Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the Immune Response of Elderly Subjects to COVID-19 Vaccination: A Randomized Controlled Trial
et al., Nutrients, doi:10.3390/nu14010228, NCT04756466, Jan 2022
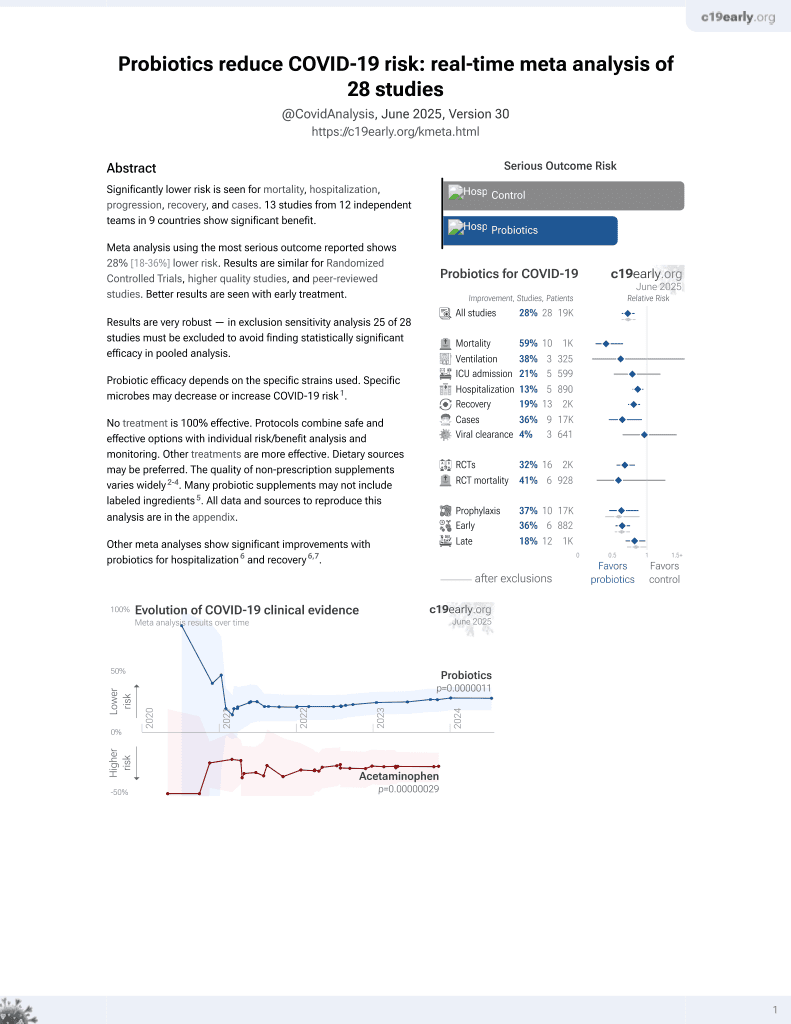
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 200 nursing home residents over 60 years old in Spain showing Loigolactobacillus coryniformis K8 probiotic administration enhanced IgG antibody response in subjects previously infected with SARS-CoV-2 and tended to improve IgA antibody response in those over 85 years old not previously infected, in the context of COVID-19 vaccination. There was no significant difference in incidence of COVID-19 infection between the probiotic and placebo groups during the study. The probiotic group had a higher percentage of asymptomatic COVID-19 cases compared to placebo, without statistical significance.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
|
risk of death, 2.0% higher, RR 1.02, p = 1.00, treatment 1 of 98 (1.0%), control 1 of 100 (1.0%).
|
|
recovery time, 37.8% higher, relative time 1.38, p = 0.56, treatment mean 8.45 (±9.69) n=10, control mean 6.13 (±4.22) n=7.
|
|
risk of severe case, 27.6% higher, RR 1.28, p = 0.75, treatment 5 of 98 (5.1%), control 4 of 100 (4.0%).
|
|
risk of symptomatic case, 2.0% higher, RR 1.02, p = 1.00, treatment 7 of 98 (7.1%), control 7 of 100 (7.0%).
|
|
risk of case, 35.1% higher, RR 1.35, p = 0.53, treatment 11 of 98 (11.2%), control 8 of 100 (8.0%), adjusted per study.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Fernández-Ferreiro et al., 5 Jan 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Spain, peer-reviewed, mean age 83.1, 9 authors, study period January 2021 - April 2021, trial NCT04756466 (history).
Contact: rblanco@biosearchlife.com (corresponding author), anxordes@gmail.com, roi.veiga.gutierrez@sergas.es, ana.maria.hermida.cao@sergas.es, fjformigo@hotmail.com, jamaldonado@biosearchlife.com, crodriguez@biosearchlife.com, obanuelos@biosearchlife.com, molivares@biosearchlife.com.
Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the Immune Response of Elderly Subjects to COVID-19 Vaccination: A Randomized Controlled Trial
Nutrients, doi:10.3390/nu14010228
Elderly people are particularly vulnerable to COVID-19, with a high risk of developing severe disease and a reduced immune response to the COVID-19 vaccine. A randomized, placebocontrolled, double-blind trial to assess the effect of the consumption of the probiotic Loigolactobacillus coryniformis K8 CECT 5711 on the immune response generated by the COVID-19 vaccine in an elderly population was performed. Two hundred nursing home residents >60 yrs that had not COVID-19 were randomized to receive L. coryniformis K8 or a placebo daily for 3 months. All volunteers received a complete vaccination schedule of a mRNA vaccine, starting the intervention ten days after the first dose. Specific IgG and IgA antibody levels were analyzed 56 days after the end of the immunization process. No differences between the groups were observed in the antibody levels. During the intervention, 19 subjects had COVID-19 (11 receiving K8 vs. 8 receiving placebo, p = 0.457). Subgroup analysis in these patients showed that levels of IgG were significantly higher in those receiving K8 compared to placebo (p = 0.038). Among subjects >85 yrs that did not get COVID-19, administration of K8 tended to increase the IgA levels (p = 0.082). The administration of K8 may enhance the specific immune response against COVID-19 and may improve the COVID-19 vaccine-specific responses in elderly populations.
postvaccine immune response in the oldest subjects who were not infected with the virus. These results add evidence to previous clinical data [21, 22] corroborating the capability of the probiotic strain L. coryniformis K8 to enhance the immune response. Probiotic administration may be a natural and safe strategy to improve the efficacy of vaccines, especially in vulnerable populations such as the elderly. Future studies should be performed to determine the role of probiotics in the prevention and mitigation of COVID-19 infection.
Supplementary Materials: The following are available online at https://www.mdpi.com/article/10 .3390/nu14010228/s1, Figure S1 : Flow chart of study procedures, Table S1 : Baseline medication of the subjects participating in the study, Table S2 : Summary of statistics for COVID-19 S1 RBD IgG and IgA antibodies' response, Table S3 . Summary of statistics for cytokines levels.
References
Akatsu, Exploring the Effect of Probiotics, Prebiotics, and Postbiotics in Strengthening Immune Activity in the Elderly, Vaccines, doi:10.3390/vaccines9020136
Anderson, Rouphael, Widge, Jackson, Roberts et al., Safety and Immunogenicity of SARS-CoV-2 MRNA-1273 Vaccine in Older Adults, N. Engl. J. Med, doi:10.1056/NEJMoa2028436
Bellino, COVID-19 Vaccines Approved in the European Union: Current Evidence and Perspectives, Expert Rev. Vaccines, doi:10.1080/14760584.2021.1962304
Boge, Rémigy, Vaudaine, Tanguy, Bourdet-Sicard et al., A Probiotic Fermented Dairy Drink Improves Antibody Response to Influenza Vaccination in the Elderly in Two Randomised Controlled Trials, Vaccine, doi:10.1016/j.vaccine.2009.06.094
Canaday, Carias, Oyebanji, Keresztesy, Wilk et al., Reduced BNT162b2 Messenger RNA Vaccine Response in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Naive Nursing Home Residents, Clin. Infect. Dis, doi:10.1093/cid/ciab447
Ciabattini, Garagnani, Santoro, Rappuoli, Franceschi et al., Shelter from the Cytokine Storm: Pitfalls and Prospects in the Development of SARS-CoV-2 Vaccines for an Elderly Population, doi:10.1007/s00281-020-00821-0
Collier, Ferreira, Kotagiri, Datir, Lim et al., Age-Related Heterogeneity in Immune Responses to SARS-CoV-2 Vaccine BNT162b2, Nature, doi:10.1038/s41586-021-03739-1
Crooke, Ovsyannikova, Poland, Kennedy, Immunosenescence and Human Vaccine Immune Responses, Immun. Ageing, doi:10.1186/s12979-019-0164-9
Crotty, Hybrid Immunity, Science, doi:10.1126/science.abj2258
Demaret, Corroyer-Simovic, Alidjinou, Goffard, Trauet et al., Impaired Functional T-Cell Response to SARS-CoV-2 After Two Doses of BNT162b2 mRNA Vaccine in Older People, Front Immunol, doi:10.3389/fimmu.2021.778679
Docherty, Harrison, Green, Hardwick, Pius et al., Features of 16,749 Hospitalised UK Patients with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol
Ebinger, Fert-Bober, Printsev, Wu, Sun et al., Antibody Responses to the BNT162b2 MRNA Vaccine in Individuals Previously Infected with SARS-CoV-2, Nat. Med, doi:10.1038/s41591-021-01325-6
Fonollá, Gracián, Maldonado-Lobón, Romero, Bédmar et al., Effects of Lactobacillus Coryniformis K8 CECT5711 on the Immune Response to Influenza Vaccination and the Assessment of Common Respiratory Symptoms in Elderly Subjects: A Randomized Controlled Trial, Eur. J. Nutr, doi:10.1007/s00394-017-1573-1
Fortner, Schumacher, First COVID-19 Vaccines Receiving the US FDA and EMA Emergency Use Authorization, Discoveries, doi:10.15190/d.2021.1
Gaebler, Wang, Lorenzi, Muecksch, Finkin et al., Evolution of Antibody Immunity to SARS-CoV-2, Nature, doi:10.1038/s41586-021-03207-w
Garnier-Crussard, Forestier, Gilbert, Krolak-Salmon, Novel Coronavirus (COVID-19) Epidemic: What Are the Risks for Older Patients?, J. Am. Geriatr. Soc, doi:10.1111/jgs.16407
Hevia, Delgado, Sánchez, Margolles, Molecular Players Involved in the Interaction between Beneficial Bacteria and the Immune System, Front. Microbiol, doi:10.3389/fmicb.2015.01285
Kissling, Hooiveld, Martín, Martínez-Baz, William et al., Vaccine Effectiveness against Symptomatic SARS-CoV-2 Infection in Adults Aged 65 Years and Older in Primary Care: I-MOVE-COVID-19 Project, Europe, Eurosurveillance, doi:10.2807/1560-7917.ES.2021.26.29.2100670
Kurian, Unnikrishnan, Miraj, Bagchi, Banerjee et al., Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects, Arch. Med. Res, doi:10.1016/j.arcmed.2021.03.002
Lara-Villoslada, Sierra, Boza, Efectos beneficiosos en niños sanos del consumo de un producto lácteo que contiene dos cepas probióticas. Lactobacillus coryniformis CECT5711 y lactobacillus gasseri CECT5714, Nutr. Hosp
Lee, Knabl, Knabl, Kapferer, Pateter et al., Robust Immune Response to the BNT162b MRNA Vaccine in an Elderly Population Vaccinated 15 Months after Recovery from COVID-19, Infectious Diseases (except HIV/AIDS, doi:10.1101/2021.09.08.21263284v1
Mahooti, Miri, Abdolalipour, Ghaemi, The Immunomodulatory Effects of Probiotics on Respiratory Viral Infections: A Hint for COVID-19 Treatment?, Microb. Pathog, doi:10.1016/j.micpath.2020.104452
Manisty, Otter, Treibel, Mcknight, Altmann et al., Antibody Response to First BNT162b2 Dose in Previously SARS-CoV-2-Infected Individuals, Lancet, doi:10.1016/S0140-6736(21)00501-8
Markewitz, Pauli, Dargvainiene, Steinhagen, Engel et al., The Temporal Course of T-and B-Cell-Responses to Vaccination with BNT162b2 and MRNA-1273, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2021.09.006
Martínez-Cañavate, Sierra, Lara-Villoslada, Romero, Maldonado et al., A Probiotic Dairy Product Containing L. Gasseri CECT5714 and L. Coryniformis CECT5711 Induces Immunological Changes in Children Suffering from Allergy, Pediatr. Allergy Immunol, doi:10.1111/j.1399-3038.2008.00833.x
Mirzaei, Attar, Papizadeh, Jeda, Hosseini-Fard et al., The Emerging Role of Probiotics as a Mitigation Strategy against Coronavirus Disease 2019 (COVID-19), Arch. Virol, doi:10.1007/s00705-021-05036-8
Monge, Olmedo, Alejos, Lapeña, Sierra et al., Direct and Indirect Effectiveness of MRNA Vaccination against Severe Acute Respiratory Syndrome Coronavirus 2 in Long-Term Care Facilities, Spain, Emerg. Infect. Dis, doi:10.3201/eid2710.211184
Moradi-Kalbolandi, Majidzadeh-A, Abdolvahab, Jalili, Farahmand, The Role of Mucosal Immunity and Recombinant Probiotics in SARS-CoV2 Vaccine Development, Probiotics Antimicrob Proteins, doi:10.1007/s12602-021-09773-9
Mueller, Mcnamara, Sinclair, Why Does COVID-19 Disproportionately Affect Older People? Aging, doi:10.18632/aging.103344
Müller, Andrée, Moskorz, Drexler, Walotka et al., Age-Dependent Immune Response to the Biontech/Pfizer BNT162b2 COVID-19 Vaccination, Clin. Infect. Dis, doi:10.1093/cid/ciab381
Nunes, Rodrigues, Kislaya, Cruz, Peralta-Santos et al., MRNA Vaccine Effectiveness against COVID-19-Related Hospitalisations and Deaths in Older Adults: A Cohort Study Based on Data Linkage of National Health Registries in Portugal, February to August 2021, Eurosurveillance, doi:10.2807/1560-7917.ES.2021.26.38.2100833
Olivares, Díaz-Ropero, Gómez, Lara-Villoslada, Sierra et al., The Consumption of Two New Probiotic Strains, Lactobacillus Gasseri CECT 5714 and Lactobacillus Coryniformis CECT 5711, Boosts the Immune System of Healthy Humans, Int. Microbiol
Olivares, Díaz-Ropero, Sierra, Lara-Villoslada, Fonollá et al., Oral Intake of Lactobacillus Fermentum CECT5716 Enhances the Effects of Influenza Vaccination, Nutrition, doi:10.1016/j.nut.2007.01.004
Peroni, Morelli, Probiotics as Adjuvants in Vaccine Strategy: Is There More Room for Improvement?, Vaccines, doi:10.3390/vaccines9080811
Redondo, Nova, Gheorghe, Díaz, Hernández et al., Evaluation of Lactobacillus Coryniformis CECT5711 Strain as a Coadjuvant in a Vaccination Process: A Randomised Clinical Trial in Healthy Adults, Nutr. Metab, doi:10.1186/s12986-016-0154-2
Sakai, Hosoya, Ono-Ohmachi, Ukibe, Ogawa et al., Lactobacillus Gasseri SBT2055 Induces TGF-β Expression in Dendritic Cells and Activates TLR2 Signal to Produce IgA in the Small Intestine, PLoS ONE, doi:10.1371/journal.pone.0105370
Schwarz, Tober-Lau, Hillus, Helbig, Lippert et al., Delayed Antibody and T-Cell Response to BNT162b2 Vaccination in the Elderly, Germany, Emerg. Infect. Dis, doi:10.3201/eid2708.211145
Singh, Rao, Probiotics: A Potential Immunomodulator in COVID-19 Infection Management, Nutr. Res, doi:10.1016/j.nutres.2020.12.014
Sterlin, Mathian, Miyara, Mohr, Anna et al., IgA Dominates the Early Neutralizing Antibody Response to SARS-CoV-2, Sci. Transl. Med, doi:10.1126/scitranslmed.abd2223
Takahashi, Fukudome, Ueno, Watanabe-Matsuhashi, Nakano et al., Effects of Probiotic Supplementation on TGF-B1, TGF-B2, and IgA Levels in the Milk of Japanese Women: An Open-Label Pilot Study, Front. Nutr, doi:10.3389/fnut.2019.00128
Tenforde, Effectiveness of Pfizer-BioNTech and Moderna Vaccines against COVID-19 among Hospitalized Adults Aged ≥65 Years-United States, January-March 2021, MMWR Morb. Mortal. Wkly. Rep, doi:10.15585/mmwr.mm7018e1
Tisminetzky, Delude, Hebert, Carr, Goldberg et al., Multiple Chronic Conditions, and COVID-19: A Literature Review, J. Gerontol. Ser. A, doi:10.1093/gerona/glaa320
Urbanowicz, Tsoleridis, Jackson, Cusin, Duncan et al., Two Doses of the SARS-CoV-2 BNT162b2 Vaccine Enhance Antibody Responses to Variants in Individuals with Prior SARS-CoV-2 Infection, Sci. Transl. Med, doi:10.1126/scitranslmed.abj0847
Van Belle, Martin, Sample Size as a Function of Coefficient of Variation and Ratio of Means, Am. Stat, doi:10.1080/00031305.1993.10475968
Van Praet, Vandecasteele, De Roo, De Vriese, Reynders, Humoral and Cellular Immunogenicity of the BNT162b2 MRNA Covid-19 Vaccine in Nursing Home Residents, Clin. Infect. Dis, doi:10.1093/cid/ciab300
Van Praet, Vandecasteele, De Roo, Vynck, De Vriese et al., Dynamics of the Cellular and Humoral Immune Response after BNT162b2 MRNA Covid-19 Vaccination in Covid-19 Naive Nursing Home Residents, J. Infect. Dis, doi:10.1093/infdis/jiab458
Yanez, Weiss, Romand, Treggiari, COVID-19 Mortality Risk for Older Men and Women, BMC Public Health, doi:10.1186/s12889-020-09826-8
Zheng, Wittouck, Salvetti, Franz, Harris et al., A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae, Int. J. Syst. Evol. Microbiol, doi:10.1099/ijsem.0.004107
DOI record:
{
"DOI": "10.3390/nu14010228",
"ISSN": [
"2072-6643"
],
"URL": "http://dx.doi.org/10.3390/nu14010228",
"abstract": "<jats:p>Elderly people are particularly vulnerable to COVID-19, with a high risk of developing severe disease and a reduced immune response to the COVID-19 vaccine. A randomized, placebo-controlled, double-blind trial to assess the effect of the consumption of the probiotic Loigolactobacillus coryniformis K8 CECT 5711 on the immune response generated by the COVID-19 vaccine in an elderly population was performed. Two hundred nursing home residents >60 yrs that had not COVID-19 were randomized to receive L. coryniformis K8 or a placebo daily for 3 months. All volunteers received a complete vaccination schedule of a mRNA vaccine, starting the intervention ten days after the first dose. Specific IgG and IgA antibody levels were analyzed 56 days after the end of the immunization process. No differences between the groups were observed in the antibody levels. During the intervention, 19 subjects had COVID-19 (11 receiving K8 vs. 8 receiving placebo, p = 0.457). Subgroup analysis in these patients showed that levels of IgG were significantly higher in those receiving K8 compared to placebo (p = 0.038). Among subjects >85 yrs that did not get COVID-19, administration of K8 tended to increase the IgA levels (p = 0.082). The administration of K8 may enhance the specific immune response against COVID-19 and may improve the COVID-19 vaccine-specific responses in elderly populations.</jats:p>",
"alternative-id": [
"nu14010228"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7348-7337",
"affiliation": [
{
"name": "Pharmacy Department, University Clinical Hospital of Santiago de Compostela (SERGAS), 15706 Santiago de Compostela, Spain"
},
{
"name": "Pharmacology Group, Health Research Institute Santiago Compostela (IDIS), 15706 Santiago de Compostela, Spain"
}
],
"authenticated-orcid": false,
"family": "Fernández-Ferreiro",
"given": "Anxo",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Medicina Familiar y Comunitaria, 15706 Santiago de Compostela, Spain"
}
],
"family": "Formigo-Couceiro",
"given": "Francisco J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacy Department, University Clinical Hospital of Santiago de Compostela (SERGAS), 15706 Santiago de Compostela, Spain"
},
{
"name": "Pharmacology Group, Health Research Institute Santiago Compostela (IDIS), 15706 Santiago de Compostela, Spain"
}
],
"family": "Veiga-Gutierrez",
"given": "Roi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research and Development Department, Biosearch Life, a Kerry Company, 18004 Granada, Spain"
}
],
"family": "Maldonado-Lobón",
"given": "Jose A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacy Department, University Clinical Hospital of Santiago de Compostela (SERGAS), 15706 Santiago de Compostela, Spain"
},
{
"name": "Pharmacology Group, Health Research Institute Santiago Compostela (IDIS), 15706 Santiago de Compostela, Spain"
}
],
"family": "Hermida-Cao",
"given": "Ana M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research and Development Department, Biosearch Life, a Kerry Company, 18004 Granada, Spain"
}
],
"family": "Rodriguez",
"given": "Carlos",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7418-5955",
"affiliation": [
{
"name": "Research and Development Department, Biosearch Life, a Kerry Company, 18004 Granada, Spain"
}
],
"authenticated-orcid": false,
"family": "Bañuelos",
"given": "Oscar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research and Development Department, Biosearch Life, a Kerry Company, 18004 Granada, Spain"
}
],
"family": "Olivares",
"given": "Mónica",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6159-3597",
"affiliation": [
{
"name": "Research and Development Department, Biosearch Life, a Kerry Company, 18004 Granada, Spain"
}
],
"authenticated-orcid": false,
"family": "Blanco-Rojo",
"given": "Ruth",
"sequence": "additional"
}
],
"container-title": "Nutrients",
"container-title-short": "Nutrients",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
10
]
],
"date-time": "2022-01-10T01:21:56Z",
"timestamp": 1641777716000
},
"deposited": {
"date-parts": [
[
2024,
7,
24
]
],
"date-time": "2024-07-24T04:18:26Z",
"timestamp": 1721794706000
},
"funder": [
{
"DOI": "10.13039/501100004587",
"award": [
"JR18/00014"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100004587",
"id-type": "DOI"
}
],
"name": "Instituto de Salud Carlos III"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
5
]
],
"date-time": "2024-08-05T18:42:19Z",
"timestamp": 1722883339092
},
"is-referenced-by-count": 20,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
1,
5
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
5
]
],
"date-time": "2022-01-05T00:00:00Z",
"timestamp": 1641340800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2072-6643/14/1/228/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "228",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
1,
5
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
5
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref_1",
"unstructured": "(2021, October 15). WHO COVID-19 Dashboard. Available online: https://covid19.who.int/."
},
{
"DOI": "10.1080/14760584.2021.1962304",
"article-title": "COVID-19 Vaccines Approved in the European Union: Current Evidence and Perspectives",
"author": "Bellino",
"doi-asserted-by": "crossref",
"first-page": "1195",
"journal-title": "Expert Rev. Vaccines",
"key": "ref_2",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.15190/d.2021.1",
"article-title": "First COVID-19 Vaccines Receiving the US FDA and EMA Emergency Use Authorization",
"author": "Fortner",
"doi-asserted-by": "crossref",
"first-page": "e122",
"journal-title": "Discoveries",
"key": "ref_3",
"volume": "9",
"year": "2021"
},
{
"article-title": "Why Does COVID-19 Disproportionately Affect Older People?",
"author": "Mueller",
"first-page": "9959",
"journal-title": "Aging (Albany N. Y.)",
"key": "ref_4",
"volume": "12",
"year": "2020"
},
{
"key": "ref_5",
"unstructured": "Docherty, A., Harrison, E., Green, C., Hardwick, H., Pius, R., Norman, L., Holden, K., Read, J., Dondelinger, F., and Carson, G. (2021, October 15). Features of 16,749 Hospitalised UK Patients with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol, Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7243036/."
},
{
"DOI": "10.1186/s12889-020-09826-8",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Yanez, N.D., Weiss, N.S., Romand, J.-A., and Treggiari, M.M. (2020). COVID-19 Mortality Risk for Older Men and Women. BMC Public Health, 20."
},
{
"DOI": "10.1111/jgs.16407",
"article-title": "Novel Coronavirus (COVID-19) Epidemic: What Are the Risks for Older Patients?",
"author": "Forestier",
"doi-asserted-by": "crossref",
"first-page": "939",
"journal-title": "J. Am. Geriatr. Soc.",
"key": "ref_7",
"volume": "68",
"year": "2020"
},
{
"DOI": "10.1093/gerona/glaa320",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Tisminetzky, M., Delude, C., Hebert, T., Carr, C., Goldberg, R.J., and Gurwitz, J.H. (2020). Age, Multiple Chronic Conditions, and COVID-19: A Literature Review. J. Gerontol. Ser. A."
},
{
"DOI": "10.3201/eid2710.211184",
"article-title": "Direct and Indirect Effectiveness of MRNA Vaccination against Severe Acute Respiratory Syndrome Coronavirus 2 in Long-Term Care Facilities, Spain",
"author": "Monge",
"doi-asserted-by": "crossref",
"first-page": "2595",
"journal-title": "Emerg. Infect. Dis.",
"key": "ref_9",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.2807/1560-7917.ES.2021.26.29.2100670",
"article-title": "Vaccine Effectiveness against Symptomatic SARS-CoV-2 Infection in Adults Aged 65 Years and Older in Primary Care: I-MOVE-COVID-19 Project, Europe, December 2020 to May 2021",
"author": "Kissling",
"doi-asserted-by": "crossref",
"first-page": "2100670",
"journal-title": "Eurosurveillance",
"key": "ref_10",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03739-1",
"article-title": "Age-Related Heterogeneity in Immune Responses to SARS-CoV-2 Vaccine BNT162b2",
"author": "Collier",
"doi-asserted-by": "crossref",
"first-page": "417",
"journal-title": "Nature",
"key": "ref_11",
"volume": "596",
"year": "2021"
},
{
"DOI": "10.3201/eid2708.211145",
"article-title": "Delayed Antibody and T-Cell Response to BNT162b2 Vaccination in the Elderly, Germany",
"author": "Schwarz",
"doi-asserted-by": "crossref",
"first-page": "2174",
"journal-title": "Emerg. Infect. Dis.",
"key": "ref_12",
"volume": "27",
"year": "2021"
},
{
"article-title": "Age-Dependent Immune Response to the Biontech/Pfizer BNT162b2 COVID-19 Vaccination",
"author": "Moskorz",
"first-page": "2065",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_13",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1186/s12979-019-0164-9",
"article-title": "Immunosenescence and Human Vaccine Immune Responses",
"author": "Crooke",
"doi-asserted-by": "crossref",
"first-page": "25",
"journal-title": "Immun. Ageing",
"key": "ref_14",
"volume": "16",
"year": "2019"
},
{
"DOI": "10.1099/ijsem.0.004107",
"article-title": "A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "2782",
"journal-title": "Int. J. Syst. Evol. Microbiol.",
"key": "ref_15",
"volume": "70",
"year": "2020"
},
{
"DOI": "10.3389/fmicb.2015.01285",
"article-title": "Molecular Players Involved in the Interaction between Beneficial Bacteria and the Immune System",
"author": "Hevia",
"doi-asserted-by": "crossref",
"first-page": "1285",
"journal-title": "Front. Microbiol.",
"key": "ref_16",
"volume": "6",
"year": "2015"
},
{
"DOI": "10.1016/j.nut.2007.01.004",
"article-title": "Oral Intake of Lactobacillus Fermentum CECT5716 Enhances the Effects of Influenza Vaccination",
"author": "Olivares",
"doi-asserted-by": "crossref",
"first-page": "254",
"journal-title": "Nutrition",
"key": "ref_17",
"volume": "23",
"year": "2007"
},
{
"DOI": "10.1016/j.vaccine.2009.06.094",
"article-title": "A Probiotic Fermented Dairy Drink Improves Antibody Response to Influenza Vaccination in the Elderly in Two Randomised Controlled Trials",
"author": "Boge",
"doi-asserted-by": "crossref",
"first-page": "5677",
"journal-title": "Vaccine",
"key": "ref_18",
"volume": "27",
"year": "2009"
},
{
"article-title": "The Consumption of Two New Probiotic Strains, Lactobacillus Gasseri CECT 5714 and Lactobacillus Coryniformis CECT 5711, Boosts the Immune System of Healthy Humans",
"author": "Olivares",
"first-page": "47",
"journal-title": "Int. Microbiol.",
"key": "ref_19",
"volume": "9",
"year": "2006"
},
{
"article-title": "Efectos beneficiosos en niños sanos del consumo de un producto lácteo que contiene dos cepas probióticas. Lactobacillus coryniformis CECT5711 y lactobacillus gasseri CECT5714",
"author": "Sierra",
"first-page": "496",
"journal-title": "Nutr. Hosp.",
"key": "ref_20",
"volume": "22",
"year": "2007"
},
{
"DOI": "10.1111/j.1399-3038.2008.00833.x",
"article-title": "A Probiotic Dairy Product Containing L. Gasseri CECT5714 and L. Coryniformis CECT5711 Induces Immunological Changes in Children Suffering from Allergy",
"author": "Sierra",
"doi-asserted-by": "crossref",
"first-page": "592",
"journal-title": "Pediatr. Allergy Immunol.",
"key": "ref_21",
"volume": "20",
"year": "2009"
},
{
"DOI": "10.1186/s12986-016-0154-2",
"article-title": "Evaluation of Lactobacillus Coryniformis CECT5711 Strain as a Coadjuvant in a Vaccination Process: A Randomised Clinical Trial in Healthy Adults",
"author": "Redondo",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Nutr. Metab.",
"key": "ref_22",
"volume": "14",
"year": "2017"
},
{
"DOI": "10.1007/s00394-017-1573-1",
"article-title": "Effects of Lactobacillus Coryniformis K8 CECT5711 on the Immune Response to Influenza Vaccination and the Assessment of Common Respiratory Symptoms in Elderly Subjects: A Randomized Controlled Trial",
"author": "Romero",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Eur. J. Nutr.",
"key": "ref_23",
"volume": "58",
"year": "2019"
},
{
"DOI": "10.3390/vaccines9020136",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Akatsu, H. (2021). Exploring the Effect of Probiotics, Prebiotics, and Postbiotics in Strengthening Immune Activity in the Elderly. Vaccines, 9."
},
{
"DOI": "10.3390/vaccines9080811",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Peroni, D.G., and Morelli, L. (2021). Probiotics as Adjuvants in Vaccine Strategy: Is There More Room for Improvement?. Vaccines, 9."
},
{
"DOI": "10.1056/NEJMoa2028436",
"article-title": "Safety and Immunogenicity of SARS-CoV-2 MRNA-1273 Vaccine in Older Adults",
"author": "Anderson",
"doi-asserted-by": "crossref",
"first-page": "2427",
"journal-title": "N. Engl. J. Med.",
"key": "ref_26",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1080/00031305.1993.10475968",
"article-title": "Sample Size as a Function of Coefficient of Variation and Ratio of Means",
"author": "Martin",
"doi-asserted-by": "crossref",
"first-page": "165",
"journal-title": "Am. Stat.",
"key": "ref_27",
"volume": "47",
"year": "1993"
},
{
"key": "ref_28",
"unstructured": "Centers for Disease Control and Prevention (2021, September 27). COVID-19 (coronavirus disease): Clinical Spectrum of SARS-CoV-2 Infection, Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/."
},
{
"DOI": "10.1007/s00281-020-00821-0",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Ciabattini, A., Garagnani, P., Santoro, F., Rappuoli, R., Franceschi, C., and Medaglini, D. (2020). Shelter from the Cytokine Storm: Pitfalls and Prospects in the Development of SARS-CoV-2 Vaccines for an Elderly Population. Seminars in Immunopathology, Springer."
},
{
"DOI": "10.2807/1560-7917.ES.2021.26.38.2100833",
"article-title": "MRNA Vaccine Effectiveness against COVID-19-Related Hospitalisations and Deaths in Older Adults: A Cohort Study Based on Data Linkage of National Health Registries in Portugal, February to August 2021",
"author": "Nunes",
"doi-asserted-by": "crossref",
"first-page": "2100833",
"journal-title": "Eurosurveillance",
"key": "ref_30",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7018e1",
"article-title": "Effectiveness of Pfizer-BioNTech and Moderna Vaccines against COVID-19 among Hospitalized Adults Aged ≥65 Years—United States, January–March 2021",
"author": "Tenforde",
"doi-asserted-by": "crossref",
"first-page": "674",
"journal-title": "MMWR Morb. Mortal. Wkly. Rep.",
"key": "ref_31",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01325-6",
"article-title": "Antibody Responses to the BNT162b2 MRNA Vaccine in Individuals Previously Infected with SARS-CoV-2",
"author": "Ebinger",
"doi-asserted-by": "crossref",
"first-page": "981",
"journal-title": "Nat. Med.",
"key": "ref_32",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abj0847",
"article-title": "Two Doses of the SARS-CoV-2 BNT162b2 Vaccine Enhance Antibody Responses to Variants in Individuals with Prior SARS-CoV-2 Infection",
"author": "Urbanowicz",
"doi-asserted-by": "crossref",
"first-page": "eabj0847",
"journal-title": "Sci. Transl. Med.",
"key": "ref_33",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00501-8",
"article-title": "Antibody Response to First BNT162b2 Dose in Previously SARS-CoV-2-Infected Individuals",
"author": "Manisty",
"doi-asserted-by": "crossref",
"first-page": "1057",
"journal-title": "Lancet",
"key": "ref_34",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1101/2021.09.08.21263284",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Lee, H.K., Knabl, L., Knabl, L., Kapferer, S., Pateter, B., Walter, M., Furth, P.A., and Hennighausen, L. (2021, October 21). Robust Immune Response to the BNT162b MRNA Vaccine in an Elderly Population Vaccinated 15 Months after Recovery from COVID-19. Available online: https://www.medrxiv.org/content/10.1101/2021.09.08.21263284v1."
},
{
"DOI": "10.3389/fimmu.2021.778679",
"article-title": "Impaired Functional T-Cell Response to SARS-CoV-2 After Two Doses of BNT162b2 mRNA Vaccine in Older People",
"author": "Demaret",
"doi-asserted-by": "crossref",
"first-page": "778679",
"journal-title": "Front Immunol.",
"key": "ref_36",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1126/science.abj2258",
"article-title": "Hybrid Immunity",
"author": "Crotty",
"doi-asserted-by": "crossref",
"first-page": "1392",
"journal-title": "Science",
"key": "ref_37",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1007/s12602-021-09773-9",
"article-title": "The Role of Mucosal Immunity and Recombinant Probiotics in SARS-CoV2 Vaccine Development",
"author": "Abdolvahab",
"doi-asserted-by": "crossref",
"first-page": "1239",
"journal-title": "Probiotics Antimicrob Proteins",
"key": "ref_38",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2021.09.006",
"doi-asserted-by": "crossref",
"key": "ref_39",
"unstructured": "Markewitz, R., Pauli, D., Dargvainiene, J., Steinhagen, K., Engel, S., Herbst, V., Zapf, D., Krüger, C., Sharifzadeh, S., and Schomburg, B. (2021). The Temporal Course of T- and B-Cell-Responses to Vaccination with BNT162b2 and MRNA-1273. Clin. Microbiol. Infect."
},
{
"DOI": "10.1126/scitranslmed.abd2223",
"article-title": "IgA Dominates the Early Neutralizing Antibody Response to SARS-CoV-2",
"author": "Sterlin",
"doi-asserted-by": "crossref",
"first-page": "eabd2223",
"journal-title": "Sci. Transl. Med.",
"key": "ref_40",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03207-w",
"article-title": "Evolution of Antibody Immunity to SARS-CoV-2",
"author": "Gaebler",
"doi-asserted-by": "crossref",
"first-page": "639",
"journal-title": "Nature",
"key": "ref_41",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab447",
"article-title": "Reduced BNT162b2 Messenger RNA Vaccine Response in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)–Naive Nursing Home Residents",
"author": "Canaday",
"doi-asserted-by": "crossref",
"first-page": "2112",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_42",
"volume": "73",
"year": "2021"
},
{
"article-title": "Dynamics of the Cellular and Humoral Immune Response after BNT162b2 MRNA Covid-19 Vaccination in Covid-19 Naive Nursing Home Residents",
"author": "Vandecasteele",
"first-page": "1690",
"journal-title": "J. Infect. Dis.",
"key": "ref_43",
"volume": "224",
"year": "2021"
},
{
"article-title": "Humoral and Cellular Immunogenicity of the BNT162b2 MRNA Covid-19 Vaccine in Nursing Home Residents",
"author": "Vandecasteele",
"first-page": "2145",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_44",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.3389/fnut.2019.00128",
"article-title": "Effects of Probiotic Supplementation on TGF-Β1, TGF-Β2, and IgA Levels in the Milk of Japanese Women: An Open-Label Pilot Study",
"author": "Takahashi",
"doi-asserted-by": "crossref",
"first-page": "128",
"journal-title": "Front. Nutr.",
"key": "ref_45",
"volume": "6",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0105370",
"doi-asserted-by": "crossref",
"key": "ref_46",
"unstructured": "Sakai, F., Hosoya, T., Ono-Ohmachi, A., Ukibe, K., Ogawa, A., Moriya, T., Kadooka, Y., Shiozaki, T., Nakagawa, H., and Nakayama, Y. (2014). Lactobacillus Gasseri SBT2055 Induces TGF-β Expression in Dendritic Cells and Activates TLR2 Signal to Produce IgA in the Small Intestine. PLoS ONE, 9."
},
{
"key": "ref_47",
"unstructured": "(2016). EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Guidance on the Scientific Requirements for Health Claims Related to the Immune System, the Gastrointestinal Tract and Defence against Pathogenic Microorganisms. EFSA J., 14, 4369."
},
{
"DOI": "10.1016/j.arcmed.2021.03.002",
"article-title": "Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects",
"author": "Kurian",
"doi-asserted-by": "crossref",
"first-page": "582",
"journal-title": "Arch. Med. Res.",
"key": "ref_48",
"volume": "52",
"year": "2021"
},
{
"DOI": "10.1016/j.nutres.2020.12.014",
"article-title": "Probiotics: A Potential Immunomodulator in COVID-19 Infection Management",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Nutr. Res.",
"key": "ref_49",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.1016/j.micpath.2020.104452",
"article-title": "The Immunomodulatory Effects of Probiotics on Respiratory Viral Infections: A Hint for COVID-19 Treatment?",
"author": "Mahooti",
"doi-asserted-by": "crossref",
"first-page": "104452",
"journal-title": "Microb. Pathog.",
"key": "ref_50",
"volume": "148",
"year": "2020"
},
{
"DOI": "10.1007/s00705-021-05036-8",
"article-title": "The Emerging Role of Probiotics as a Mitigation Strategy against Coronavirus Disease 2019 (COVID-19)",
"author": "Mirzaei",
"doi-asserted-by": "crossref",
"first-page": "1819",
"journal-title": "Arch. Virol.",
"key": "ref_51",
"volume": "166",
"year": "2021"
}
],
"reference-count": 51,
"references-count": 51,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2072-6643/14/1/228"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effects of Loigolactobacillus coryniformis K8 CECT 5711 on the Immune Response of Elderly Subjects to COVID-19 Vaccination: A Randomized Controlled Trial",
"type": "journal-article",
"volume": "14"
}