Evaluation of outpatient treatment for non-hospitalised patients with COVID-19: The experience of a regional centre in the UK
et al., PLOS ONE, doi:10.1371/journal.pone.0281915, Mar 2023
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
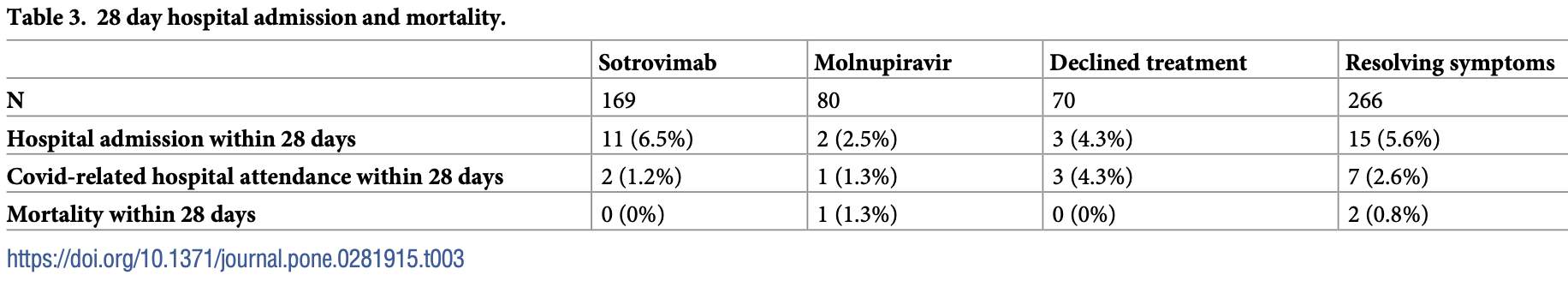
Retrospective 604 outpatients in the UK, showing lower risk of hospitalization with sotrovimab treatment, without statistical significance due to the small number of hospitalizations.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.11-3, BA.4, BA.54, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.15, and no efficacy for BA.26, XBB, XBB.1.5, ХВВ.1.9.17, XBB.1.16, BQ.1.1.45, and CL.15. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments8.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
Study covers molnupiravir and sotrovimab.
|
risk of death, 75.0% lower, RR 0.25, p = 0.55, treatment 0 of 169 (0.0%), control 2 of 336 (0.6%), NNT 168, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 60.2% lower, RR 0.40, p = 0.35, treatment 2 of 169 (1.2%), control 10 of 336 (3.0%), NNT 56, COVID-19 related.
|
|
risk of hospitalization, 21.5% higher, RR 1.21, p = 0.69, treatment 11 of 169 (6.5%), control 18 of 336 (5.4%), all cause.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Goodwin et al., 15 Mar 2023, retrospective, United Kingdom, peer-reviewed, 3 authors, study period 22 December, 2021 - 20 February, 2022.
Contact: amanda.goodwin@nottingham.ac.uk.
Evaluation of outpatient treatment for non-hospitalised patients with COVID-19: The experience of a regional centre in the UK
PLOS ONE, doi:10.1371/journal.pone.0281915
Introduction Antivirals, such as molnupiravir, and SARS-CoV-2 neutralising monoclonal antibodies (nMAbs), such as sotrovimab, reduced the risk of hospitalisation and death in clinical trials of high-risk non-hospitalised patients with Covid-19. However, the real-world benefits of these drugs are unclear.
Aims To evaluate the characteristics and outcomes of high-risk patients referred for outpatient antiviral or nMAb treatment for symptomatic Covid-19.
Methods The records of patients referred to a large UK Covid Medicines Delivery Unit (CMDU) over nine weeks (December 2021-February 2022) were reviewed. Data were collected on demographics, referral indications, vaccination, deprivation, treatment, complications, hospital admission, and mortality.
Results 1820 patients were referred to the CMDU, with 604 (33.2%) suitable for further assessment. 169 patients received sotrovimab, 80 patients received molnupiravir, 70 patients declined treatment, and 266 were ineligible for treatment because of resolving symptoms. There were trends towards higher proportions of female and white patients, lower deprivation scores, and malignancy-or transplant-related indications in the groups receiving treatment compared with untreated patients. Covid-19-related hospitalisations occurred in 1.2% of the treated group and 3.0% of the untreated group indicating a potential treatment effect, however Covid-related hospitalisations were lower than reported in the original clinical trials (2.2% compared with 7-10%).
Conclusion The referral pathways for outpatient treatment of Covid-19 are inefficient, and the UK system may not be serving all groups equitably. Hospitalisation with Covid-19 was rare
References
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Eng J Med, doi:10.1056/NEJMoa2116044
Bruel, Hadjadj, Maes, Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nat Med, doi:10.1038/s41591-022-01792-5
Chavarot, Melenotte, Amrouche, Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with Omicron infection, Kidney Int, doi:10.1016/j.kint.2022.04.003
Deng, Heybati, Ba, Differential efficacy and safety of anti-SARS-CoV-2 antibody therapies for the management of COVID-19: a systematic review and network meta-analysis, Infection, doi:10.1007/s15010-022-01825-8
Dhand, Okumura, Wolfe, Sotrovimab for treatment of COVID-19 in solid organ transplant recipients, Transplantation, doi:10.1097/TP.0000000000004136
Gupta, Gonzales-Rojas, Juarez, Early treatment for Covid-19 with SARS-CoV-2 Neutralizing antibody sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Gupta, Gonzales-Rojas, Juarez, Effect of sotrovimab on hospitalisation or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2022.2832
Nhs England, Statistical work areas: COVID-19 therapeutics (antivirals, neutralising monoclonal antibodies, and interleukin 6 inhibitors
Scobie, Morris, Quality and inequality: digging deeper
DOI record:
{
"DOI": "10.1371/journal.pone.0281915",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0281915",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Introduction</jats:title>\n<jats:p>Antivirals, such as molnupiravir, and SARS-CoV-2 neutralising monoclonal antibodies (nMAbs), such as sotrovimab, reduced the risk of hospitalisation and death in clinical trials of high-risk non-hospitalised patients with Covid-19. However, the real-world benefits of these drugs are unclear.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Aims</jats:title>\n<jats:p>To evaluate the characteristics and outcomes of high-risk patients referred for outpatient antiviral or nMAb treatment for symptomatic Covid-19.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Methods</jats:title>\n<jats:p>The records of patients referred to a large UK Covid Medicines Delivery Unit (CMDU) over nine weeks (December 2021-February 2022) were reviewed. Data were collected on demographics, referral indications, vaccination, deprivation, treatment, complications, hospital admission, and mortality.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Results</jats:title>\n<jats:p>1820 patients were referred to the CMDU, with 604 (33.2%) suitable for further assessment. 169 patients received sotrovimab, 80 patients received molnupiravir, 70 patients declined treatment, and 266 were ineligible for treatment because of resolving symptoms. There were trends towards higher proportions of female and white patients, lower deprivation scores, and malignancy- or transplant-related indications in the groups receiving treatment compared with untreated patients. Covid-19-related hospitalisations occurred in 1.2% of the treated group and 3.0% of the untreated group indicating a potential treatment effect, however Covid-related hospitalisations were lower than reported in the original clinical trials (2.2% compared with 7–10%).</jats:p>\n</jats:sec>\n<jats:sec id=\"sec005\">\n<jats:title>Conclusion</jats:title>\n<jats:p>The referral pathways for outpatient treatment of Covid-19 are inefficient, and the UK system may not be serving all groups equitably. Hospitalisation with Covid-19 was rare regardless of treatment. Ongoing service evaluation is required to ensure efficient use of resources for the outpatient management of Covid-19.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1488-6549",
"affiliation": [],
"authenticated-orcid": true,
"family": "Goodwin",
"given": "Amanda T.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Thompson",
"given": "Jonathan S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hall",
"given": "Ian P.",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2023,
3,
15
]
],
"date-time": "2023-03-15T17:25:42Z",
"timestamp": 1678901142000
},
"deposited": {
"date-parts": [
[
2023,
3,
15
]
],
"date-time": "2023-03-15T17:25:57Z",
"timestamp": 1678901157000
},
"editor": [
{
"affiliation": [],
"family": "Lee",
"given": "Seung-Hwa",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/501100000272",
"award": [
"I.P. Hall holds a NIHR Senior Investigator award (NF-SI-0617-10096)"
],
"doi-asserted-by": "publisher",
"name": "National Institute for Health Research"
},
{
"DOI": "10.13039/501100000272",
"award": [
"ATG holds an NIHR-funded Academic Clinical Lecturer post (CL-2020-12-003)"
],
"doi-asserted-by": "publisher",
"name": "National Institute for Health Research"
}
],
"indexed": {
"date-parts": [
[
2023,
3,
16
]
],
"date-time": "2023-03-16T04:42:29Z",
"timestamp": 1678941749610
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2023,
3,
15
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2023,
3,
15
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
15
]
],
"date-time": "2023-03-15T00:00:00Z",
"timestamp": 1678838400000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0281915",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0281915",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2023,
3,
15
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
15
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"key": "pone.0281915.ref001",
"unstructured": "World Health Organisation (WHO) Coronavirus (COVID-19) dashboard. https://covid19.who.int Accessed 1/12/22."
},
{
"article-title": "Differential efficacy and safety of anti-SARS-CoV-2 antibody therapies for the management of COVID-19: a systematic review and network meta-analysis",
"author": "J Deng",
"journal-title": "Infection",
"key": "pone.0281915.ref002",
"year": "2022"
},
{
"key": "pone.0281915.ref003",
"unstructured": "NHS interim clinical commissioning policy: neutralising monoclonal antibodies or antivirals for non-hospitalised patients with COVID-19. C1603. Published 24th February 2022. https://www.england.nhs.uk/coronavirus/documents/c1603-interim-clinical-commissioning-policy-antivirals-or-neutralising-monoclonal-antibodies-for-non-hospitalised-patients-with-covid-19-version-5. Accessed 21st April 2022"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for Covid-19 with SARS-CoV-2 Neutralizing antibody sotrovimab",
"author": "A Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "pone.0281915.ref004",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2022.2832",
"article-title": "Effect of sotrovimab on hospitalisation or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "A Gupta",
"doi-asserted-by": "crossref",
"first-page": "1236",
"issue": "13",
"journal-title": "JAMA",
"key": "pone.0281915.ref005",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "A Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"issue": "6",
"journal-title": "N Eng J Med",
"key": "pone.0281915.ref006",
"volume": "386",
"year": "2022"
},
{
"key": "pone.0281915.ref007",
"unstructured": "NHS England. Statistical work areas: COVID-19 therapeutics (antivirals, neutralising monoclonal antibodies, and interleukin 6 inhibitors). https://www.england.nhs.uk/statistics/statistical-work-areas/covid-therapeutics-antivirals-and-neutralising-monoclonal-antibodies/. Accessed 14th May 2022."
},
{
"key": "pone.0281915.ref008",
"unstructured": "UK Government. Indices of multiple deprivation maps 2015 and 2019. http://dclgapps.communities.gov.uk/imd/iod_index.html"
},
{
"key": "pone.0281915.ref009",
"unstructured": "Ministry of Housing, Communities and Local Government. English indices of deprivation 2019 –LOSA level. https://opendatacommunities.org/resource?uri=http%3A%2F%2Fopendatacommunities.org%2Fdata%2Fsocietal-wellbeing%2Fimd2019%2Findices. Accessed April 2022"
},
{
"key": "pone.0281915.ref010",
"unstructured": "UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England: Technical briefing 37. Published 25th February 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1057359/Technical-Briefing-37-25February2022.pdf"
},
{
"DOI": "10.1016/j.kint.2022.04.003",
"article-title": "Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with Omicron infection",
"author": "N Chavarot",
"doi-asserted-by": "crossref",
"first-page": "1290",
"issue": "6",
"journal-title": "Kidney Int",
"key": "pone.0281915.ref011",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000004136",
"article-title": "Sotrovimab for treatment of COVID-19 in solid organ transplant recipients",
"author": "A Dhand",
"doi-asserted-by": "crossref",
"first-page": "e336",
"issue": "7",
"journal-title": "Transplantation",
"key": "pone.0281915.ref012",
"volume": "106",
"year": "2022"
},
{
"author": "S Scobie",
"key": "pone.0281915.ref013",
"volume-title": "Quality and inequality: digging deeper",
"year": "2020"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"article-title": "Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies",
"author": "T Bruel",
"doi-asserted-by": "crossref",
"first-page": "1297",
"issue": "6",
"journal-title": "Nat Med",
"key": "pone.0281915.ref014",
"volume": "28",
"year": "2022"
},
{
"key": "pone.0281915.ref015",
"unstructured": "UK Health Security Agency. SARS-CoV-2 therapeutics technical briefing 3: Genomic surveillance. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1074186/therapeutics-programme-technical-briefing-3.pdf. Accessed 13th July 2022."
},
{
"key": "pone.0281915.ref016",
"unstructured": "US Food and Drug Administration. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization. Accessed 22nd April 2022"
}
],
"reference-count": 16,
"references-count": 16,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0281915"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Evaluation of outpatient treatment for non-hospitalised patients with COVID-19: The experience of a regional centre in the UK",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "18"
}
