Role of H2 receptor blocker famotidine over the clinical recovery of COVID-19 patients: A randomized controlled trial
et al., World Journal of Clinical Cases, doi:10.12998/wjcc.v10.i23.8170, NCT04504240, Aug 2022
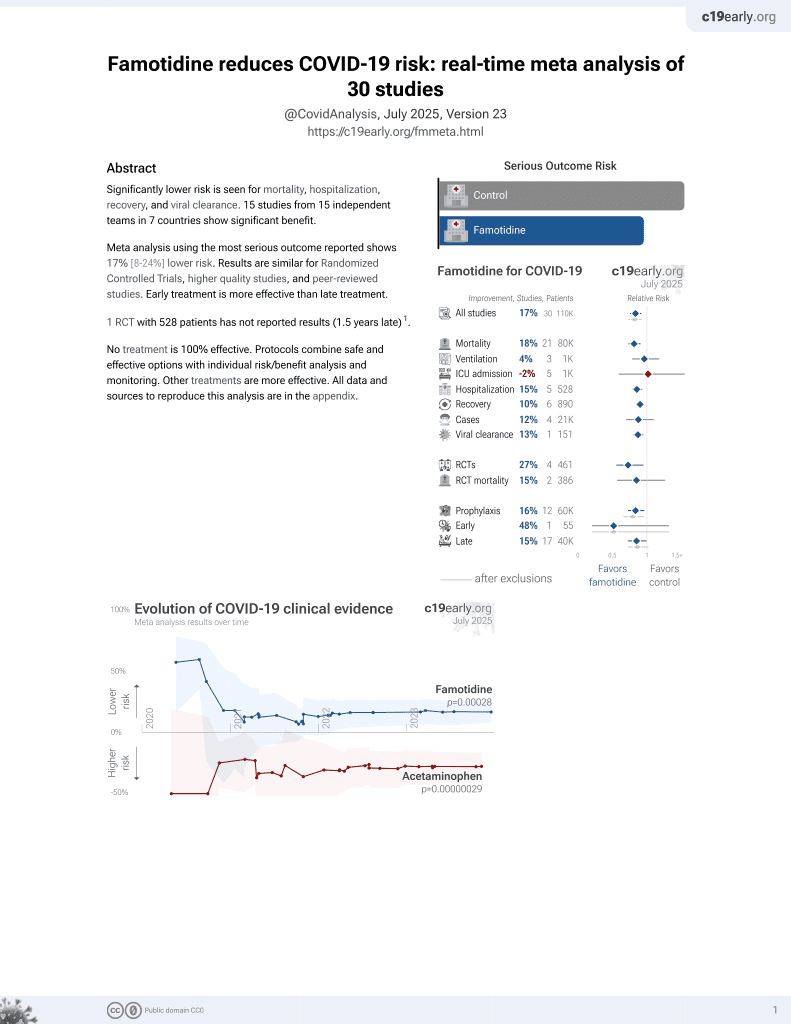
Famotidine for COVID-19
29th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
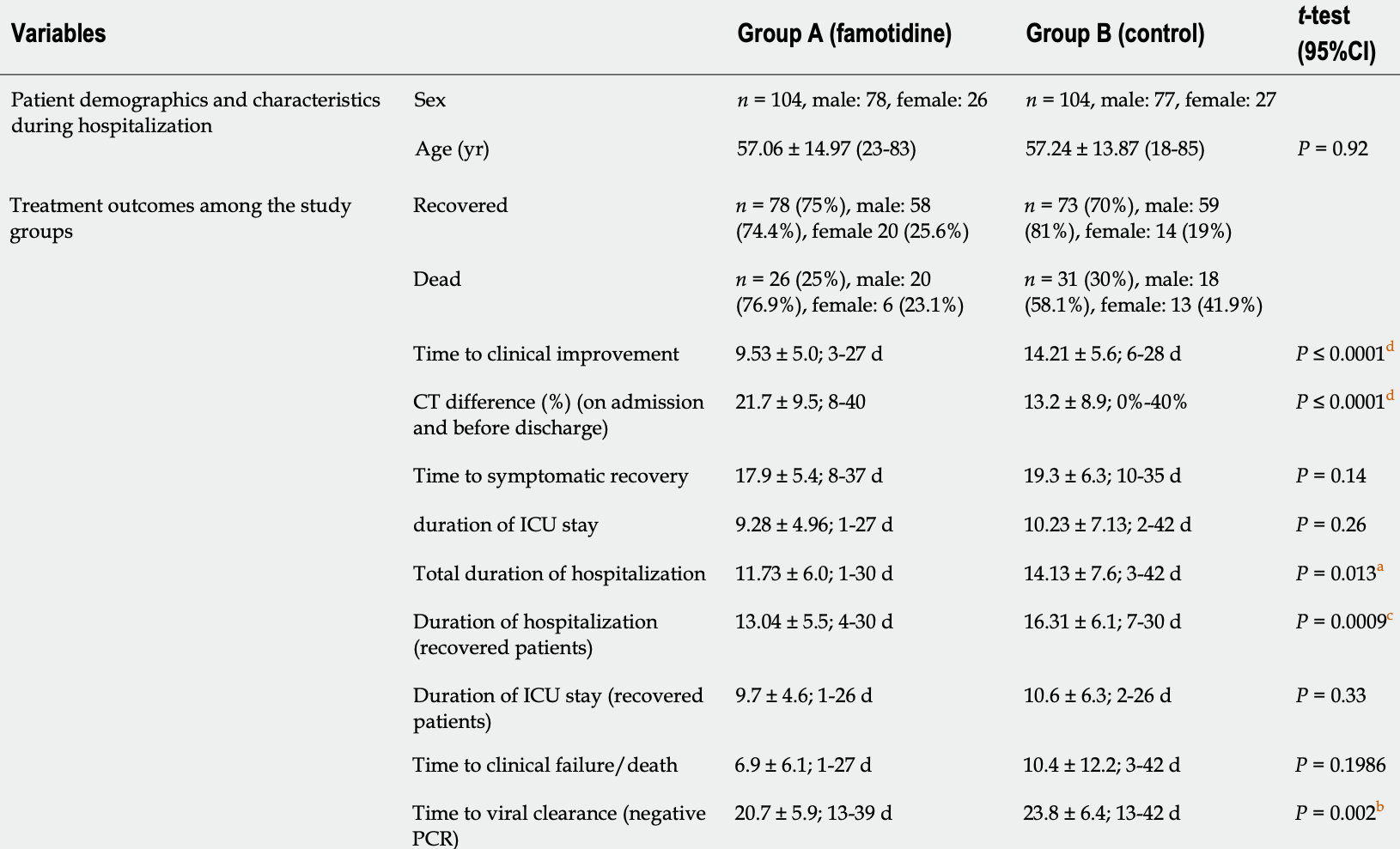
RCT 208 ICU patients in Bangladesh, showing improved recovery with famotidine. Famotidine 40mg (<60kg) or 60mg every 8 hours.
|
risk of death, 16.1% lower, RR 0.84, p = 0.53, treatment 26 of 104 (25.0%), control 31 of 104 (29.8%), NNT 21.
|
|
ICU time, 9.3% lower, relative time 0.91, p = 0.33, treatment 78, control 73.
|
|
time to improvement, 32.9% lower, relative time 0.67, p < 0.001, treatment mean 9.53 (±5.0) n=78, control mean 14.21 (±5.6) n=73, time to clinical improvement.
|
|
recovery time, 7.3% lower, relative time 0.93, p = 0.14, treatment mean 17.9 (±5.4) n=78, control mean 19.3 (±6.3) n=73, time to symptomatic recovery.
|
|
hospitalization time, 17.0% lower, relative time 0.83, p = 0.01, treatment 78, control 73.
|
|
time to viral-, 13.0% lower, relative time 0.87, p = 0.002, treatment 78, control 73.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Chowdhury et al., 16 Aug 2022, Randomized Controlled Trial, Bangladesh, peer-reviewed, mean age 57.1, 11 authors, study period 1 August, 2020 - 15 April, 2021, trial NCT04504240 (history).
Contact: 39485572@qq.com, bpgoffice@wjgnet.com, dyyyjxk@mail.xjtu.edu.cn.
Role of H2 receptor blocker famotidine over the clinical recovery of COVID-19 patients: A randomized controlled trial
World Journal of Clinical Cases, doi:10.12998/wjcc.v10.i23.8170
The primary aim of World Journal of Clinical Cases (WJCC, World J Clin Cases) is to provide scholars and readers from various fields of clinical medicine with a platform to publish high-quality clinical research articles and communicate their research findings online. WJCC mainly publishes articles reporting research results and findings obtained in the field of clinical medicine and covering a wide range of topics, including case control studies, retrospective cohort studies, retrospective studies, clinical trials studies, observational studies, prospective studies, randomized controlled trials, randomized clinical trials, systematic reviews, meta-analysis, and case reports.
Research motivation COVID-19 is a worldwide pandemic. Hence SARS-CoV-2 is a novel virus; there is no specific medication against it. Thus, clinicians and scientists all over the world are struggling with the treatment of this disease. Besides antiviral drugs, immunosuppressive agents and symptomatic therapy like the H 2 receptor blocker famotidine came to the limelight due to its role in reducing the symptoms of COVID-19 patients.
Research objectives To evaluate the role of H 2 receptor blocker "famotidine" in COVID-19 illness.
Research methods
Research results (1) The recovery (75% in group A and 70% in group B and death (25% in group A and 30% in group B) were found preferable in group A than that in group B; (2) Superior improvement of the computed tomography (CT) chest findings was observed in the famotidine treatment group; (3) Among the group A survivors, the duration of ICU and hospital stays were low; (4) However, the difference between the time to symptomatic recovery, ICU stay duration and the time to clinical failure/death among the groups were not significant, P ≥ 0.05; ( 5 ) Group A achieved a reduction of hospital stay and rapid recovery; ( 6 ) Viral recovery was delayed in the control group; and (7) The Kaplan Meier survival analysis was performed. The difference involving survival among the two study groups did not show any statistical significance (P = 0.989).
Research conclusions The famotidine treatment group demonstrated a..
References
Bozkurt, Bilen, Gastrointestinal symptoms in COVID-19 could be associated with severe lung involvement and increased readmission rates, Eur J Inflamm, doi:10.1177/20587392211048259
Báez-Santos, John, Mesecar, Mielech, Chen et al., Nidovirus papain-like proteases: multifunctional enzymes with protease, deubiquitinating and deISGylating activities, Antiviral Res, doi:10.1016/j.virusres.2014.01.025
Chowdhury, Karim, Mehedi, Shahbaz, Chowdhury et al., Analysis of the primary presenting symptoms and hematological findings of COVID-19 patients in Bangladesh, J Infect Dev Ctries, doi:10.3855/jidc.13692
Chowdhury, Shahbaz, Karim, Islam, Dan et al., A Comparative Study on Ivermectin-Doxycycline and Hydroxychloroquine-Azithromycin Therapy on COVID-19 Patients, Eurasian J Med Oncol, doi:10.14744/EJMO.2021.16263
Freedberg, Conigliaro, Wang, Tracey, Callahan et al., Famotidine Use Is Associated With Improved Clinical Outcomes in Hospitalized COVID-19 Patients: A Propensity Score Matched Retrospective Cohort Study, Gastroenterology, doi:10.1053/j.gastro.2020.05.053
Freedberg, Wang, Abrams, Famotidine and Coronavirus Disease 2019, Gastroenterology, doi:10.1053/j.gastro.2020.12.044
Janowitz, Gablenz, Pattinson, Wang, Conigliaro et al., Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series, Gut, doi:10.1136/gutjnl-2020-321852
Langtry, Grant, Goa, Famotidine. An updated review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in peptic ulcer disease and other allied diseases, Drugs, doi:10.2165/00003495-198938040-00005
Lechien, Chiesa-Estomba, Siati, Horoi, Bon et al., Olfactory and gustatory dysfunctions as a clinical presentation of mildto-moderate forms of the coronavirus disease (COVID-19): a multicenter European study, Eur Arch Otorhinolaryngol, doi:10.1007/s00405-020-05965-1
Malone, Tisdall, Smith, Liu, Huang et al., COVID-19: Famotidine, Histamine, Mast Cells, and Mechanisms, Front Pharmacol, doi:10.3389/fphar.2021.633680
Mather, Seip, Mckay, Impact of Famotidine Use on Clinical Outcomes of Hospitalized Patients With COVID-19, Am J Gastroenterol, doi:10.14309/ajg.0000000000000832
Samimagham, Azad, Haddad, Arabi, Hooshyar et al., The Efficacy of Famotidine in improvement of outcomes in Hospitalized COVID-19 Patients: A structured summary of a study protocol for a randomised controlled trial, Trials, doi:10.1186/s13063-020-04773-6
Sun, Chen, Hu, Wu, Liang et al., Does Famotidine Reduce the Risk of Progression to Severe Disease, Death, and Intubation for COVID-19 Patients?, Dig Dis Sci, doi:10.1007/s10620-021-06872-z
Xie, Ding, Li, Wang, Guo et al., Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test, J Med Virol, doi:10.1002/jmv.25930
Yousefi, Mashouri, Okpechi, Alahari, Alahari, Repurposing existing drugs for the treatment of COVID-19/SARS-CoV-2 infection: A review describing drug mechanisms of action, Biochem Pharmacol, doi:10.1016/j.bcp.2020.114296
Zheng, Yang, Zhou, Li, Liu et al., Recommendations and guidance for providing pharmaceutical care services during COVID-19 pandemic: A China perspective, Res Social Adm Pharm, doi:10.1016/j.sapharm.2020.03.012
Zhou, Wang, Lee, Wu, Cheung et al., Proton pump inhibitor or famotidine use and severe COVID-19 disease: a propensity score-matched territory-wide study, Gut, doi:10.1136/gutjnl-2020-323668
DOI record:
{
"DOI": "10.12998/wjcc.v10.i23.8170",
"ISSN": [
"2307-8960"
],
"URL": "http://dx.doi.org/10.12998/wjcc.v10.i23.8170",
"author": [
{
"affiliation": [],
"family": "Chowdhury",
"given": "Abu Taiub Mohammed Mohiuddin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kamal",
"given": "Aktar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abbas",
"given": "Md. Kafil Uddin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karim",
"given": "Md Rezaul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Md. Ahsan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Talukder",
"given": "Shubhashis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mehedi",
"given": "H.M. Hamidullah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hassan",
"given": "Hamid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shahin",
"given": "Abul Hossain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yarui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "He",
"given": "Shuixiang",
"sequence": "additional"
}
],
"container-title": "World Journal of Clinical Cases",
"container-title-short": "WJCC",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
25
]
],
"date-time": "2022-07-25T18:32:43Z",
"timestamp": 1658773963000
},
"deposited": {
"date-parts": [
[
2022,
7,
25
]
],
"date-time": "2022-07-25T18:33:35Z",
"timestamp": 1658774015000
},
"indexed": {
"date-parts": [
[
2022,
7,
25
]
],
"date-time": "2022-07-25T19:11:12Z",
"timestamp": 1658776272546
},
"is-referenced-by-count": 0,
"issue": "23",
"issued": {
"date-parts": [
[
2022,
8,
16
]
]
},
"journal-issue": {
"issue": "23"
},
"link": [
{
"URL": "https://www.wjgnet.com/2307-8960/full/v10/i23/8170.htm",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "2060",
"original-title": [],
"page": "8170-8185",
"prefix": "10.12998",
"published": {
"date-parts": [
[
2022,
8,
16
]
]
},
"published-print": {
"date-parts": [
[
2022,
8,
16
]
]
},
"publisher": "Baishideng Publishing Group Inc.",
"reference": [
{
"DOI": "10.1002/jmv.25930",
"doi-asserted-by": "publisher",
"key": "B1"
},
{
"DOI": "10.1016/j.sapharm.2020.03.012",
"doi-asserted-by": "publisher",
"key": "B2"
},
{
"DOI": "10.3389/fphar.2021.633680",
"doi-asserted-by": "publisher",
"key": "B3"
},
{
"DOI": "10.1136/gutjnl-2020-321852",
"doi-asserted-by": "publisher",
"key": "B4"
},
{
"DOI": "10.14309/ajg.0000000000000832",
"doi-asserted-by": "publisher",
"key": "B5"
},
{
"DOI": "10.3855/jidc.13692",
"doi-asserted-by": "publisher",
"key": "B6"
},
{
"DOI": "10.1007/s00405-020-05965-1",
"doi-asserted-by": "publisher",
"key": "B7"
},
{
"key": "B8",
"unstructured": "Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID–19). [cited 5 March 2022]. Available from: https://stacks.cdc.gov/view/cdc/88624"
},
{
"DOI": "10.14744/EJMO.2021.16263",
"doi-asserted-by": "publisher",
"key": "B9"
},
{
"DOI": "10.2165/00003495-198938040-00005",
"doi-asserted-by": "publisher",
"key": "B10"
},
{
"DOI": "10.1016/j.antiviral.2014.12.015",
"doi-asserted-by": "publisher",
"key": "B11"
},
{
"DOI": "10.1016/j.jmb.2017.04.011",
"doi-asserted-by": "publisher",
"key": "B12"
},
{
"DOI": "10.1016/j.virusres.2014.01.025",
"doi-asserted-by": "publisher",
"key": "B13"
},
{
"DOI": "10.1053/j.gastro.2020.12.044",
"doi-asserted-by": "publisher",
"key": "B14"
},
{
"DOI": "10.1053/j.gastro.2020.05.053",
"doi-asserted-by": "publisher",
"key": "B15"
},
{
"DOI": "10.1177/20587392211048259",
"doi-asserted-by": "publisher",
"key": "B16"
},
{
"DOI": "10.1186/s13063-020-04773-6",
"doi-asserted-by": "publisher",
"key": "B17"
},
{
"DOI": "10.1136/gutjnl-2020-323668",
"doi-asserted-by": "publisher",
"key": "B18"
},
{
"DOI": "10.1016/j.bcp.2020.114296",
"doi-asserted-by": "publisher",
"key": "B19"
},
{
"DOI": "10.1007/s10620-021-06872-z",
"doi-asserted-by": "publisher",
"key": "B20"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.wjgnet.com/2307-8960/full/v10/i23/8170.htm"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Role of H<sub>2 </sub>receptor blocker famotidine over the clinical recovery of COVID-19 patients: A randomized controlled trial",
"type": "journal-article",
"volume": "10"
}