Efficacy of Prolonged-Release Melatonin 2 mg (PRM 2 mg) Prescribed for Insomnia in Hospitalized Patients for COVID-19: A Retrospective Observational Study
et al., Journal of Clinical Medicine, doi:10.3390/jcm10245857, Dec 2021
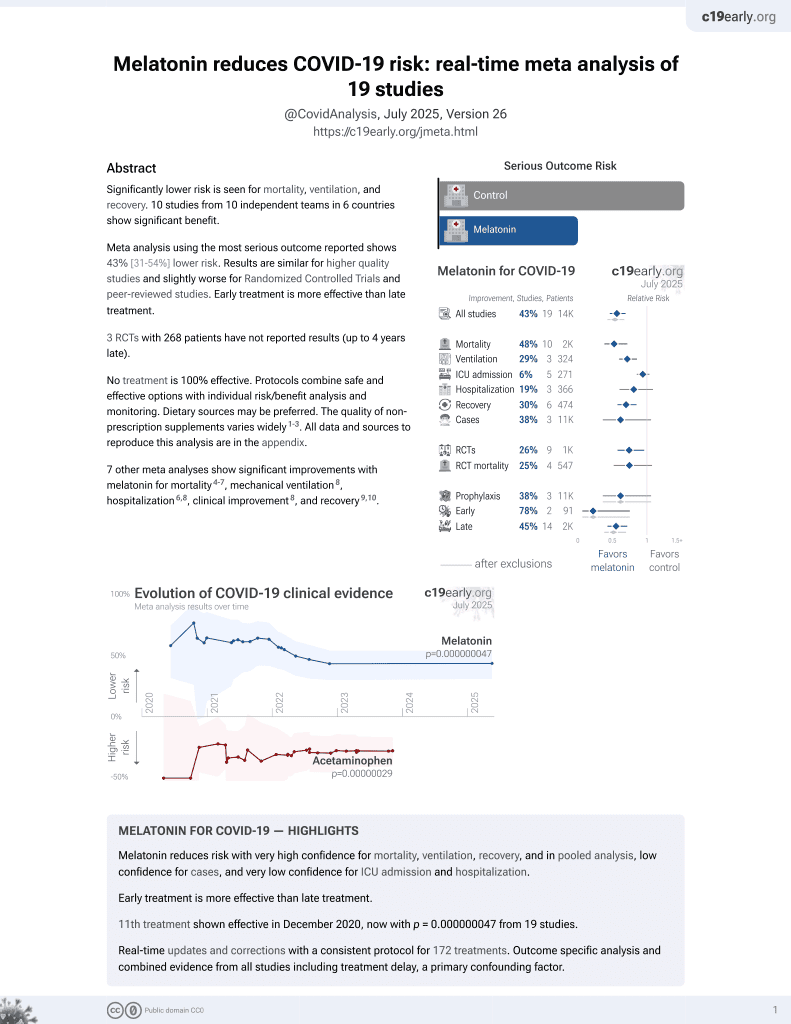
Melatonin for COVID-19
12th treatment shown to reduce risk in
December 2020, now with p = 0.0000000099 from 19 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
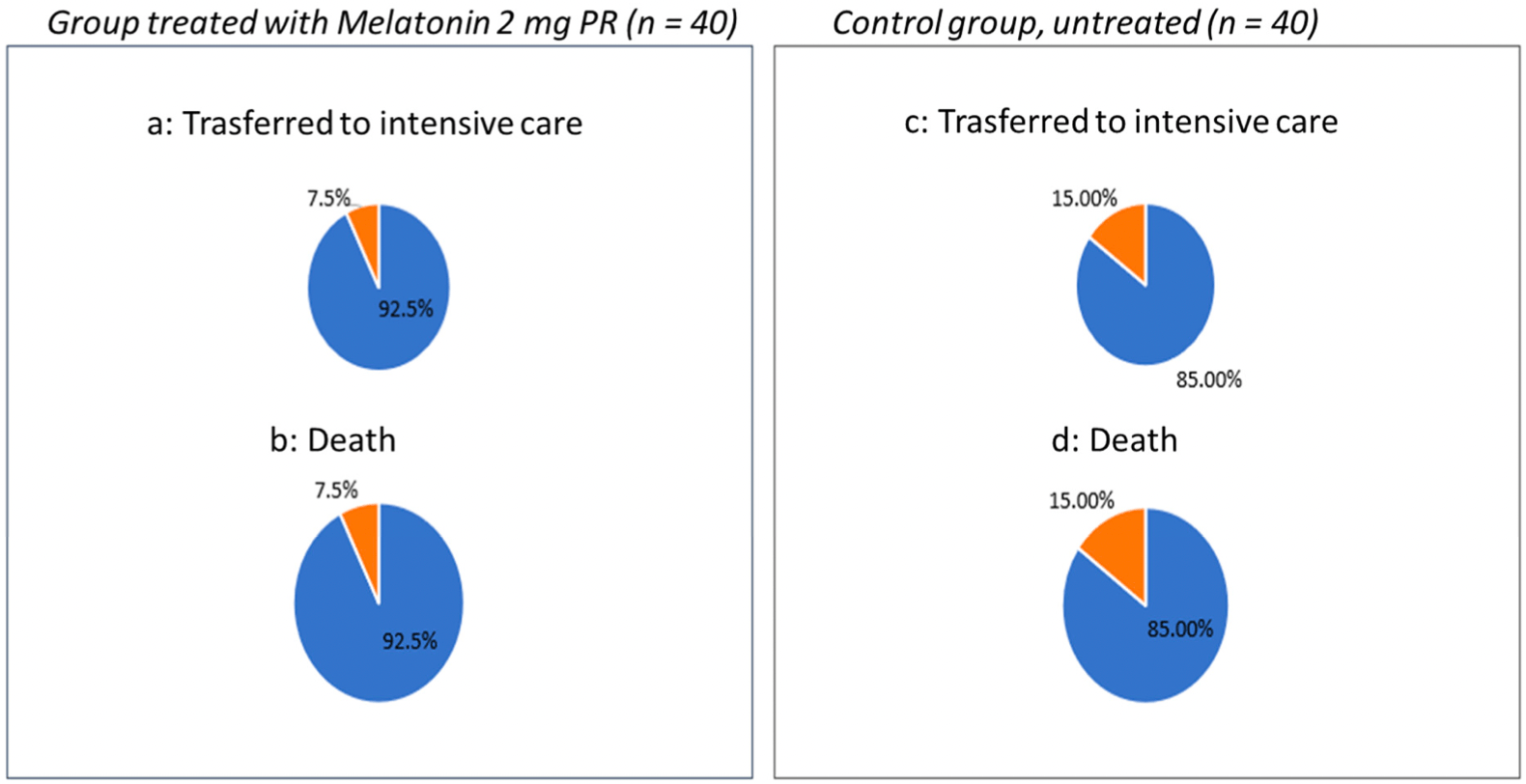
Retrospective 40 hospitalized patients in Italy treated with melatonin and 40 control patients, showing improved sleep, reduced delirium, shorter hospitalization and oxygen times, and reduced ICU admission and mortality (not statistically significant).
|
risk of death, 50.0% lower, RR 0.50, p = 0.48, treatment 3 of 40 (7.5%), control 6 of 40 (15.0%), NNT 13.
|
|
risk of ICU admission, 50.0% lower, RR 0.50, p = 0.48, treatment 3 of 40 (7.5%), control 6 of 40 (15.0%), NNT 13.
|
|
hospitalization time, 8.7% lower, relative time 0.91, p = 0.05, treatment mean 31.3 (±6.8) n=40, control mean 34.3 (±6.9) n=40.
|
|
relative sub-intensive hospitalization time, 38.8% better, relative time 0.61, p < 0.001, treatment mean 12.3 (±3.0) n=40, control mean 20.1 (±6.1) n=40.
|
|
relative NIV time, 58.4% better, relative time 0.42, p < 0.001, treatment mean 5.2 (±3.0) n=40, control mean 12.5 (±4.2) n=40.
|
|
relative high flow oxygen time, 7.8% better, relative time 0.92, p = 0.35, treatment mean 7.1 (±2.5) n=40, control mean 7.7 (±3.2) n=40.
|
|
relative sleep time, 18.2% better, RR 0.82, p < 0.001, treatment mean 5.5 (±0.8) n=40, control mean 4.5 (±1.2) n=40.
|
|
delirium, 33.3% lower, RR 0.67, p < 0.001, treatment mean 2.2 (±1.1) n=40, control mean 3.3 (±1.3) n=40.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bologna et al., 14 Dec 2021, retrospective, Italy, peer-reviewed, 3 authors.
Contact: carolina.bologna@libero.it.
Efficacy of Prolonged-Release Melatonin 2 mg (PRM 2 mg) Prescribed for Insomnia in Hospitalized Patients for COVID-19: A Retrospective Observational Study
Journal of Clinical Medicine, doi:10.3390/jcm10245857
Background: we have observed the effect of insomnia treatment in clinical and prognostic differences of patients admitted for COVID-19 pneumonia in respiratory sub-intensive units that were administered a prolonged-release melatonin 2 mg (PRM 2 mg) therapy versus a group of patients out of therapy. Materials and Methods: We evaluated 40 patients on prolonged-release melatonin 2 mg (PRM 2 mg) therapy versus a control group of 40 patients out of therapy. Results: patients in the PRM 2 mg group had a shorter duration of therapy with non-invasive ventilation (5.2 ± 3.0 vs. 12.5 ± 4.2; p < 0.001), with a shorter stay in sub-intensive care (12.3 ± 3.2 vs. 20.1 ± 6.1; p < 0.001), and, therefore, a shorter overall duration of hospitalization (31.3 ± 6.8 vs. 34.3 ± 6.9 p = 0.03). In addition, a lower incidence of delirium was found (2.2 ± 1.1 vs. 3.3 ± 1.3; p < 0.001). Conclusions: A significant increase in sleep hours and a reduction in delirium episodes occurs in hospitalized insomniac patients treated with PRM 2 mg, compared to untreated patients. Based on these preliminary results, we can assume that there are benefits of prolonged-release melatonin 2 mg in COVID-19 therapy.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: The authors declare no conflict of interest.
References
Anderson, Reiter, Melatonin: Roles in influenza, COVID-19, and other viral infections, R. J. Rev. Med. Virol
Arbon, Knurowska, Dijk, Randomised clinical trial of the effects of prolonged-release melatonin, temazepam and zolpidem on slow-wave activity during sleep in healthy people, J. Psychopharmacol, doi:10.1177/0269881115581963
Bahrampour, Juybari, Pourhanifeh, Hosseinzadeh, Hemati et al., Melatonin potentials against viral infections including COVID-19: Current evidence and new findings, Virus Res, doi:10.1016/j.virusres.2020.198108
Baller, Hogan, Fusunyan, Ivkovic, Luccarelli et al., Neurocovid: Pharmacological Recommendations for Delirium Associated With COVID-19, Psychosomatics, doi:10.1016/j.psym.2020.05.013
Cardinali, Brown, Pandi-Perumal, Can Melatonin Be a Potential "Silver Bullet" in Treating COVID-19 Patients, Diseases, doi:10.3390/diseases8040044
Cerezo, Leal, Álvarez-Fernández, Hornedo-Ortega, Troncoso et al., Quality control and determination of melatonin in food supplements, J. Food Compos. Anal, doi:10.1016/j.jfca.2015.09.013
Duggan, Van, Ely, Assessment in Critically Ill Older Adults: Considerations During the COVID-19 Pandemic, Crit. Care Clin, doi:10.1016/j.ccc.2020.08.009
Erland, Saxena, Melatonin Natural Health Products and Supplements: Presence of Serotonin and Significant Variability of Melatonin Content, J. Clin. Sleep Med, doi:10.5664/jcsm.6462
Lemoine, Laudon, Nir, Zisapel, Prolonged-release melatonin for insomnia-An open-label long-term study of efficacy, safety, and withdrawal, Ther. Clin. Risk Manag, doi:10.2147/TCRM.S23036
Luthringer, Muzet, Zisapel, Staner, The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia, Int. Clin. Psychopharmacol, doi:10.1097/YIC.0b013e32832e9b08
Martín Giménez, Inserra, Tajer, Mariani, Ferder et al., Lungs as target of COVID-19 infection: Protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment, Life Sci, doi:10.1016/j.lfs.2020.117808
Missiry, El-Missiry, Othman, Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of COVID-19, J. Pharmacol, doi:10.1016/j.ejphar.2020.173329
Otmani, Demazieres, Staner, Jacob, Nir et al., Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers, Hum. Psychopharmacol. Clin. Exp, doi:10.1002/hup.980
Otmani, Metzger, Guichard, Danjou, Nir et al., Effects of prolonged-release melatonin and zolpidem on postural stability in older adults, Hum. Psychopharmacol. Clin. Exp, doi:10.1002/hup.2219
Palagini, Manni, Aguglia, Amore, Brugnoli et al., Expert Opinions and Consensus Recommendations for the Evaluation and Management of Insomnia in Clinical Practice: Joint Statements of Five Italian Scientific Societies, Front. Psychiatry, doi:10.3389/fpsyt.2020.00558
Patel, Steinberg, Patel, Insomnia in the Elderly: A Review, J. Clin. Sleep Med, doi:10.5664/jcsm.7172
Pinto, Ferri, Pengo, Lombardi, Pucci et al., The Italian Society of Hypertension. Diagnostic and Therapeutic Approach to Sleep Disorders, High Blood Pressure and Cardiovascular Diseases: A Consensus Document by the Italian Society of Hypertension (SIIA), High Blood Press Cardiovasc. Prev, doi:10.1007/s40292-021-00436-y
Reiter, Abreu-Gonzalez, Marik, Dominguez-Rodriguez, Therapeutic Algorithm for Use of Melatonin in Patients With COVID-19, Front. Med, doi:10.3389/fmed.2020.00226
Shneider, Kudriavtsev, Vakhrusheva, Can melatonin reduce the severity of COVID-19 pandemic?, Int. Rev. Immunol, doi:10.1080/08830185.2020.1756284
Wade, Ford, Crawford, Mcmahon, Nir et al., Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: Quality of sleep and next-day alertness outcomes, Curr. Med. Res. Opin, doi:10.1185/030079907X233098
Wilson, Anderson, British Association for Psycopharmacology consensus statement on evidence based treatment of insomnia, parasomnias and citrcadian rhythm disorders: An update, J. Psychopharmacol, doi:10.1177/0269881119855343
Wiwanitkit, Delirium, sleep, COVID-19 and melatonin, Sleep Med, doi:10.1016/j.sleep.2020.05.028
Zhang, Wang, Ni, Di, Ma et al., COVID-19: Melatonin as a potential adjuvant treatment, Life Sci, doi:10.1016/j.lfs.2020.117583
Zisapel, New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation, Br. J. Pharmacol, doi:10.1111/bph.14116
Öztürk, Akbulut, Güney, Turk Melatonin, aging, and COVID-19: Could melatonin be beneficial for COVID-19 treatment in the elderly?, Med. Sci, doi:10.3906/sag-2005-356
DOI record:
{
"DOI": "10.3390/jcm10245857",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm10245857",
"abstract": "<jats:p>Background: we have observed the effect of insomnia treatment in clinical and prognostic differences of patients admitted for COVID-19 pneumonia in respiratory sub-intensive units that were administered a prolonged-release melatonin 2 mg (PRM 2 mg) therapy versus a group of patients out of therapy. Materials and Methods: We evaluated 40 patients on prolonged-release melatonin 2 mg (PRM 2 mg) therapy versus a control group of 40 patients out of therapy. Results: patients in the PRM 2 mg group had a shorter duration of therapy with non-invasive ventilation (5.2 ± 3.0 vs. 12.5 ± 4.2; p < 0.001), with a shorter stay in sub-intensive care (12.3 ± 3.2 vs. 20.1 ± 6.1; p < 0.001), and, therefore, a shorter overall duration of hospitalization (31.3 ± 6.8 vs. 34.3 ± 6.9 p = 0.03). In addition, a lower incidence of delirium was found (2.2 ± 1.1 vs. 3.3 ± 1.3; p < 0.001). Conclusions: A significant increase in sleep hours and a reduction in delirium episodes occurs in hospitalized insomniac patients treated with PRM 2 mg, compared to untreated patients. Based on these preliminary results, we can assume that there are benefits of prolonged-release melatonin 2 mg in COVID-19 therapy.</jats:p>",
"alternative-id": [
"jcm10245857"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2007-3835",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bologna",
"given": "Carolina",
"sequence": "first"
},
{
"affiliation": [],
"family": "Madonna",
"given": "Pasquale",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pone",
"given": "Eduardo",
"sequence": "additional"
}
],
"container-title": [
"Journal of Clinical Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
14
]
],
"date-time": "2021-12-14T16:11:35Z",
"timestamp": 1639498295000
},
"deposited": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T03:16:05Z",
"timestamp": 1639538165000
},
"indexed": {
"date-parts": [
[
2021,
12,
16
]
],
"date-time": "2021-12-16T06:47:30Z",
"timestamp": 1639637250710
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2077-0383"
}
],
"issue": "24",
"issued": {
"date-parts": [
[
2021,
12,
14
]
]
},
"journal-issue": {
"issue": "24",
"published-online": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
14
]
],
"date-time": "2021-12-14T00:00:00Z",
"timestamp": 1639440000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/10/24/5857/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "5857",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
12,
14
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
14
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/j.lfs.2020.117583",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1002/rmv.2109",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1080/08830185.2020.1756284",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.3390/diseases8040044",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.5664/jcsm.7172",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/j.jfca.2015.09.013",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.5664/jcsm.6462",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.3389/fpsyt.2020.00558",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1111/bph.14116",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1185/030079907X233098",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1097/YIC.0b013e32832e9b08",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.2147/TCRM.S23036",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1177/0269881115581963",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1002/hup.980",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1002/hup.2219",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1177/0269881119855343",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1007/s40292-021-00436-y",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1016/j.psym.2020.05.013",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1016/j.ccc.2020.08.009",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.lfs.2020.117808",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/j.virusres.2020.198108",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1016/j.sleep.2020.05.028",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1016/j.ejphar.2020.173329",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.3389/fmed.2020.00226",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.3906/sag-2005-356",
"doi-asserted-by": "publisher",
"key": "ref25"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"score": 1,
"short-container-title": [
"JCM"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Efficacy of Prolonged-Release Melatonin 2 mg (PRM 2 mg) Prescribed for Insomnia in Hospitalized Patients for COVID-19: A Retrospective Observational Study"
],
"type": "journal-article",
"volume": "10"
}