Discovery and Intranasal Administration of a SARS-CoV-2 Broadly-Acting Neutralizing Antibody with Activity against multiple Omicron sub variants
et al., Med, doi:10.1016/j.medj.2022.08.002, Aug 2022
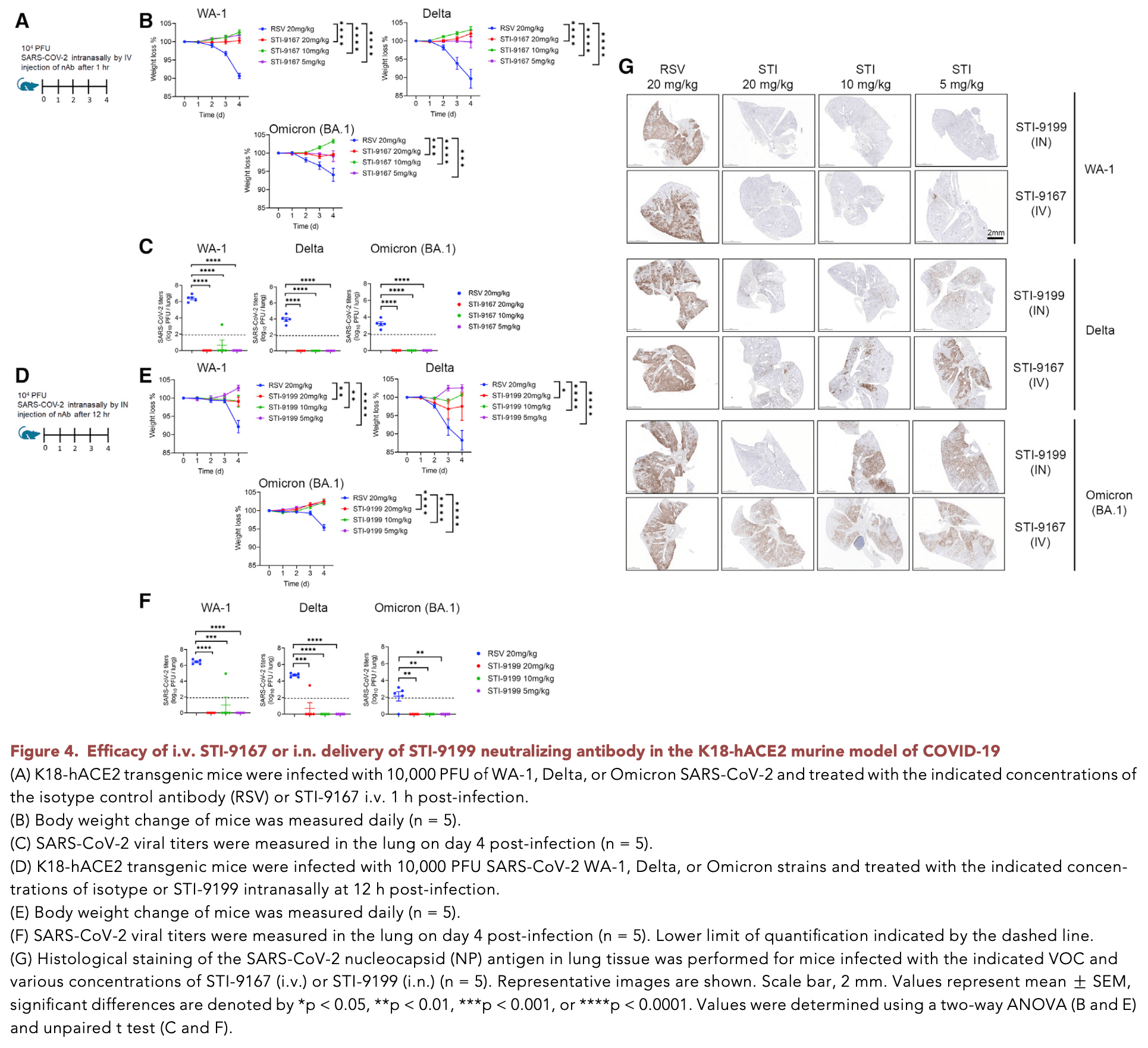
In vitro and mouse study showing broad neutralizing activity of a novel human monoclonal antibody STI-9167 against SARS-CoV-2 variants, including omicron subvariants. Authors demonstrated that STI-9167 effectively neutralized pseudotyped and live virus particles of multiple SARS-CoV-2 variants, including delta and omicron BA.1, BA.1.1, and BA.2. The antibody bound strongly to spike proteins from various variants and showed neutralizing potency within 10-fold of that measured against the original Wuhan strain. In K18-hACE2 transgenic mice infected with WA-1, delta, or omicron BA.1 strains, both intravenous and intranasal administration of STI-9167 provided protection against weight loss and reduced lung viral titers to below detectable levels. Intranasal delivery resulted in higher antibody concentrations in lung lavage fluid compared to intravenous delivery. The study suggests STI-9167 could be a promising therapeutic candidate for COVID-19, potentially deliverable through both intravenous and intranasal routes.
Duty et al., 31 Aug 2022, USA, peer-reviewed, 49 authors.
Contact: hji@sorrentotherapeutics.com.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Discovery and intranasal administration of a SARS-CoV-2 broadly acting neutralizing antibody with activity against multiple Omicron subvariants
Med, doi:10.1016/j.medj.2022.08.002
The burden of COVID-19 and the emergence of virus variants necessitates continued exploration of neutralizing antibody therapies and methods of treatment. Duty et al. identify a human monoclonal antibody that neutralizes recently described SARS-CoV-2 variants and, when administered intranasally or intravenously, offers protection in a mouse model of SARS-CoV-2 disease.
Detailed methods are provided in the online version of this paper and include the following:
SUPPLEMENTAL INFORMATION Supplemental information can be found online at https://doi.org/10.1016/j.medj. 2022.08.002.
AUTHOR CONTRIBUTIONS
DECLARATION OF INTERESTS The A.G.-S. laboratory has received research support from Pfizer, Senhwa Biosciences, Kenall Manufacturing, Avimex, Johnson & Johnson, Dynavax, 7Hills Pharma, Pharmamar, ImmunityBio, Accurius, Nanocomposix, Hexamer, N-fold LLC, Model Medicines, Atea Pharma, and Merck, outside of the reported work. A.G.-S. has consulting agreements for the following companies involving cash and/or stock: Vivaldi Biosciences, Contrafect, 7Hills Pharma, Avimex, Vaxalto, Pagoda, Accurius, Esperovax, Farmak, Applied Biological Laboratories, Pharmamar, Paratus, CureLab Oncology, CureLab Veterinary, and Pfizer, outside of the reported work. A.G.-S. is inventor on patents and patent applications on the use of antivirals and vaccines for the treatment and prevention of virus infections and cancer, owned by the Icahn School of Medicine at Mount Sinai, New York, outside of the reported work. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and NDV-based SARS-CoV-2 vaccines, which list F.K. as co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. F.K. has consulted for Merck and Pfizer (before 2020) and is currently consulting..
References
Abdelnabi, Foo, Zhang, Lemmens, Maes et al., does not readily infect Syrian hamsters, doi:10.1101/2021.12.24.474086
Aggarwal, Stella, Walker, Akerman, Milogiannakis et al., SARS-CoV-2 Omicron: evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern, doi:10.1101/2021.12.14.21267772
Ahn, Shin, Kim, Lee, Kim et al., Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19), J. Microbiol. Biotechnol, doi:10.4014/jmb.2003.03011
Amanat, Krammer, SARS-CoV-2 vaccines: status report, Immunity, doi:10.1016/j.immuni.2020.03.007
Arce, Costoya, SARS-CoV-2 infection in K18-ACE2 transgenic mice replicates human pulmonary disease in COVID-19, Cell. Mol. Immunol, doi:10.1038/s41423-020-00616-1(2021
Bedford, Enria, Giesecke, Heymann, Ihekweazu et al., COVID-19: towards controlling of a pandemic, Lancet, doi:10.1073/pnas.88.18.7978
Cameroni, Saliba, Bowen, Rosen, Culap et al., Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift, doi:10.1101/2021.12.12.472269
Cao, Maruyama, Zhou, Kerwin, Sattler et al., Discovery and development of human SARS-CoV-2 neutralizing antibodies using an unbiased phage display library approach, doi:10.1101/2020.09.27.316174
Cao, Wang, Jian, Xiao, Song et al., Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, doi:10.1101/2021.12.07.470392
Cao, Yisimayi, Jian, Song, Xiao et al., and BA.5 escape antibodies elicited by Omicron infection, BA, doi:10.1101/2022.04.30.489997
Cobb, Nkolola, Gilchuk, Chandrashekar, Yu et al., A combination of two human neutralizing antibodies prevents SARS-CoV-2 infection in cynomolgus macaques, Med, doi:10.1016/j.medj.2022.01.004
Diamond, Halfmann, Maemura, Iwatsuki-Horimoto, Iida et al., The SARS-CoV-2 B.1.1.529 Omicron virus causes attenuated infection and disease in mice and hamsters, Res. Sq, doi:10.21203/rs.3.rs-1211792/v1
Donoghue, Hsieh, Baronas, Godbout, Gosselin et al., A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9, Circ. Res, doi:10.1161/01.res.87.5.e1
Elbe, Buckland-Merrett, Data, disease and diplomacy: GISAID's innovative contribution to global health, Glob. Chall, doi:10.1002/gch2.1018
Fu, Maruyama, Singh, Lim, Ledesma et al., Protective effects of STI-2020 antibody delivered postinfection by the intranasal or intravenous route in a Syrian golden hamster COVID-19 model, doi:10.1101/2020.10.28.359836
Gardner, Stein, Duty, Schwarz, Noriega et al., Functional screening for anti-CMV biologics identifies a broadly neutralizing epitope of an essential envelope protein, Nat. Commun, doi:10.1038/ncomms13627
Gupta, Gonzalez-Rojas, Juarez, Crespo Casal, Moya et al., Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N. Engl. J. Med, doi:10.1056/NEJMoa2107934
Hadfield, Megill, Bell, Huddleston, Potter et al., Nextstrain: real-time tracking of pathogen evolution, Bioinformatics, doi:10.1093/bioinformatics/bty407
Halwe, Kupke, Vanshylla, Liberta, Gruell et al., Intranasal administration of a monoclonal neutralizing antibody protects mice against SARS-CoV-2 infection, Viruses
Hamming, Timens, Bulthuis, Lely, Navis et al., Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis, J. Pathol, doi:10.1002/path.1570
Hansen, Baum, Pascal, Russo, Giordano et al., Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail, Science, doi:10.1126/science.abd0827
Harmer, Gilbert, Borman, Clark, Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme, FEBS Lett, doi:10.1016/s0014-5793(02)03640-2
Hoffmann, Kru ¨ger, Schulz, Cossmann, Rocha et al., The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic, Cell, doi:10.1016/j.cell.2021.12.032
Ikegame, Siddiquey, Hung, Haas, Brambilla et al., Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants, Nat. Commun, doi:10.1038/s41467-021-24909-9(2021
Iketani, Liu, Guo, Liu, Chan et al., Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature, doi:10.1038/s41586-022-04594-4
Korber, Fischer, Gnanakaran, Yoon, Theiler et al., Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus, Cell, doi:10.1038/s41577-020-00434-6
Lee, Wheatley, Kent, Dekosky, Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies, Nat. Microbiol, doi:10.1038/s41564-020-00789-5
Leyva-Grado, Tan, Leon, Yondola, Palese, Direct administration in the respiratory tract improves efficacy of broadly neutralizing anti-influenza virus monoclonal antibodies, Antimicrob. Agents Chemother, doi:10.1128/aac.00290-15
Li, Edwards, Manne, Martinez, Scha ¨fer et al., In vitro and in vivo functions of SARS-CoV-2 infectionenhancing and neutralizing antibodies, Cell, doi:10.1128/aac.00290-15
Liu, Iketani, Guo, Chan, Wang et al., Striking antibody evasion manifested by the omicron variant of SARS-CoV-2, doi:10.1101/2021.12.14.472719
Lu, Hwang, Liu, Lee, Tsai et al., Development of therapeutic antibodies for the treatment of diseases, J. Biomed. Sci, doi:10.1186/s12929-019-0592-z
Ly-, bebtelovimab) potently neutralizes SARS-CoV-2 variants, doi:10.1101/2021.04.30.442182
Mascola, Graham, Fauci, SARS-CoV-2 viral variants-tackling a moving target, JAMA, doi:10.1001/jama.2021.2088
Mcmahan, Giffin, Tostanoski, Chung, Siamatu et al., Reduced pathogenicity of the SARS-CoV-2 omicron variant in hamsters, doi:10.1101/2022.01.02.474743
Mullen, Latif, Alkuzweny, Cano, Haag et al., None
Mun ˜oz-Fontela, Dowling, Funnell, Gsell, Riveros-Balta et al., Animal models for COVID-19, Nature, doi:10.1038/s41586-020-2787-6
Piepenbrink, Park, Oladunni, Deshpande, Basu et al., Therapeutic activity of an inhaled potent SARS-CoV-2 neutralizing human monoclonal antibody in hamsters, Cell Rep. Med, doi:10.1038/s41467-020-15562-9(2021
Pinto, Park, Beltramello, Walls, Tortorici et al., Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody, Nature, doi:10.1038/s41586-020-2349-y
Planas, Saunders, Maes, Guivel-Benhassine, Planchais et al., Considerable escape of SARS-CoV-2 variant Omicron to antibody neutralization, doi:10.1101/2021.12.14.472630
Poh, Carissimo, Wang, Amrun, Lee et al., Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients, Nat. Commun, doi:10.1038/s41467-020-16638-2
Pulliam, Van Schalkwyk, Govender, Gottberg, Cohen et al., Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa, doi:10.1101/2021.11.11.21266068
Radvak, Kwon, Kosikova, Ortega-Rodriguez, Xiang et al., SARS-CoV-2 B.1.1.7 (alpha) and B.1.351 (beta) variants induce pathogenic patterns in K18-hACE2 transgenic mice distinct from early strains, Nat. Commun, doi:10.1038/s41467-021-26803-w(2021
Ramakrishnan, Determination of 50% endpoint titer using a simple formula, World J. Virol, doi:10.5501/wjv.v5.i2.85
Schlothauer, Herter, Koller, Grau-Richards, Steinhart et al., Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions, Protein Eng. Des. Sel, doi:10.1093/protein/gzw040
Scott, Hsiao, Moyo, Singh, Tegally et al., None
Shi, Shan, Duan, Chen, Liu et al., A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2, Nature, doi:10.1038/s41586-020-2381-y
Tan, O'dell, Hernandez, Sordillo, Kahn et al., Human anti-neuraminidase antibodies reduce airborne transmission of clinical influenza virus isolates in the Guinea pig model, J. Virol, doi:10.1128/jvi.01421-21
Uriu, Kimura, Shirakawa, Takaori-Kondo, Nakada et al., Neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine serum, N. Engl. J. Med, doi:10.1056/NEJMc2114706
Vanblargan, Errico, Halfmann, Zost, Crowe et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, doi:10.1101/2021.12.15.472828
Wang, Guo, Iketani, Li, Mohri et al., .5 subvariants evolved to extend antibody evasion, doi:10.1101/2022.05.26.493517
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2035002
Weltzin, Hsu, Mittler, Georgakopoulos, Monath, Intranasal monoclonal immunoglobulin A against respiratory syncytial virus protects against upper and lower respiratory tract infections in mice, Antimicrob. Agents Chemother, doi:10.1128/aac.38.12.2785
Weltzin, Monath, Intranasal antibody prophylaxis for protection against viral disease, Clin. Microbiol. Rev, doi:10.1128/CMR.12.3.383
Weltzin, Traina-Dorge, Soike, Zhang, Mack et al., Intranasal monoclonal IgA antibody to respiratory syncytial virus protects rhesus monkeys against upper and lower respiratory tract infection, J. Infect. Dis, doi:10.1093/infdis/174.2.256
Wen, Cheng, Ling, Dai, Huang et al., Antibody-dependent enhancement of coronavirus, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.09.015
Westendorf, Zentelis, Wang, Foster, Vaillancourt et al., None
Widjaja, Wang, Van Haperen, Gutie ´rrez-A ´lvarez, Van Dieren et al., Towards a solution to MERS: protective human monoclonal antibodies targeting different domains and functions of the MERS-coronavirus spike glycoprotein, Emerg. Microbes Infect, doi:10.1080/22221751.2019.1597644
Winkler, Bailey, Kafai, Nair, Mccune et al., SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function, Nat. Immunol, doi:10.1038/s41590-020-0778-2
Yinda, Port, Bushmaker, Offei Owusu, Purushotham et al., K18-hACE2 mice develop respiratory disease resembling severe COVID-19, PLoS Pathog, doi:10.1371/journal.ppat.1009195
Zhang, Yang, Xiang, Cui, Liu et al., Intranasal administration of SARS-CoV-2 neutralizing human antibody prevents infection in mice, doi:10.1101/2020.12.08.416677
Zhu, Zhang, Wang, Li, Yang et al., A novel coronavirus from patients with pneumonia in China, N. Engl. J. Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.1016/j.medj.2022.08.002",
"ISSN": [
"2666-6340"
],
"URL": "http://dx.doi.org/10.1016/j.medj.2022.08.002",
"alternative-id": [
"S266663402200321X"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Discovery and Intranasal Administration of a SARS-CoV-2 Broadly-Acting Neutralizing Antibody with Activity against multiple Omicron sub variants"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Med"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.medj.2022.08.002"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Elsevier Inc."
}
],
"author": [
{
"affiliation": [],
"family": "Duty",
"given": "J. Andrew",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kraus",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhou",
"given": "Heyue",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Yanliang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shaabani",
"given": "Namir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yildiz",
"given": "Soner",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Du",
"given": "Na",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Alok",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miorin",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Donghui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stegman",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ophir",
"given": "Sabrina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cao",
"given": "Xia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Atanasoff",
"given": "Kristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Reyna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mena",
"given": "Ignacio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouvier",
"given": "Nicole M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kowdle",
"given": "Shreyas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carreño",
"given": "Juan Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rivero-Nava",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raskin",
"given": "Ariel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson",
"given": "Sachi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rathnasinghe",
"given": "Raveen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pai",
"given": "Chin I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kehrer",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cabral",
"given": "Elizabeth Paz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jangra",
"given": "Sonia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Healy",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Gagandeep",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Warang",
"given": "Prajakta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simon",
"given": "Viviana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sordillo",
"given": "Emilia Mia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van Bakel",
"given": "Harm",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Yonghong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Weina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kerwin",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teijaro",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schotsaert",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krammer",
"given": "Florian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bresson",
"given": "Damien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Sastre",
"given": "Adolfo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fu",
"given": "Yanwen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Benhur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Powers",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moran",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ji",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tortorella",
"given": "Domenico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allen",
"given": "Robert",
"sequence": "additional"
}
],
"container-title": "Med",
"container-title-short": "Med",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
8,
8
]
],
"date-time": "2022-08-08T22:51:32Z",
"timestamp": 1659999092000
},
"deposited": {
"date-parts": [
[
2022,
8,
8
]
],
"date-time": "2022-08-08T22:51:32Z",
"timestamp": 1659999092000
},
"indexed": {
"date-parts": [
[
2022,
8,
8
]
],
"date-time": "2022-08-08T23:12:30Z",
"timestamp": 1660000350255
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T00:00:00Z",
"timestamp": 1659312000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S266663402200321X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S266663402200321X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
8
]
]
},
"published-print": {
"date-parts": [
[
2022,
8
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S266663402200321X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Discovery and Intranasal Administration of a SARS-CoV-2 Broadly-Acting Neutralizing Antibody with Activity against multiple Omicron sub variants",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}