Comparative effectiveness of sotrovimab versus no treatment in non-hospitalised high-risk COVID-19 patients in north west London: a retrospective cohort study
et al., BMJ Open Respiratory Research, doi:10.1136/bmjresp-2023-002238, Jul 2023
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
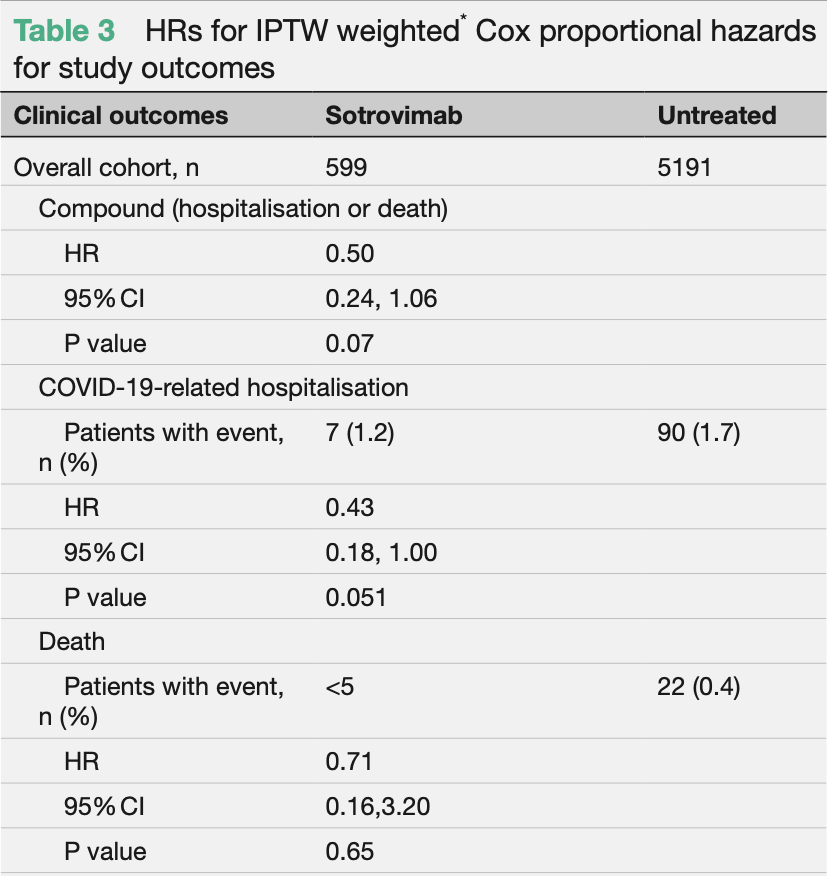
Retrospective 599 high-risk sotrovimab patients and 5,191 untreated controls, showing lower hospitalization/mortality with treatment, without statistical significance in the overall cohort. Efficacy was better for those ≥65, and efficacy was lower in later time periods.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending sotrovimab also recommended them, or
because the patient seeking out sotrovimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.14-6, BA.4, BA.57, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.18, and no efficacy for BA.29, XBB, XBB.1.5, ХВВ.1.9.110, XBB.1.16, BQ.1.1.45, and CL.18. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments11.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 29.0% lower, HR 0.71, p = 0.65, treatment 599, control 5,191, propensity score weighting, Cox proportional hazards.
|
|
risk of death, 45.0% lower, HR 0.55, p = 0.55, ≥65 years old, propensity score weighting, Cox proportional hazards.
|
|
risk of death, 98.0% higher, HR 1.98, p = 0.55, <65 years old, propensity score weighting, Cox proportional hazards.
|
|
risk of death, 41.0% lower, HR 0.59, p = 0.62, period 2, propensity score weighting, Cox proportional hazards.
|
|
risk of death, 4.0% higher, HR 1.04, p = 0.97, period 3, propensity score weighting, Cox proportional hazards.
|
|
risk of death/hospitalization, 50.0% lower, HR 0.50, p = 0.07, treatment 599, control 5,191, propensity score weighting, Cox proportional hazards.
|
|
risk of hospitalization, 57.0% lower, HR 0.43, p = 0.05, treatment 599, control 5,191, propensity score weighting, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
5.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
6.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
7.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
8.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
9.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Drysdale et al., 27 Jul 2023, retrospective, United Kingdom, peer-reviewed, 14 authors, study period August 2020 - March 2021.
Contact: myriam.g.drysdale@gsk.com.
Comparative effectiveness of sotrovimab versus no treatment in non-hospitalised high-risk COVID-19 patients in north west London: a retrospective cohort study
BMJ Open Respiratory Research, doi:10.1136/bmjresp-2023-002238
Background We assessed the effectiveness of sotrovimab vs no early COVID-19 treatment in highest-risk COVID-19 patients during Omicron predominance. Methods Retrospective cohort study using the Discover dataset in North West London. Included patients were nonhospitalised, aged ≥12 years and met ≥1 National Health Service highest-risk criterion for sotrovimab treatment. We used Cox proportional hazards models to compare HRs of 28-day COVID-19-related hospitalisation/death between highest-risk sotrovimab-treated and untreated patients. Age, renal disease and Omicron subvariant subgroup analyses were performed. Results We included 599 sotrovimab-treated patients and 5191 untreated patients. Compared with untreated patients, the risk of COVID-19 hospitalisation/death (HR 0.50, 95% CI 0.24, 1.06; p=0.07) and the risk of COVID-19 hospitalisation (HR 0.43, 95% CI 0.18, 1.00; p=0.051) were both lower in the sotrovimab-treated group; however, statistical significance was not reached. In the ≥65 years and renal disease subgroups, sotrovimab was associated with a significantly reduced risk of COVID-19 hospitalisation, by 89% (HR 0.11, 95% CI 0.02, 0.82; p=0.03) and 82% (HR 0.18, 95% CI 0.05, 0.62; p=0.007), respectively. Conclusions Risk of COVID-19 hospitalisation in sotrovimab-treated patients aged ≥65 years and with renal disease was significantly lower compared with untreated patients. Overall, risk of hospitalisation was also lower for sotrovimab-treated patients, but statistical significance was not reached. ⇒ This study begins to fill the evidence gap in relation to early treatments for mild-to-moderate COVID-19, particularly their effectiveness against disease caused by Omicron variants to date. on April 14,
Contributors MD, MJY, VP, BL, DCG, JDW, SY, BFP, HJB, TK and SJB designed the study; ERG performed inverse probability of treatment weighted survival analysis; MJY performed descriptive analysis; MD, ERG, MJY, BL, DCG, JDW, SY, BFP, EJL, WK, TK and SJB interpreted results. MD is responsible for the overall content as the guarantor. All authors took part in drafting, revising or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Competing interests MD, DCG, EJL, WK and HJB are employees of, and/or shareholders in, GSK. VP was an employee of GSK at the time of the study and is now an employee of KVM Analytics. ERG, MJY, JDW, SY, BFP and TK are (or were at time of study) employees of Imperial College Health Partners, which received funding from GSK and Vir Biotechnology to conduct the study. BL is an employee of OPEN Health, which received funding from GSK and Vir Biotechnology, Inc, to conduct the study. A consultancy fee was paid to SJB's institutional account. Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not applicable. Provenance and peer review Not commissioned; externally peer reviewed. Data availability statement Data are available upon reasonable request. The..
References
Austin, Stuart, Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies, Stat Med, doi:10.1002/sim.6607
Bottle, Cohen, Lucas, How an electronic health record became a real-world research resource: comparison between London's whole systems integrated care database and the clinical practice research Datalink, BMC Med Inform Decis Mak, doi:10.1186/s12911-020-1082-7
Bruel, Hadjadj, Maes, Serum neutralization of SARS-Cov-2 Omicron Sublineages BA.1 and BA.2 in patients receiving Monoclonal antibodies, Nat Med, doi:10.1038/s41591-022-01792-5
Case, Mackin, Errico, Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-Cov-2 Omicron lineage strains, Nat Commun, doi:10.1038/s41467-022-31615-7
Cathcart, Havenar-Daughton, Lempp, The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-Cov-2
Cheng, Reyes, Satram, Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-Cov-2 Delta and Omicron waves in the USA, Infect Dis Ther, doi:10.1007/s40121-022-00755-0
Drysdale, Gibbons, Singh, Real-world effectiveness of Sotrovimab for the treatment of SARS-Cov-2 infection during Omicron BA.2 Subvariant predominance: a systematic literature review, Infection, doi:10.1007/s15010-023-02098-5
Drysdale, for SARS-Cov-2 infection during the Omicron wave in England (Qcovid4): cohort study, BMJ Open Respir Res, doi:10.1136/bmj-2022-072976
Gaudinski, Coates, Houser, Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults, PLoS Med, doi:10.1371/journal.pmed.1002493
Gupta, Gonzalez-Rojas, Juarez, Effect of Sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2022.2832
Harman, Nash, Webster, Comparison of the risk of hospitalisation among BA.1 and BA.2 COVID-19 cases treated with sotrovimab in the community in England, Influenza Other Respir Viruses, doi:10.1111/irv.13150
Hippisley-Cox, Khunti, Sheikh, Risk prediction of COVID-19 related death or hospital admission in adults testing on April 14, 2024 by guest
Ko, Pegu, Rudicell, Enhanced neonatal Fc receptor function improves protection against primate SHIV infection, Nature, doi:10.1038/nature13612
Mendiola-Pastrana, López-Ortiz, De La Loza-Zamora, SARS-Cov-2 variants and clinical outcomes: a systematic review, Life, doi:10.3390/life12020170
Paraskevis, Gkova, Mellou, Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir as treatments for COVID-19 in patients at high risk, J Infect Dis, doi:10.1093/infdis/jiad324
Park, Pinto, Walls, Imprinted antibody responses against SARS-Cov-2 Omicron sublineages, Science, doi:10.1126/science.adc9127
Patel, Levick, Boult, Characteristics and outcomes of COVID-19 patients presumed to be treated with sotrovimab in Nhs hospitals in England
Patel, Yarwood, Levick, Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving Sotrovimab, oral Antivirals or no treatment in England
Pinto, Park, Beltramello, Cross-neutralization of SARS-Cov-2 by a human Monoclonal SARS-Cov antibody, Nature, doi:10.1038/s41586-020-2349-y
Tazare, Nab, Zheng, Effectiveness of Sotrovimab and Molnupiravir in community settings in England across the Omicron Ba.1 and Ba.2 Sublineages: emulated target trials using the OpenSAFELY platform
Wai, Chan, Cheung, Association of molnupiravir and nirmatrelvir-ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19, Lancet Reg Health West Pac, doi:10.1016/j.lanwpc.2022.100602
Xu, Ross, Raebel, Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals, Value Health, doi:10.1111/j.1524-4733.2009.00671.x
Yip, Lui, Lai, Impact of the use of oral antiviral agents on the risk of hospitalization in community Coronavirus disease, Clinical Infectious Diseases, doi:10.1093/cid/ciac687
Young-Xu, Korves, Zwain, Effectiveness of sotrovimab in preventing COVID-19-related hospitalizations or deaths among U.S. veterans, Open Forum Infect Dis, doi:10.1093/ofid/ofad605
Zheng, Campbell, Carr, Comparative effectiveness of Sotrovimab and Molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients on kidney replacement therapy: observational cohort study using the Opensafely-UKRR linked platform and SRR database, Clinical Kidney Journal, doi:10.1093/ckj/sfad184
Zheng, Green, Tazare, Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in patients in the community: observational cohort study with the opensafely platform, BMJ, doi:10.1136/bmj-2022-071932
Zheng, Tazare, Nab, Comparative effectiveness of Nirmatrelvir/Ritonavir versus Sotrovimab and Molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised high-risk patients during Omicron waves: observational cohort study using the Opensafely platform, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2023.100741
DOI record:
{
"DOI": "10.1136/bmjresp-2023-002238",
"ISSN": [
"2052-4439"
],
"URL": "http://dx.doi.org/10.1136/bmjresp-2023-002238",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>We assessed the effectiveness of sotrovimab vs no early COVID-19 treatment in highest-risk COVID-19 patients during Omicron predominance.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Retrospective cohort study using the Discover dataset in North West London. Included patients were non-hospitalised, aged ≥12 years and met ≥1 National Health Service highest-risk criterion for sotrovimab treatment. We used Cox proportional hazards models to compare HRs of 28-day COVID-19-related hospitalisation/death between highest-risk sotrovimab-treated and untreated patients. Age, renal disease and Omicron subvariant subgroup analyses were performed.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>We included 599 sotrovimab-treated patients and 5191 untreated patients. Compared with untreated patients, the risk of COVID-19 hospitalisation/death (HR 0.50, 95% CI 0.24, 1.06; p=0.07) and the risk of COVID-19 hospitalisation (HR 0.43, 95% CI 0.18, 1.00; p=0.051) were both lower in the sotrovimab-treated group; however, statistical significance was not reached. In the ≥65 years and renal disease subgroups, sotrovimab was associated with a significantly reduced risk of COVID-19 hospitalisation, by 89% (HR 0.11, 95% CI 0.02, 0.82; p=0.03) and 82% (HR 0.18, 95% CI 0.05, 0.62; p=0.007), respectively.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Risk of COVID-19 hospitalisation in sotrovimab-treated patients aged ≥65 years and with renal disease was significantly lower compared with untreated patients. Overall, risk of hospitalisation was also lower for sotrovimab-treated patients, but statistical significance was not reached.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2024,
3,
14
]
]
},
"alternative-id": [
"10.1136/bmjresp-2023-002238"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8994-2816",
"affiliation": [],
"authenticated-orcid": false,
"family": "Drysdale",
"given": "Myriam",
"sequence": "first"
},
{
"affiliation": [],
"family": "Galimov",
"given": "Evgeniy R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yarwood",
"given": "Marcus James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Vishal",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6371-8744",
"affiliation": [],
"authenticated-orcid": false,
"family": "Levick",
"given": "Bethany",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibbons",
"given": "Daniel C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watkins",
"given": "Jonathan D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Young",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pierce",
"given": "Benjamin F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lloyd",
"given": "Emily J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kerr",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Birch",
"given": "Helen J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamalati",
"given": "Tahereh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brett",
"given": "Stephen J",
"sequence": "additional"
}
],
"container-title": "BMJ Open Respiratory Research",
"container-title-short": "BMJ Open Resp Res",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2024,
4,
4
]
],
"date-time": "2024-04-04T17:35:17Z",
"timestamp": 1712252117000
},
"deposited": {
"date-parts": [
[
2024,
4,
4
]
],
"date-time": "2024-04-04T17:36:05Z",
"timestamp": 1712252165000
},
"funder": [
{
"DOI": "10.13039/100019650",
"doi-asserted-by": "publisher",
"name": "Vir Biotechnology"
},
{
"award": [
"219543"
],
"name": "GSK"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
5
]
],
"date-time": "2024-04-05T00:44:30Z",
"timestamp": 1712277870202
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
4
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
4,
4
]
]
},
"published-print": {
"date-parts": [
[
2024,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 3,
"start": {
"date-parts": [
[
2024,
4,
4
]
],
"date-time": "2024-04-04T00:00:00Z",
"timestamp": 1712188800000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjresp-2023-002238",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e002238",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2024,
4
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
4
]
]
},
"published-print": {
"date-parts": [
[
2024,
4
]
]
},
"publisher": "BMJ",
"reference": [
{
"key": "2024040410350981000_11.1.e002238.1",
"unstructured": "World Health Organization . Coronavirus disease (COVID-19) pandemic. Available: https://www.who.int/europe/emergencies/situations/covid-19 [Accessed 5 Jul 2023]."
},
{
"key": "2024040410350981000_11.1.e002238.2",
"unstructured": "Department of Health and Social Care . Higher-risk patients eligible for COVID-19 treatments: independent advisory group report. Available: https://www.gov.uk/government/publications/higher-risk-patients-eligible-for-covid-19-treatments-independent-advisory-group-report [Accessed 5 Jul 2023]."
},
{
"key": "2024040410350981000_11.1.e002238.3",
"unstructured": "Department of Health and . Interim clinical commissioning policy: Antivirals or Neutralising Monoclonal antibodies for non-hospitalised patients with COVID-19. Available: https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAttachment.aspx?Attachment_id=104044 [Accessed 5 Jul 2023]."
},
{
"key": "2024040410350981000_11.1.e002238.4",
"unstructured": "Medicines and Healthcare products Regulatory Agency . Summary of product characteristics for Xevudy. Available: https://www.gov.uk/government/publications/regulatory-approval-of-xevudy-sotrovimab/summary-of-product-characteristics-for-xevudy [Accessed 5 Jul 2023]."
},
{
"key": "2024040410350981000_11.1.e002238.5",
"unstructured": "Medicines and Healthcare products Regulatory Agency . First oral antiviral for COVID-19, Lagevrio (Molnupiravir), approved by MHRA. available at. n.d. Available: https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra#:~:text=and%20licensing%20guidance-,First%20oral%20antiviral%20for%20COVID%2D19%2C%20Lagevrio%20(molnupiravir),review%20of%20the%20available%20evidence"
},
{
"key": "2024040410350981000_11.1.e002238.6",
"unstructured": "Medicines and Healthcare products Regulatory Agency . Oral COVID-19 antiviral, Paxlovid, approved by UK regulator. Available: https://www.gov.uk/government/news/oral-covid-19-antiviral-paxlovid-approved-by-uk-regulator [Accessed 5 Jul 2023]."
},
{
"DOI": "10.1371/journal.pmed.1002493",
"article-title": "Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults",
"author": "Gaudinski",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Med",
"key": "2024040410350981000_11.1.e002238.7",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1038/nature13612",
"doi-asserted-by": "publisher",
"key": "2024040410350981000_11.1.e002238.8"
},
{
"DOI": "10.1038/s41586-020-2349-y",
"article-title": "Cross-neutralization of SARS-Cov-2 by a human Monoclonal SARS-Cov antibody",
"author": "Pinto",
"doi-asserted-by": "crossref",
"first-page": "290",
"journal-title": "Nature",
"key": "2024040410350981000_11.1.e002238.9",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1101/2021.03.09.434607",
"doi-asserted-by": "crossref",
"key": "2024040410350981000_11.1.e002238.10",
"unstructured": "Cathcart AL , Havenar-Daughton C , Lempp FA , et al . The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-Cov-2. bioRxiv [Preprint]. doi:10.1101/2021.03.09.434607"
},
{
"DOI": "10.1001/jama.2022.2832",
"doi-asserted-by": "publisher",
"key": "2024040410350981000_11.1.e002238.11"
},
{
"key": "2024040410350981000_11.1.e002238.12",
"unstructured": "National Institute for Health and Care Excellence . Final draft guidance: Therapeutics for people with COVID-19. Available: https://www.nice.org.uk/guidance/ta878/documents/final-appraisal-determination-document [Accessed 5 Jul 2023]."
},
{
"article-title": "SARS-Cov-2 variants and clinical outcomes: a systematic review",
"author": "Mendiola-Pastrana",
"journal-title": "Life (Basel)",
"key": "2024040410350981000_11.1.e002238.13",
"volume": "12",
"year": "2022"
},
{
"key": "2024040410350981000_11.1.e002238.14",
"unstructured": "World Health Organization . Weekly epidemiological update on COVID-19. 2022. Available: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-january-2022 [Accessed 5 Jul 2023]."
},
{
"key": "2024040410350981000_11.1.e002238.15",
"unstructured": "World Health Organization . Weekly epidemiological update on COVID-19. 2022. Available: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---22-march-2022 [Accessed 5 2023]."
},
{
"DOI": "10.1126/science.adc9127",
"doi-asserted-by": "publisher",
"key": "2024040410350981000_11.1.e002238.16"
},
{
"key": "2024040410350981000_11.1.e002238.17",
"unstructured": "UK Health Security Agency . SARS-Cov-2 variants of concern and variants under investigation in England: technical briefing 49. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1129169/variant-technical-briefing-49-11-january-2023.pdf [Accessed 5 2023]."
},
{
"DOI": "10.1007/s15010-023-02098-5",
"article-title": "Real-world effectiveness of Sotrovimab for the treatment of SARS-Cov-2 infection during Omicron BA.2 Subvariant predominance: a systematic literature review",
"author": "Drysdale",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Infection",
"key": "2024040410350981000_11.1.e002238.18",
"volume": "52",
"year": "2024"
},
{
"DOI": "10.1101/2022.11.28.22282808",
"doi-asserted-by": "crossref",
"key": "2024040410350981000_11.1.e002238.19",
"unstructured": "Patel V , Yarwood MJ , Levick B , et al . Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving Sotrovimab, oral Antivirals or no treatment in England. medRxiv [Preprint]. doi:10.1101/2022.11.28.22282808"
},
{
"DOI": "10.1186/s12911-020-1082-7",
"article-title": "How an electronic health record became a real-world research resource: comparison between London’s whole systems integrated care database and the clinical practice research Datalink",
"author": "Bottle",
"doi-asserted-by": "crossref",
"journal-title": "BMC Med Inform Decis Mak",
"key": "2024040410350981000_11.1.e002238.20",
"volume": "20",
"year": "2020"
},
{
"key": "2024040410350981000_11.1.e002238.21",
"unstructured": "Discover-NOW . Our Dataset: linked data. Available: https://discover-now.co.uk/the-data [Accessed 5 2023]."
},
{
"key": "2024040410350981000_11.1.e002238.22",
"unstructured": "UK Health Security Agency . SARS-Cov-2 variants of concern and variants under investigation in England: technical briefing 45. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1115071/Technical-Briefing-45-9September2022.pdf [Accessed 5 2023]."
},
{
"DOI": "10.1111/j.1524-4733.2009.00671.x",
"doi-asserted-by": "publisher",
"key": "2024040410350981000_11.1.e002238.23"
},
{
"DOI": "10.1002/sim.6607",
"doi-asserted-by": "publisher",
"key": "2024040410350981000_11.1.e002238.24"
},
{
"DOI": "10.1136/bmj-2022-072976",
"article-title": "Risk prediction of COVID-19 related death or hospital admission in adults testing positive for SARS-Cov-2 infection during the Omicron wave in England (Qcovid4): cohort study",
"author": "Hippisley-Cox",
"doi-asserted-by": "crossref",
"journal-title": "BMJ",
"key": "2024040410350981000_11.1.e002238.25",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.1093/ckj/sfad184",
"article-title": "Comparative effectiveness of Sotrovimab and Molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients on kidney replacement therapy: observational cohort study using the Opensafely-UKRR linked platform and SRR database",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "2048",
"journal-title": "Clinical Kidney Journal",
"key": "2024040410350981000_11.1.e002238.26",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1007/s40121-022-00755-0",
"article-title": "Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-Cov-2 Delta and Omicron waves in the USA",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "607",
"journal-title": "Infect Dis Ther",
"key": "2024040410350981000_11.1.e002238.27",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1093/ofid/ofad605",
"article-title": "Effectiveness of sotrovimab in preventing COVID-19-related hospitalizations or deaths among U.S. veterans",
"author": "Young-Xu",
"doi-asserted-by": "crossref",
"journal-title": "Open Forum Infect Dis",
"key": "2024040410350981000_11.1.e002238.28",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1111/irv.13150",
"article-title": "Comparison of the risk of hospitalisation among BA.1 and BA.2 COVID-19 cases treated with sotrovimab in the community in England",
"author": "Harman",
"doi-asserted-by": "crossref",
"journal-title": "Influenza Other Respir Viruses",
"key": "2024040410350981000_11.1.e002238.29",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1101/2023.02.08.23285654",
"doi-asserted-by": "crossref",
"key": "2024040410350981000_11.1.e002238.30",
"unstructured": "Patel V , Levick B , Boult S , et al . Characteristics and outcomes of COVID-19 patients presumed to be treated with sotrovimab in Nhs hospitals in England. medRxiv [Preprint]. doi:10.1101/2023.02.08.23285654"
},
{
"DOI": "10.1136/bmj-2022-071932",
"article-title": "Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in patients in the community: observational cohort study with the opensafely platform",
"author": "Zheng",
"doi-asserted-by": "crossref",
"journal-title": "BMJ",
"key": "2024040410350981000_11.1.e002238.31",
"volume": "379",
"year": "2022"
},
{
"DOI": "10.1016/j.lanepe.2023.100741",
"article-title": "Comparative effectiveness of Nirmatrelvir/Ritonavir versus Sotrovimab and Molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised high-risk patients during Omicron waves: observational cohort study using the Opensafely platform",
"author": "Zheng",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Reg Health Eur",
"key": "2024040410350981000_11.1.e002238.32",
"volume": "34",
"year": "2023"
},
{
"DOI": "10.1101/2023.05.12.23289914",
"doi-asserted-by": "crossref",
"key": "2024040410350981000_11.1.e002238.33",
"unstructured": "Tazare J , Nab L , Zheng B , et al . Effectiveness of Sotrovimab and Molnupiravir in community settings in England across the Omicron Ba.1 and Ba.2 Sublineages: emulated target trials using the OpenSAFELY platform. Epidemiology [Preprint]. doi:10.1101/2023.05.12.23289914"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"article-title": "Serum neutralization of SARS-Cov-2 Omicron Sublineages BA.1 and BA.2 in patients receiving Monoclonal antibodies",
"author": "Bruel",
"doi-asserted-by": "crossref",
"first-page": "1297",
"journal-title": "Nat Med",
"key": "2024040410350981000_11.1.e002238.34",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-31615-7",
"article-title": "Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-Cov-2 Omicron lineage strains",
"author": "Case",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "2024040410350981000_11.1.e002238.35",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1093/infdis/jiad324",
"article-title": "Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir as treatments for COVID-19 in patients at high risk",
"author": "Paraskevis",
"doi-asserted-by": "crossref",
"first-page": "1667",
"journal-title": "J Infect Dis",
"key": "2024040410350981000_11.1.e002238.36",
"volume": "228",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac687",
"article-title": "Impact of the use of oral antiviral agents on the risk of hospitalization in community Coronavirus disease",
"author": "Yip",
"doi-asserted-by": "crossref",
"first-page": "e26",
"journal-title": "Clinical Infectious Diseases",
"key": "2024040410350981000_11.1.e002238.37",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/j.lanwpc.2022.100602",
"article-title": "Association of molnupiravir and nirmatrelvir-ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19",
"author": "Wai",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Reg Health West Pac",
"key": "2024040410350981000_11.1.e002238.38",
"volume": "30",
"year": "2023"
},
{
"key": "2024040410350981000_11.1.e002238.39",
"unstructured": "Office for National Statistics . Coronavirus (COVID-19) latest insights: hospitals. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/hospitals [Accessed 5 2023]."
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmjopenrespres.bmj.com/lookup/doi/10.1136/bmjresp-2023-002238"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Comparative effectiveness of sotrovimab versus no treatment in non-hospitalised high-risk COVID-19 patients in north west London: a retrospective cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "11"
}
