Effectiveness and Optimal Timing of Azvudine in COVID-19 Patients: A Multi-center Retrospective Study in Beijing, China
et al., Research Square, doi:10.21203/rs.3.rs-3145554/v1, Jul 2023
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
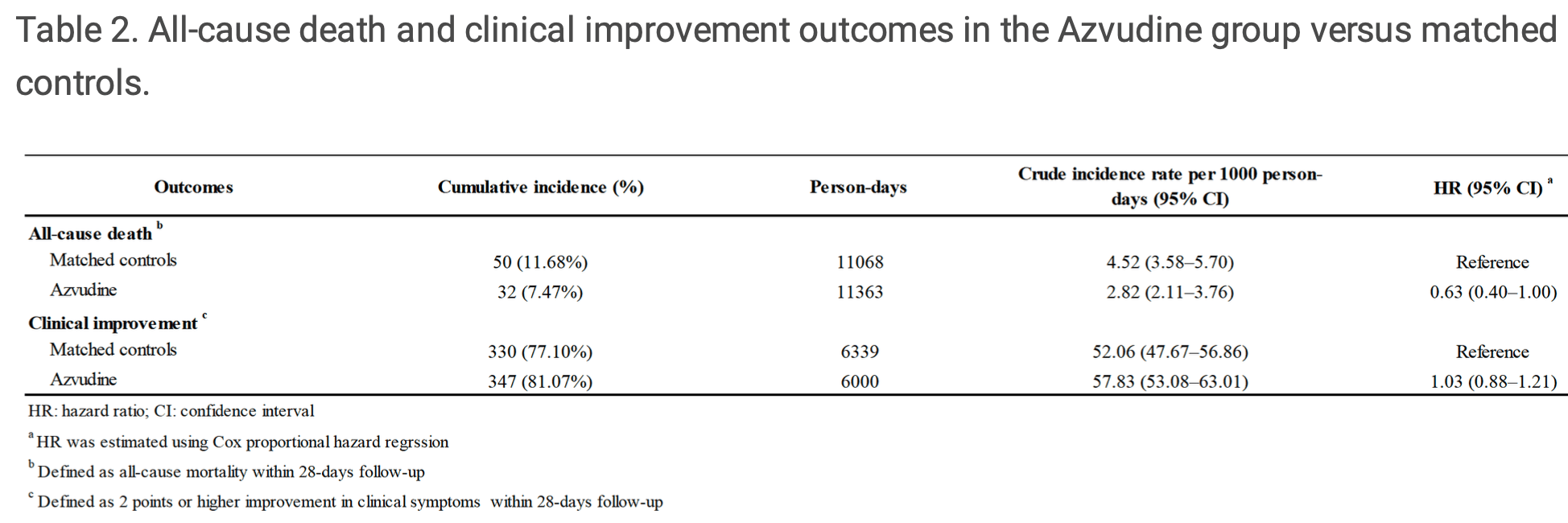
PSM retrospective 6,218 hospitalized COVID-19 patients in China showing lower 28-day all-cause mortality with azvudine treatment compared to controls (HR 0.63, 95% CI 0.40-1.00). Subgroup analysis found significantly faster clinical improvement when azvudine was initiated within 5 days of symptom onset compared to controls.
Although the 37% lower mortality is not statistically significant, it is consistent with the significant 30% lower mortality [20‑39%] from meta-analysis of the 28 mortality results to date.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of death, 37.0% lower, HR 0.63, p = 0.048, treatment 428, control 428, propensity score matching.
|
|
risk of no improvement, 2.9% lower, HR 0.97, p = 0.73, treatment 428, control 428, inverted to make HR<1 favor treatment, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Han et al., 14 Jul 2023, retrospective, China, preprint, 22 authors, study period 10 December, 2022 - 20 February, 2023.
Contact: xielx301@126.com.
Effectiveness and Optimal Timing of Azvudine in COVID-19 Patients: A Multi-center Retrospective Study in Beijing, China
doi:10.21203/rs.3.rs-3145554/v1
Background: Clinical effectiveness of Azvudine against coronavirus infection and optimal time for initiation of Azvudine treatment to hospitalized COVID-19 patients are not fully understood. Methods: This is a multi-center retrospective cohort study, and ve clinical centers of the Chinese People's Liberation Army General Hospital participated. From omicron pandemics, 6218 hospitalized patients con rmed with COVID-19 from December 10, 2022, to February 20, 2023, were retrieved for this study. After exclusions and propensity score matching , 428 Azvudine recipients and 428 controls were included with a follow-up of 28 days. The primary outcome was all-cause mortality during 28 days of hospitalization, and the secondary outcome was the proportion of patients with clinical improvement up to day 28. Results: The Azvudine group had a lower crude all-cause death rate when compared to the control group (2.82 per 1000 person-days vs. 4.52 per 1000 person-days; HR: 0.63, 95%CI: 0.40-1.00; P=0.038). Notably, the incidence rate of clinical improvement outcome was signi cantly higher in patients who received Azvudine within 5 days from the onset of symptoms, compared to the control group (Median days: 9 vs. 10; P=0.007). Subgroup analyses showed that chronic lung disease and corticosteroid treatment acted as protective factors (P=0.010; P=0.050). Conclusions: Clinical effectiveness of Azvudine in improving all-cause mortality in COVID-19 patients was seen, and initiation of Azvudine treatment within 5 days of the onset of symptoms was found to be signi cant. Additionally, the ndings revealed the protective effect of Azvudine in COVID-19 patients with chronic lung disease.
Ethical Approval This research was approved by the Ethics Committee of People's Liberation Army General Hospital (NO. 309202302230712). Informed consent was not required due to the retrospective nature of the study design.
Competing interests All authors in this study declare no competing con icts.
Authors' contributions Han Xinjie, Han Xiaobo, WY, WZ, XW and XL reviewed the literature, designed statistical analysis, conducted analyses, wrote the manuscript; CJ, ZW, MG, LY, Zheng Mengli, XF, WK, MJ, YX, HZ, and CH collected and analyzed clinical data, also contributed to the interpretation of the analysis; XK, PP, SJ, Zhang Mingyue and ZX prepared some paragraphs of the manuscript. All authors contributed to the interpretation of the analysis, critically reviewed and revised the manuscript, and approved the nal manuscript as submitted. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. This retrospective study was supported by China National Nature Science Foundation (82172109). Figure 4 The subgroup analysis of the effectiveness of Azvudine treatment in reducing all-cause death (A) and improving clinical improvement (B) Supplementary Files This is a list of supplementary les associated with this preprint. Click to download.
References
Chan, Yam, Zhang, Globally e cient non-parametric inference of average treatment effects by empirical balancing calibration weighting, J R Stat Soc Series B Stat Methodol, doi:10.1111/rssb.12129
Chang, Chen, Huang, Clinical effectiveness of molnupiravir in patients with COVID-19 undergoing haemodialysis, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2023.106834
Deng, Li, Sun, Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study, J Med Virol, doi:10.1002/jmv.28756
Deng, Li, Sun, Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study, J Med Virol, doi:10.1002/jmv.28756
Dian, Meng, Sun, Azvudine versus Paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities, J Infect, doi:10.1016/j.jinf.2023.05.012
Gal-Oz, Maier, Yoshida, ImmGen report: sexual dimorphism in the immune system transcriptome, Nat Commun, doi:10.1038/s41467-019-12348-6
Ganatra, Dani, Ahmad, Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19, Clin Infect Dis
General O Ce, of the National Health Commission. Notice on the issuance of Diagnosis and Treatment Protocol for novel coronavirus infection (trial version 10)
Kale, Shelke, Dagar, How to use COVID-19 antiviral drugs in patients with chronic kidney disease, Front Pharmacol, doi:10.3389/fphar.2023.1053814
Liu, Chen, Lin, Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients, J Infect, doi:10.1016/j.jinf.2020.03.005
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Radcliffe, Palacios, Azar, Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge, Am J Transplant, doi:10.1111/ajt.17098
Ren, Luo, Yu, Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study, Adv Sci (Weinh), doi:2001435.10.1002/advs.202001435
Ren, Luo, Yu, Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study, Adv Sci (Weinh), doi:2001435.10.1002/advs.202001435
Shao, Fan, Guo, Composite interventions on outcomes of severely and critically ill patients with COVID-19, doi:10.1101/2023.05.10.23289325
Silva, Abreu Cabral, Souza, Serial viral load analysis by DDPCR to evaluate FNC e cacy and safety in the treatment of mild cases of COVID-19, Front Med, doi:10.3389/fmed.2023.1143485
Sun, Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study, EClinicalMedicine, doi:10.1016/j.eclinm.2023.101981
Therneau, Grambsch, Pankratz, Penalized survival models and frailty, J Comput Graphical Stat
Xu, Yang, Zheng, The Pyrimidine Analog FNC Potently Inhibits the Replication of Multiple Enteroviruses, J Virol, doi:10.1128/JVI.00204-20
Ye, Wang, Mao, The pathogenesis and treatment of the `Cytokine Storm' in COVID-19, J Infect, doi:10.1016/j.jinf.2020.03.037
Yu, Chang, The rst Chinese oral anti-COVID-19 drug Azvudine launched, Innovation (Camb), doi:10.1016/j.xinn.2022.100321
Zheng, Peng, Xu, Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis, J Infect, doi:10.1016/j.jinf.2020.04.021
DOI record:
{
"DOI": "10.21203/rs.3.rs-3145554/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-3145554/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p><jats:bold>Background: </jats:bold>Clinical effectiveness of Azvudine against coronavirus infection and optimal time for initiation of Azvudine treatment to hospitalized COVID-19 patients are not fully understood.\n<jats:bold>Methods: </jats:bold>This is a multi-center retrospective cohort study, and five clinical centers of the Chinese People’s Liberation Army General Hospital participated. From omicron pandemics, 6218 hospitalized patients confirmed with COVID-19 from December 10, 2022, to February 20, 2023, were retrieved for this study. After exclusions and propensity score matching , 428 Azvudine recipients and 428 controls were included with a follow-up of 28 days. The primary outcome was all-cause mortality during 28 days of hospitalization, and the secondary outcome was the proportion of patients with clinical improvement up to day 28.\n<jats:bold>Results: </jats:bold>The Azvudine group had a lower crude all-cause death rate when compared to the control group (2.82 per 1000 person-days vs. 4.52 per 1000 person-days; HR: 0.63, 95%CI: 0.40-1.00; <jats:italic>P</jats:italic>=0.038). Notably, the incidence rate of clinical improvement outcome was significantly higher in patients who received Azvudine within 5 days from the onset of symptoms, compared to the control group (Median days: 9 vs. 10; <jats:italic>P</jats:italic>=0.007). Subgroup analyses showed that chronic lung disease and corticosteroid treatment acted as protective factors <jats:italic>(P</jats:italic>=0.010; <jats:italic>P</jats:italic>=0.050).\n<jats:bold>Conclusions: </jats:bold>Clinical effectiveness of Azvudine in improving all-cause mortality in COVID-19 patients was seen, and initiation of Azvudine treatment within 5 days of the onset of symptoms was found to be significant. Additionally, the findings revealed the protective effect of Azvudine in COVID-19 patients with chronic lung disease.</jats:p>",
"accepted": {
"date-parts": [
[
2023,
7,
6
]
]
},
"author": [
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Han",
"given": "Xinjie",
"sequence": "first"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Han",
"given": "Xiaobo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Peking University First Hospital"
}
],
"family": "Wang",
"given": "Yongqian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chinese PLA Medical School"
}
],
"family": "Wang",
"given": "Ze",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Cui",
"given": "Junchang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Zhao",
"given": "Weiguo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Mo",
"given": "Guoxin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Liu",
"given": "Yuhong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Zheng",
"given": "Mengli",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "First Affiliated Hospital of Chinese PLA General Hospital"
}
],
"family": "Xie",
"given": "Fei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "First Affiliated Hospital of Chinese PLA General Hospital"
}
],
"family": "Wang",
"given": "Kaifei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Forth Affiliated Hospital of Chinese PLA General Hospital"
}
],
"family": "Meng",
"given": "Jiguang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fifth Affiliated Hospital of Chinese PLA General Hospital"
}
],
"family": "Yuan",
"given": "Xin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sixth Affiliated Hospital of Chinese PLA General Hospital"
}
],
"family": "Han",
"given": "Zhihai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Xiao",
"given": "Kun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Pan",
"given": "Pan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Sun",
"given": "Junping",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "First Affiliated Hospital of Chinese PLA General Hospital"
}
],
"family": "Zhang",
"given": "Mingyue",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Zhang",
"given": "Xinxin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shandong Future Network Research Institute, Jiangsu Future Network Group Co., Ltd"
}
],
"family": "Cheng",
"given": "Haibo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Peking University First Hospital"
}
],
"family": "Xie",
"given": "Wuxiang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The 8 th Medical Center, Chinese PLA General Hospital"
}
],
"family": "Xie",
"given": "Lixin",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
14
]
],
"date-time": "2023-07-14T15:03:11Z",
"timestamp": 1689346991000
},
"deposited": {
"date-parts": [
[
2023,
7,
14
]
],
"date-time": "2023-07-14T15:03:28Z",
"timestamp": 1689347008000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2023,
7,
15
]
],
"date-time": "2023-07-15T04:29:39Z",
"timestamp": 1689395379169
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
7,
14
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
7,
14
]
],
"date-time": "2023-07-14T00:00:00Z",
"timestamp": 1689292800000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-3145554/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-3145554/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
7,
14
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2023,
7,
14
]
]
},
"publisher": "Research Square Platform LLC",
"reference": [
{
"DOI": "10.1128/JVI.00204-20",
"article-title": "The Pyrimidine Analog FNC Potently Inhibits the Replication of Multiple Enteroviruses",
"author": "Xu N",
"doi-asserted-by": "publisher",
"first-page": "e00204",
"issue": "9",
"journal-title": "J Virol 2020 Apr",
"key": "ref1",
"unstructured": "Xu N, Yang J, Zheng B et al. The Pyrimidine Analog FNC Potently Inhibits the Replication of Multiple Enteroviruses. J Virol 2020 Apr 16;94(9):e00204–20. doi: 10.1128/JVI.00204-20. PMID: 32075935; PMCID: PMC7163137.",
"volume": "16"
},
{
"DOI": "10.3389/fmed.2023.1143485",
"article-title": "Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19. Front Med (Lausanne)",
"author": "Silva RM",
"doi-asserted-by": "publisher",
"first-page": "1143485",
"key": "ref2",
"unstructured": "Da Silva RM, Gebe Abreu Cabral P, De Souza SB et al. Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19. Front Med (Lausanne). 2023 Mar 14;10:1143485. doi:10.3389/fmed.2023.1143485. PMID: 37007788; PMCID: PMC10053779.",
"volume": "14;10",
"year": "2023"
},
{
"DOI": "10.1002/advs.202001435",
"article-title": "Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study",
"author": "Ren Z",
"doi-asserted-by": "publisher",
"first-page": "e2001435",
"issue": "19",
"journal-title": "Adv Sci (Weinh)",
"key": "ref3",
"unstructured": "Ren Z, Luo H, Yu Z, Randomized A, et al. Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study. Adv Sci (Weinh). 2020 Oct;7(19):e2001435. 10.1002/advs.202001435. Epub 2020 Aug 13. PMID: 35403380; PMCID: PMC7404576.",
"volume": "7"
},
{
"DOI": "10.1002/jmv.28756",
"author": "Deng G",
"doi-asserted-by": "publisher",
"key": "ref4",
"unstructured": "Deng G, Li D, Sun Y et al. Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study. J Med Virol. 2023 Apr;95(4):e28756. doi: 10.1002/jmv.28756. PMID: 37185838."
},
{
"key": "ref5",
"unstructured": "General Office of the National Health Commission. Notice on the issuance of Diagnosis and Treatment Protocol for novel coronavirus infection (trial version 10). (2023)."
},
{
"DOI": "10.1111/rssb.12129",
"article-title": "Globally efficient non-parametric inference of average treatment effects by empirical balancing calibration weighting. J R Stat Soc Series B Stat Methodol",
"author": "Chan KC",
"doi-asserted-by": "publisher",
"first-page": "673",
"issue": "3",
"key": "ref6",
"unstructured": "Chan KC, Yam SC, Zhang Z. Globally efficient non-parametric inference of average treatment effects by empirical balancing calibration weighting. J R Stat Soc Series B Stat Methodol. 2016 Jun;78(3):673–700. doi: 10.1111/rssb.12129. Epub 2015 Nov 8. PMID: 27346982; PMCID: PMC4915747.",
"volume": "78"
},
{
"DOI": "10.1198/1061860031365",
"article-title": "Penalized survival models and frailty",
"author": "Therneau T",
"doi-asserted-by": "crossref",
"first-page": "156",
"journal-title": "J Comput Graphical Stat",
"key": "ref7",
"unstructured": "Therneau T, Grambsch P, Pankratz VS. Penalized survival models and frailty. J Comput Graphical Stat. 2003;12:156–75.",
"volume": "12",
"year": "2003"
},
{
"DOI": "10.1016/j.xinn.2022.100321",
"author": "Yu B",
"doi-asserted-by": "publisher",
"key": "ref8",
"unstructured": "Yu B, Chang J. The first Chinese oral anti-COVID-19 drug Azvudine launched. Innovation (Camb). 2022 Nov 8;3(6):100321. doi: 10.1016/j.xinn.2022.100321. Epub 2022 Sep 9. PMID: 36106026; PMCID: PMC9461232.",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"article-title": "Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. EClinicalMedicine",
"author": "Sun Y",
"doi-asserted-by": "publisher",
"first-page": "101981",
"key": "ref9",
"unstructured": "Sun Y, Jin L, Dian Y et al. Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. EClinicalMedicine. 2023 May 5;59:101981. doi: 10.1016/j.eclinm.2023.101981. PMID: 37193346; PMCID: PMC10167478.",
"volume": "5",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac443",
"author": "Najjar-Debbiny R",
"doi-asserted-by": "publisher",
"key": "ref10",
"unstructured": "Najjar-Debbiny R, Gronich N, Weber G et al. Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients. Clin Infect Dis. 2023 Feb 8;76(3):e342-e349. doi: 10.1093/cid/ciac443. Erratum in: Clin Infect Dis. 2023 Mar 21;76(6):1158–1159. PMID: 35653428; PMCID: PMC9214014.",
"year": "2023"
},
{
"DOI": "10.1002/advs.202001435",
"article-title": "Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study",
"author": "Ren Z",
"doi-asserted-by": "publisher",
"first-page": "e2001435",
"issue": "19",
"journal-title": "Adv Sci (Weinh)",
"key": "ref11",
"unstructured": "Ren Z, Luo H, Yu Z, Randomized A, et al. Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study. Adv Sci (Weinh). 2020 Oct;7(19):e2001435. 10.1002/advs.202001435. Epub 2020 Aug 13. PMID: 35403380; PMCID: PMC7404576.",
"volume": "7"
},
{
"DOI": "10.1002/jmv.28756",
"author": "Deng G",
"doi-asserted-by": "publisher",
"key": "ref12",
"unstructured": "Deng G, Li D, Sun Y et al. Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study. J Med Virol. 2023 Apr;95(4):e28756. doi: 10.1002/jmv.28756. PMID: 37185838."
},
{
"DOI": "10.1016/j.jinf.2023.05.012",
"author": "Dian Y",
"doi-asserted-by": "publisher",
"key": "ref13",
"unstructured": "Dian Y, Meng Y, Sun Y et al. Azvudine versus Paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities. J Infect. 2023 May 17:S0163-4453(23)00290-6. doi: 10.1016/j.jinf.2023.05.012. Epub ahead of print. PMID: 37207823; PMCID: PMC10188372.",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2020.04.021",
"article-title": "Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis",
"author": "Zheng Z",
"doi-asserted-by": "publisher",
"first-page": "e16",
"issue": "2",
"journal-title": "J Infect",
"key": "ref14",
"unstructured": "Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020 Aug;81(2):e16–e25. 10.1016/j.jinf.2020.04.021. Epub 2020 Apr 23. PMID: 32335169; PMCID: PMC7177098.",
"volume": "81"
},
{
"DOI": "10.1038/s41467-019-12348-6",
"article-title": "ImmGen report: sexual dimorphism in the immune system transcriptome",
"author": "Gal-Oz ST",
"doi-asserted-by": "publisher",
"first-page": "4295",
"issue": "1",
"journal-title": "Nat Commun 2019 Sep",
"key": "ref15",
"unstructured": "Gal-Oz ST, Maier B, Yoshida H et al. ImmGen report: sexual dimorphism in the immune system transcriptome. Nat Commun 2019 Sep 20;10(1):4295. doi: 10.1038/s41467-019-12348-6. PMID: 31541153; PMCID: PMC6754408.",
"volume": "20"
},
{
"DOI": "10.1016/j.jinf.2020.03.005",
"article-title": "Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients",
"author": "Liu K",
"doi-asserted-by": "publisher",
"first-page": "e14",
"issue": "6",
"journal-title": "J Infect",
"key": "ref16",
"unstructured": "Liu K, Chen Y, Lin R, et al. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020 Jun;80(6):e14–8. 10.1016/j.jinf.2020.03.005. Epub 2020 Mar 27. PMID: 32171866; PMCID: PMC7102640.",
"volume": "80"
},
{
"DOI": "10.1101/2023.05.10.23289325",
"article-title": "Composite interventions on outcomes of severely and critically ill patients with COVID-19 in Shanghai, China.medRxiv",
"author": "Shao J",
"doi-asserted-by": "publisher",
"key": "ref17",
"unstructured": "Shao J, Fan R, Guo C 4 et al. Composite interventions on outcomes of severely and critically ill patients with COVID-19 in Shanghai, China.medRxiv preprint doi: https://doi.org/10.1101/2023.05.10.23289325; this version posted May 16, 2023."
},
{
"DOI": "10.1016/j.jinf.2020.03.037",
"article-title": "The pathogenesis and treatment of the `Cytokine Storm' in COVID-19",
"author": "Ye Q",
"doi-asserted-by": "publisher",
"first-page": "607",
"issue": "6",
"journal-title": "J Infect",
"key": "ref18",
"unstructured": "Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020 Jun;80(6):607–13. 10.1016/j.jinf.2020.03.037. Epub 2020 Apr 10. PMID: 32283152; PMCID: PMC7194613.",
"volume": "80"
},
{
"DOI": "10.1016/j.ijantimicag.2023.106834",
"author": "Chang YC",
"doi-asserted-by": "publisher",
"key": "ref19",
"unstructured": "Chang YC, Chen YC, Huang CC et al. Clinical effectiveness of molnupiravir in patients with COVID-19 undergoing haemodialysis. Int J Antimicrob Agents. 2023 Apr 30;62(1):106834. doi: 10.1016/j.ijantimicag.2023.106834. Epub ahead of print. PMID: 37127127; PMCID: PMC10148756.",
"year": "2023"
},
{
"DOI": "10.1111/ajt.17098",
"article-title": "Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge",
"author": "Radcliffe C",
"doi-asserted-by": "publisher",
"first-page": "2458",
"issue": "10",
"journal-title": "Am J Transplant",
"key": "ref20",
"unstructured": "Radcliffe C, Palacios CF, Azar MM, et al. Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge. Am J Transplant. 2022 Oct;22(10):2458–63. 10.1111/ajt.17098. Epub 2022 May 30. PMID: 35583664; PMCID: PMC9348251.",
"volume": "22"
},
{
"DOI": "10.3389/fphar.2023.1053814",
"article-title": "How to use COVID-19 antiviral drugs in patients with chronic kidney disease",
"author": "Kale A",
"doi-asserted-by": "publisher",
"first-page": "1053814",
"journal-title": "Front Pharmacol 2023 Feb",
"key": "ref21",
"unstructured": "Kale A, Shelke V, Dagar N et al. How to use COVID-19 antiviral drugs in patients with chronic kidney disease. Front Pharmacol 2023 Feb 9;14:1053814. doi: 10.3389/fphar.2023.1053814. PMID: 36843922; PMCID: PMC9947246.",
"volume": "9"
},
{
"article-title": "Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19",
"author": "Ganatra S",
"journal-title": "Clin Infect Dis 2022 August",
"key": "ref22",
"unstructured": "Ganatra S, Dani SS, Ahmad J et al. Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19. Clin Infect Dis 2022 August 20 (Epub ahead of print).",
"volume": "20"
},
{
"article-title": "Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. medRxiv [Preprint]. 2022 Jun 17:2022.06.14.22276393. doi: 10.1101/2022.06.14.22276393. Update in",
"author": "Dryden-Peterson S",
"journal-title": "Ann Intern Med",
"key": "ref23",
"unstructured": "Dryden-Peterson S, Kim A, Kim AY et al. Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. medRxiv [Preprint]. 2022 Jun 17:2022.06.14.22276393. doi: 10.1101/2022.06.14.22276393. Update in: Ann Intern Med. 2022 Dec 13;: PMID: 35734084; PMCID: PMC9216724.",
"year": "2022"
},
{
"author": "*Figures",
"key": "ref24",
"unstructured": "*Figures. and tables."
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-3145554/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Effectiveness and Optimal Timing of Azvudine in COVID-19 Patients: A Multi-center Retrospective Study in Beijing, China",
"type": "posted-content"
}