acids and natural polyphenols inhibit the binding of S-RBD to ACE-2. The cocktail dose of known medicines may enhance their performance. The inhibiting multiplication may be achieved using glycated compounds that bind glycoproteins and reduce glycoprotein activities.</jats:p>",
"accepted": {
"date-parts": [
[
2025,
1,
13
]
]
},
"author": [
{
"affiliation": [],
"family": "Hanai",
"given": "Toshihiko",
"sequence": "first"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
1,
16
]
],
"date-time": "2025-01-16T02:07:55Z",
"timestamp": 1736993275000
},
"deposited": {
"date-parts": [
[
2025,
1,
16
]
],
"date-time": "2025-01-16T02:09:54Z",
"timestamp": 1736993394000
},
"group-title": "Medicine and Pharmacology",
"indexed": {
"date-parts": [
[
2025,
1,
16
]
],
"date-time": "2025-01-16T05:40:35Z",
"timestamp": 1737006035084,
"version": "3.33.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
1,
14
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
14
]
],
"date-time": "2025-01-14T00:00:00Z",
"timestamp": 1736812800000
}
}
],
"member": "1968",
"original-title": [],
"posted": {
"date-parts": [
[
2025,
1,
14
]
]
},
"prefix": "10.20944",
"published": {
"date-parts": [
[
2025,
1,
14
]
]
},
"publisher": "MDPI AG",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.preprints.org/manuscript/202501.1042/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "COVID-19, Infection Inhibitors and Medicines",
"type": "posted-content"
}