Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19: Evidence from the US National COVID Cohort Collaborative (N3C)
et al., Clinical Drug Investigation, doi:10.1007/s40261-024-01344-4, Feb 2024
Sotrovimab for COVID-19
45th treatment shown to reduce risk in
August 2022, now with p = 0.00048 from 29 studies, recognized in 42 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
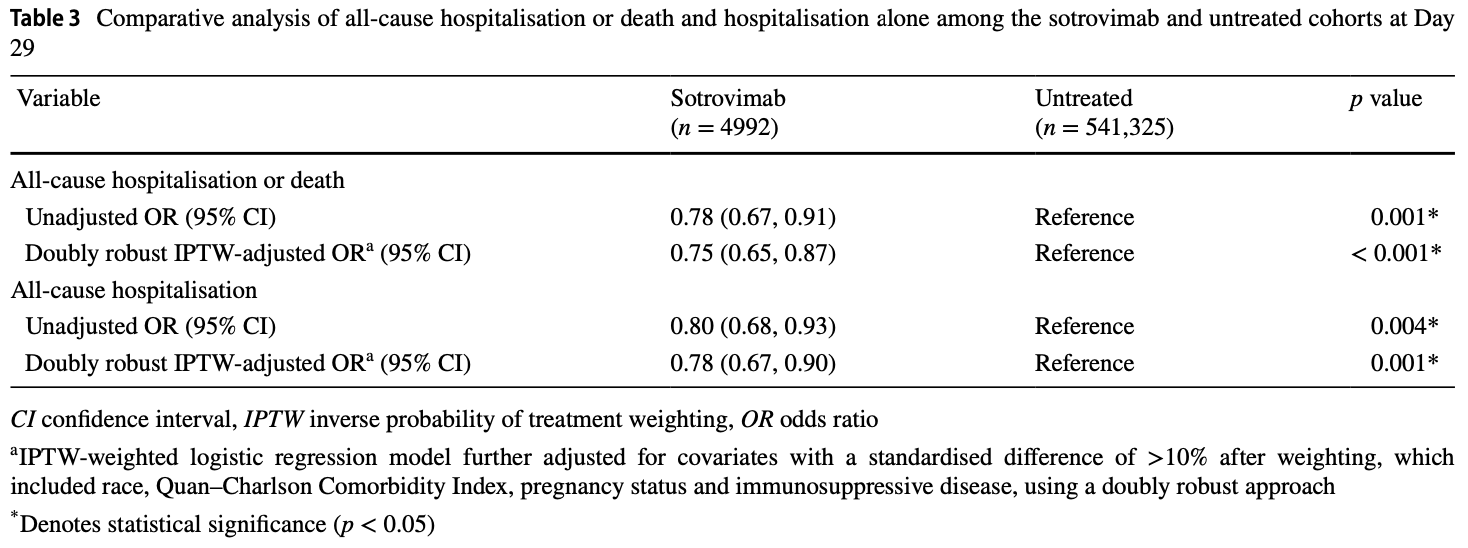
N3C retrospective 4,992 high-risk outpatients with mild-to-moderate COVID-19 showing reduced risk of hospitalization or death with sotrovimab treatment compared to 541,325 untreated controls during periods of Delta and Omicron BA.2 variant predominance in the US (September 2021-April 2022).
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending sotrovimab also recommended them, or
because the patient seeking out sotrovimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Efficacy is variant dependent. In Vitro studies predict lower efficacy for BA.14-6, BA.4, BA.57, XBB.1.9.3, XBB.1.5.24, XBB.2.9, CH.1.18, and no efficacy for BA.29, XBB, XBB.1.5, ХВВ.1.9.110, XBB.1.16, BQ.1.1.45, and CL.18. US EUA has been revoked.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments11.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death/hospitalization, 24.2% lower, RR 0.76, p < 0.001, NNT 107, odds ratio converted to relative risk, propensity score weighting, day 29.
|
|
risk of hospitalization, 21.3% lower, RR 0.79, p = 0.001, NNT 121, odds ratio converted to relative risk, propensity score weighting, day 29.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
5.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
6.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
7.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
8.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
9.
Zhou et al., SARS-CoV-2 Omicron BA.2 Variant Evades Neutralization by Therapeutic Monoclonal Antibodies, bioRxiv, doi:10.1101/2022.02.15.480166.
Bell et al., 20 Feb 2024, retrospective, USA, peer-reviewed, 12 authors, study period 27 September, 2021 - 30 April, 2022.
Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19: Evidence from the US National COVID Cohort Collaborative (N3C)
Clinical Drug Investigation, doi:10.1007/s40261-024-01344-4
Background and Objective The coronavirus disease 2019 (COVID-19) pandemic has been an unprecedented healthcare crisis, one that threatened to overwhelm health systems and prompted an urgent need for early treatment options for patients with mild-to-moderate COVID-19 at high risk for progression to severe disease. Randomised clinical trials established the safety and efficacy of monoclonal antibodies (mAbs) early in the pandemic; in vitro data subsequently led to use of the mAbs being discontinued, without clear evidence on how these data were linked to outcomes. In this study, we describe and compare real-world outcomes for patients with mild-to-moderate COVID-19 at high risk for progression to severe COVID-19 treated with sotrovimab versus untreated patients. Methods Electronic health records from the National COVID Cohort Collaborative (N3C) were used to identify US patients (aged ≥ 12 years) diagnosed with COVID-19 (positive test or ICD-10: U07.1) in an ambulatory setting (27 September 2021-30 April 2022) who met Emergency Use Authorization (EUA) high-risk criteria. Patients receiving the mAb sotrovimab within 10 days of diagnosis were assigned to the sotrovimab cohort, with the day of infusion as the index date. Untreated patients (no evidence of early mAb treatment, prophylactic mAb or oral antiviral treatment) were assigned to the untreated cohort, with an imputed index date based on the time distribution between diagnosis and sotrovimab infusion in the sotrovimab cohort. The primary endpoint was hospitalisation or death (both all-cause) within 29 days of index, reported as descriptive rate and adjusted [via inverse probability of treatment weighting (IPTW)] odds ratio (OR) and 95% confidence interval (CI). Results Of nearly 2.9 million patients diagnosed with COVID-19 during the analysis period, 4992 met the criteria for the sotrovimab cohort, and 541,325 were included in the untreated cohort. Before weighting, significant differences were noted between the cohorts; for example, patients in the sotrovimab cohort were older (60 years versus 54 years), were more likely to be white (85% versus 75%) and met more EUA criteria (mean 3.1 versus 2.2) versus the untreated cohort. The proportions of patients with 29-day hospitalisation or death were 3.5% (176/4992) and 4.5% (24,163/541,325) in the sotrovimab and untreated cohorts, respectively (unadjusted OR: 0.78; 95% CI: 0.67, 0.91; p = 0.001). In adjusted analysis, sotrovimab was associated with a 25% reduction in the odds of hospitalisation or death compared with the untreated cohort (IPTW-adjusted OR: 0.75; 95% CI: 0.61, 0.92; p = 0.005). Conclusions Sotrovimab demonstrated clinical effectiveness in preventing severe outcomes (hospitalisation, mortality) in the period 27 September 2021-30 April 2022, which included Delta and Omicron BA.1 variants and an early surge of Omicron BA.2 variant.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s40261-024-01344-4.
Acknowledgements The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave covid.cd2h.org/enclave and supported by NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data from participating organisations (covid.cd2h.org/dtas) and the organisations and scientists (covid. cd2h.org/duas) who have contributed to the ongoing development of this community resource [39] . Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors' comments for each draft, assembling tables, grammatical editing and referencing) was provided by Tony Reardon of Luna, OPEN Health Communications, in accordance with Good Publication Practice (GPP) guidelines (www. ismpp. org/ gpp-2022). The support was funded by GSK and Vir Biotechnology, Inc.
Declarations Funding This study was funded by GSK (study number 219020) and Vir Biotechnology, Inc. Authorship All named authors take responsibility for the integrity of the work as a whole and have given their approval for this version to be published. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National..
References
Addetia, Piccoli, Case, Therapeutic and vaccineinduced cross-reactive antibodies with effector function against emerging Omicron variants, bioRxiv, doi:10.1101/2023.01.17.523798
Aggarwal, Beaty, Bennett, Change in effectiveness of sotrovimab for preventing hospitalization and mortality for at-risk COVID-19 outpatients during an Omicron BA.1 and BA.1.1-predominant phase, Int J Infect Dis
Aggarwal, Beaty, Bennett, Real-world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients, J Infect Dis
Amani, Amani, Efficacy and safety of sotrovimab in patients with COVID-19: a rapid review and meta-analysis, Rev Med Virol
Barrière, Chamorey, Adjtoutah, Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors, Ann Oncol
Bennett, Moffitt, Hajagos, (N3C) Consortium. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US National COVID Cohort Collaborative, JAMA Netw Open
Bennett, Moffitt, Hajagos, The National COVID Cohort Collaborative: clinical characterization and early severity prediction, medRxiv, doi:10.1101/2021.01.12.21249511
Birk, Jain, Massoud, Real-world experience of sotrovimab in high-risk, immunocompromised COVID-19 patients, Open Forum Infect Dis
Boyarsky, Werbel, Avery, Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients, JAMA
Calderón-Parra, Guisado-Vasco, Montejano-Sánchez, Use of monoclonal antibodies in immunocompromised patients hospitalized with severe COVID-19: a retrospective multicenter cohort, J Clin Med
Case, Mackin, Errico, Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 Omicron lineage strains, Nat Commun
Cathcart, Havenar-Daughton, Lempp, The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv, doi:10.1101/2021.03.09.434607
Chaudhry, Dranitsaris, Mubashir, A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes, EClinicalMedicine
Cheng, Reyes, Satram, Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the United States, Infect Dis Ther
Dessie, Zewotir, Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients, BMC Infect Dis
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate COVID-19, N Engl J Med
Drysdale, Gibbons, Singh, Real-world effectiveness of sotrovimab for the treatment of SARS-CoV-2 infection during Omicron BA.2 subvariant predominance: a systematic literature review, medRxiv, doi:10.1101/2023.03.09.23287034
Funk, Westreich, Wiesen, Doubly robust estimation of causal effects, Am J Epidemiol
Gaudinski, Coates, Houser, Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults, PLoS Med
Gupta, Gonzalez-Rojas, Juarez, Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med
Gupta, Gonzalez-Rojas, Juarez, Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Haendel, Chute, Bennett, The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment, J Am Med Inform Assoc
Harman, Nash, Webster, Comparison of the risk of hospitalisation among BA.1 and BA.2 COVID-19 cases treated with sotrovimab in the community in England, medRxiv, doi:10.1101/2022.10.21.22281171
Hippisley-Cox, Khunti, Sheikh, QCovid 4 -predicting risk of death or hospitalisation from COVID-19 in adults testing positive for SARS-CoV-2 infection during the Omicron wave in England, medRxiv, doi:10.1101/2022.08.13.22278733
Kip, Mccreary, Collins, Evolving real-world effectiveness of monoclonal antibodies for treatment of COVID-19: a cohort study, Ann Intern Med
Ko, Pegu, Rudicell, Enhanced neonatal Fc receptor function improves protection against primate SHIV infection, Nature
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV antibody combination to prevent COVID-19, N Engl J Med
Opensafely Collaborative, Tazare, Nab, Effectiveness of sotrovimab and molnupiravir in community settings in England across the Omicron BA.1 and BA.2 sublineages: emulated target trials using the OpenSAFELY platform, medRxiv, doi:10.1101/2023.05.12.23289914
Park, Pinto, Walls, Imprinted antibody responses against SARS-CoV-2 Omicron sublineages, Science
Patel, Levick, Boult, Characteristics and outcomes of COVID-19 patients presumed to be treated with sotrovimab in NHS hospitals in England, medRxiv, doi:10.1101/2023.02.08.23285654
Patel, Yarwood, Levick, Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving sotrovimab, oral antivirals or no treatment in England, medRxiv, doi:10.1101/2022.11.28.22282808
Pinto, Park, Beltramello, Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody, Nature
Pollock, Storlie, Tande, Sampathkumar, Real-world incidence of breakthrough coronavirus disease 2019 hospitalization after vaccination vs natural infection in a large, local, empaneled primary care population using time-to-event analysis, Clin Infect Dis
Shadmi, Chen, Dourado, Health equity and COVID-19: global perspectives, Int J Equity Health
Song, Bates, Shao, Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the National COVID Cohort Collaborative, J Clin Oncol
Tao, Tzou, Kosakovsky, Susceptibility of SARS-CoV-2 omicron variants to therapeutic monoclonal antibodies: systematic review and meta-analysis, Microbiol Spectr
Thompson, Pierse, Huand, Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012-2015, Vaccine
Uraki, Kiso, Iida, Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2, Nature
Walker, Schnell, Kerr, Antiviral agents against Omicron subvariant BA.4.6 in vitro, N Engl J Med
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with COVID-19, N Engl J Med
Young-Xu, Korves, Zwain, Effectiveness of sotrovimab in preventing COVID-19-related hospitalizations or deaths among US veterans, medRxiv, doi:10.1101/2022.12.30.22284063
Zheng, Green, Tazare, Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform, BMJ
DOI record:
{
"DOI": "10.1007/s40261-024-01344-4",
"ISSN": [
"1173-2563",
"1179-1918"
],
"URL": "http://dx.doi.org/10.1007/s40261-024-01344-4",
"alternative-id": [
"1344"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "17 January 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 2,
"value": "20 February 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Funding",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study was funded by GSK (study number 219020) and Vir Biotechnology, Inc."
},
{
"group": {
"label": "Authorship",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "All named authors take responsibility for the integrity of the work as a whole and have given their approval for this version to be published. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health."
},
{
"group": {
"label": "Author Contributions",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work."
},
{
"group": {
"label": "Conflict of Interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "CFB, DCG, EJL, HJB, MD and VP (at time of study): employees of, and/or shareholders in, GSK. AZ, MDS, MSD, PB and RD: employees of Analysis Group, which received funding from GSK to conduct the study."
},
{
"group": {
"label": "Ethics Approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 6,
"value": "This study complies with all applicable laws regarding subject privacy. No direct subject contact or primary collection of individual human subject data occurred. Study results were in tabular form, and aggregate analyses that omit subject identification, therefore, informed consent, ethics committee or institutional review board (IRB) were not required. However, use of the N3C Limited Dataset did require review and approval by WCG IRB (IRB study number: 1332048/IRB tracking number: 20222051). Any publications and reports do not include subject identifiers."
},
{
"group": {
"label": "Consent to Participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 7,
"value": "Not applicable (see explanation in ‘Ethics approval’ section above)."
},
{
"group": {
"label": "Consent for Publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 8,
"value": "Not applicable."
},
{
"group": {
"label": "Availability of Data and Material",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 9,
"value": "Data archiving is not mandated but will be made available upon reasonable request."
},
{
"group": {
"label": "Code Availability",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 10,
"value": "Not applicable."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6413-2537",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bell",
"given": "Christopher F.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1343-8314",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bobbili",
"given": "Priyanka",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3010-820X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Desai",
"given": "Raj",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3769-9535",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gibbons",
"given": "Daniel C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8994-2816",
"affiliation": [],
"authenticated-orcid": false,
"family": "Drysdale",
"given": "Myriam",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5528-6531",
"affiliation": [],
"authenticated-orcid": false,
"family": "DerSarkissian",
"given": "Maral",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1517-4148",
"affiliation": [],
"authenticated-orcid": false,
"family": "Patel",
"given": "Vishal",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4924-4810",
"affiliation": [],
"authenticated-orcid": false,
"family": "Birch",
"given": "Helen J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lloyd",
"given": "Emily J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7451-7756",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Adina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5035-6687",
"affiliation": [],
"authenticated-orcid": false,
"family": "Duh",
"given": "Mei Sheng",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the N3C consortium",
"sequence": "additional"
}
],
"container-title": "Clinical Drug Investigation",
"container-title-short": "Clin Drug Investig",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
2,
20
]
],
"date-time": "2024-02-20T20:02:12Z",
"timestamp": 1708459332000
},
"deposited": {
"date-parts": [
[
2024,
2,
20
]
],
"date-time": "2024-02-20T20:09:00Z",
"timestamp": 1708459740000
},
"funder": [
{
"award": [
"219020"
],
"name": "GSK Vir Biotechnology, Inc."
}
],
"indexed": {
"date-parts": [
[
2024,
2,
21
]
],
"date-time": "2024-02-21T00:24:39Z",
"timestamp": 1708475079850
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
2,
20
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
20
]
],
"date-time": "2024-02-20T00:00:00Z",
"timestamp": 1708387200000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
20
]
],
"date-time": "2024-02-20T00:00:00Z",
"timestamp": 1708387200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40261-024-01344-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40261-024-01344-4/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40261-024-01344-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2024,
2,
20
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
20
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "1344_CR1",
"unstructured": "World Health Organization. Coronavirus disease (COVID-19) pandemic. 2022. Available from: https://www.who.int/europe/emergencies/situations/covid-19. Accessed 6 March 2023."
},
{
"key": "1344_CR2",
"unstructured": "World Health Organization. Coronavirus (COVID-19) Dashboard. February 2023. Available from: https://covid19.who.int/. Accessed 6 March 2023."
},
{
"DOI": "10.46234/ccdcw2020.032",
"author": "The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team",
"doi-asserted-by": "publisher",
"first-page": "113",
"issue": "8",
"journal-title": "China CDC Wkly.",
"key": "1344_CR3",
"unstructured": "The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) – China, 2020. China CDC Wkly. 2020;2(8):113–22.",
"volume": "2",
"year": "2020"
},
{
"key": "1344_CR4",
"unstructured": "World Health Organization. Living guidance for clinical management of COVID-19. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2. Accessed 6 March 2023."
},
{
"DOI": "10.1186/s12939-020-01218-z",
"author": "E Shadmi",
"doi-asserted-by": "publisher",
"first-page": "104",
"issue": "1",
"journal-title": "Int J Equity Health.",
"key": "1344_CR5",
"unstructured": "Shadmi E, Chen Y, Dourado I, et al. Health equity and COVID-19: global perspectives. Int J Equity Health. 2020;19(1):104.",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2020.100464",
"author": "R Chaudhry",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine.",
"key": "1344_CR6",
"unstructured": "Chaudhry R, Dranitsaris G, Mubashir T, et al. A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. EClinicalMedicine. 2020;25: 100464.",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06536-3",
"author": "ZG Dessie",
"doi-asserted-by": "publisher",
"first-page": "855",
"issue": "1",
"journal-title": "BMC Infect Dis",
"key": "1344_CR7",
"unstructured": "Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):855.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1101/2022.08.13.22278733",
"doi-asserted-by": "publisher",
"key": "1344_CR8",
"unstructured": "Hippisley-Cox J, Khunti K, Sheikh A, et al. QCovid 4 – predicting risk of death or hospitalisation from COVID-19 in adults testing positive for SARS-CoV-2 infection during the Omicron wave in England. medRxiv. 2022. https://doi.org/10.1101/2022.08.13.22278733. Accessed 6 March 2023."
},
{
"DOI": "10.1016/j.annonc.2021.04.019",
"author": "J Barrière",
"doi-asserted-by": "publisher",
"first-page": "1053",
"issue": "8",
"journal-title": "Ann Oncol",
"key": "1344_CR9",
"unstructured": "Barrière J, Chamorey E, Adjtoutah Z, et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32(8):1053–5.",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.7489",
"author": "BJ Boyarsky",
"doi-asserted-by": "publisher",
"first-page": "2204",
"issue": "21",
"journal-title": "JAMA",
"key": "1344_CR10",
"unstructured": "Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–6.",
"volume": "325",
"year": "2021"
},
{
"key": "1344_CR11",
"unstructured": "Centers for Disease Control and Prevention. COVID-19 vaccinations in the United States. 2023. Available from: https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-booster-percent-pop5. Accessed 17 August 2023."
},
{
"key": "1344_CR12",
"unstructured": "United States Food and Drug Administration. Emergency use authorizations for drugs and non-vaccine biological products. 2023. Available from: https://www.fda.gov/drugs/emergency-preparedness-drugs/emergency-use-authorizations-drugs-and-non-vaccine-biological-products. Accessed 17 August 2023."
},
{
"DOI": "10.1001/jama.2022.2832",
"author": "A Gupta",
"doi-asserted-by": "publisher",
"first-page": "1236",
"issue": "13",
"journal-title": "JAMA",
"key": "1344_CR13",
"unstructured": "Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327(13):1236–46.",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2102685",
"author": "M Dougan",
"doi-asserted-by": "publisher",
"first-page": "1382",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "1344_CR14",
"unstructured": "Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med. 2021;385(15):1382–92.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2109682",
"author": "MP O’Brien",
"doi-asserted-by": "publisher",
"first-page": "1184",
"issue": "13",
"journal-title": "N Engl J Med",
"key": "1344_CR15",
"unstructured": "O’Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N Engl J Med. 2021;385(13):1184–95.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2108163",
"author": "DM Weinreich",
"doi-asserted-by": "publisher",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "1344_CR16",
"unstructured": "Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med. 2021;385(23): e81.",
"volume": "385",
"year": "2021"
},
{
"key": "1344_CR17",
"unstructured": "American Hospital Association. HHS Reinstates Original Distribution Method for COVID-19 Monoclonal Antibody Therapies. 2021. Available from: https://www.aha.org/special-bulletin/2021-09-17-hhs-reinstates-original-distribution-method-covid-19-monoclonal. Accessed 6 December 2023."
},
{
"DOI": "10.7326/M22-1286",
"author": "KE Kip",
"doi-asserted-by": "publisher",
"first-page": "496",
"issue": "4",
"journal-title": "Ann Intern Med",
"key": "1344_CR18",
"unstructured": "Kip KE, McCreary EK, Collins K, et al. Evolving real-world effectiveness of monoclonal antibodies for treatment of COVID-19: a cohort study. Ann Intern Med. 2023;176(4):496–504.",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1371/journal.pmed.1002493",
"author": "MR Gaudinski",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "PLoS Med",
"key": "1344_CR19",
"unstructured": "Gaudinski MR, Coates EE, Houser KV, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018;15(1): e1002493.",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1038/nature13612",
"author": "SY Ko",
"doi-asserted-by": "publisher",
"first-page": "642",
"issue": "7524",
"journal-title": "Nature",
"key": "1344_CR20",
"unstructured": "Ko SY, Pegu A, Rudicell RS, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514(7524):642–5.",
"volume": "514",
"year": "2014"
},
{
"DOI": "10.1038/s41586-020-2349-y",
"author": "D Pinto",
"doi-asserted-by": "publisher",
"first-page": "290",
"issue": "7815",
"journal-title": "Nature",
"key": "1344_CR21",
"unstructured": "Pinto D, Park YJ, Beltramello M, et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–5.",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1101/2021.03.09.434607",
"doi-asserted-by": "publisher",
"key": "1344_CR22",
"unstructured": "Cathcart AL, Havenar-Daughton C, Lempp FA, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv. 2022. Available from: https://doi.org/10.1101/2021.03.09.434607. Accessed 6 March 2023."
},
{
"DOI": "10.1056/NEJMoa2107934",
"doi-asserted-by": "crossref",
"key": "1344_CR23",
"unstructured": "Gupta A, Gonzalez-Rojas Y, Juarez E, et al; COMET-ICE Investigators. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–50."
},
{
"DOI": "10.1126/science.adc9127",
"author": "YJ Park",
"doi-asserted-by": "publisher",
"first-page": "619",
"issue": "6620",
"journal-title": "Science",
"key": "1344_CR24",
"unstructured": "Park YJ, Pinto D, Walls AC, et al. Imprinted antibody responses against SARS-CoV-2 Omicron sublineages. Science. 2022;378(6620):619–27.",
"volume": "378",
"year": "2022"
},
{
"DOI": "10.1128/spectrum.00926-22",
"author": "K Tao",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "Microbiol Spectr.",
"key": "1344_CR25",
"unstructured": "Tao K, Tzou PL, Kosakovsky SL, et al. Susceptibility of SARS-CoV-2 omicron variants to therapeutic monoclonal antibodies: systematic review and meta-analysis. Microbiol Spectr. 2022;10(4): e0092622.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2216611",
"doi-asserted-by": "crossref",
"key": "1344_CR26",
"unstructured": "Walker J, Schnell G, Kerr W. Antiviral agents against Omicron subvariant BA.4.6 in vitro. N Engl J Med. 2023;388:e12."
},
{
"DOI": "10.1038/s41467-022-31615-7",
"author": "JB Case",
"doi-asserted-by": "publisher",
"first-page": "3824",
"issue": "1",
"journal-title": "Nat Commun",
"key": "1344_CR27",
"unstructured": "Case JB, Mackin S, Errico JM, et al. Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 Omicron lineage strains. Nat Commun. 2022;13(1):3824.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04856-1",
"doi-asserted-by": "crossref",
"key": "1344_CR28",
"unstructured": "Uraki R, Kiso M, Iida S, et al. Characterization and antiviral susceptibility of SARS-CoV-2 Omicron BA.2. Nature. 2022;607(7917):119–27."
},
{
"DOI": "10.1101/2023.01.17.523798",
"doi-asserted-by": "publisher",
"key": "1344_CR29",
"unstructured": "Addetia A, Piccoli L, Case JB, et al. Therapeutic and vaccine-induced cross-reactive antibodies with effector function against emerging Omicron variants. bioRxiv. 2023. https://doi.org/10.1101/2023.01.17.523798. Preprint. Accessed 3 October 2023."
},
{
"DOI": "10.1002/rmv.2402",
"author": "B Amani",
"doi-asserted-by": "publisher",
"issue": "6",
"journal-title": "Rev Med Virol",
"key": "1344_CR30",
"unstructured": "Amani B, Amani B. Efficacy and safety of sotrovimab in patients with COVID-19: a rapid review and meta-analysis. Rev Med Virol. 2022;32(6): e2402.",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1007/s40121-022-00755-0",
"author": "MM Cheng",
"doi-asserted-by": "publisher",
"first-page": "607",
"issue": "2",
"journal-title": "Infect Dis Ther",
"key": "1344_CR31",
"unstructured": "Cheng MM, Reyes C, Satram S, et al. Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the United States. Infect Dis Ther. 2023;12(2):607–21.",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1101/2023.03.09.23287034",
"doi-asserted-by": "publisher",
"key": "1344_CR32",
"unstructured": "Drysdale M, Gibbons DC, Singh M, et al. Real-world effectiveness of sotrovimab for the treatment of SARS-CoV-2 infection during Omicron BA.2 subvariant predominance: a systematic literature review. medRxiv. 2023. https://doi.org/10.1101/2023.03.09.23287034. Accessed 21 March 2023."
},
{
"DOI": "10.1101/2022.10.21.22281171",
"doi-asserted-by": "publisher",
"key": "1344_CR33",
"unstructured": "Harman K, Nash SG, Webster HH, et al. Comparison of the risk of hospitalisation among BA.1 and BA.2 COVID-19 cases treated with sotrovimab in the community in England. medRxiv. 2022. https://doi.org/10.1101/2022.10.21.22281171. Accessed 6 March 2023."
},
{
"DOI": "10.1101/2022.11.28.22282808",
"doi-asserted-by": "publisher",
"key": "1344_CR34",
"unstructured": "Patel V, Yarwood MJ, Levick B, et al. Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving sotrovimab, oral antivirals or no treatment in England. medRxiv. 2022. https://doi.org/10.1101/2022.11.28.22282808. Accessed 6 March 2023."
},
{
"DOI": "10.1101/2023.02.08.23285654",
"doi-asserted-by": "publisher",
"key": "1344_CR35",
"unstructured": "Patel V, Levick B, Boult S, et al. Characteristics and outcomes of COVID-19 patients presumed to be treated with sotrovimab in NHS hospitals in England. medRxiv. 2023. https://doi.org/10.1101/2023.02.08.23285654. Accessed 6 March 2023."
},
{
"DOI": "10.1101/2022.12.30.22284063",
"doi-asserted-by": "publisher",
"key": "1344_CR36",
"unstructured": "Young-Xu Y, Korves C, Zwain G, et al. Effectiveness of sotrovimab in preventing COVID-19-related hospitalizations or deaths among US veterans. medRxiv. 2022. https://doi.org/10.1101/2022.12.30.22284063. Accessed 21 March 2023."
},
{
"DOI": "10.1136/bmj-2022-071932",
"author": "B Zheng",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "1344_CR37",
"unstructured": "Zheng B, Green ACA, Tazare J, et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform. BMJ. 2022;379: e071932.",
"volume": "379",
"year": "2022"
},
{
"DOI": "10.1200/JCO.21.02419",
"author": "Q Song",
"doi-asserted-by": "publisher",
"first-page": "1414",
"journal-title": "J Clin Oncol",
"key": "1344_CR38",
"unstructured": "Song Q, Bates B, Shao YR, et al. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the National COVID Cohort Collaborative. J Clin Oncol. 2022;40:1414–27.",
"volume": "40",
"year": "2022"
},
{
"key": "1344_CR39",
"unstructured": "Bennett TD, Moffitt RA, Hajagos JG, et al; National COVID Cohort Collaborative (N3C) Consortium. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US National COVID Cohort Collaborative. JAMA Netw Open. 2021;4(7):e2116901."
},
{
"DOI": "10.1093/jamia/ocaa196",
"author": "MA Haendel",
"doi-asserted-by": "publisher",
"first-page": "427",
"journal-title": "J Am Med Inform Assoc",
"key": "1344_CR40",
"unstructured": "Haendel MA, Chute CG, Bennett TD, et al. The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021;28:427–43.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1101/2021.01.12.21249511",
"doi-asserted-by": "publisher",
"key": "1344_CR41",
"unstructured": "Bennett TD, Moffitt RA, Hajagos JG, et al; National COVID Cohort Collaborative (N3C) Consortium. The National COVID Cohort Collaborative: clinical characterization and early severity prediction. medRxiv. 2021. https://doi.org/10.1101/2021.01.12.21249511. Preprint. Accessed 3 October 2023."
},
{
"key": "1344_CR42",
"unstructured": "National COVID Cohort Collaborative (N3C). Available from: https://github.com/National-COVID-Cohort-Collaborative. Accessed 9 June 2023."
},
{
"key": "1344_CR43",
"unstructured": "Centers for Disease Control and Prevention. COVID Data Tracker, Nowcast. Available from: https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed 6 March 2023."
},
{
"DOI": "10.1093/aje/kwq439",
"author": "MJ Funk",
"doi-asserted-by": "publisher",
"first-page": "761",
"issue": "7",
"journal-title": "Am J Epidemiol",
"key": "1344_CR44",
"unstructured": "Funk MJ, Westreich D, Wiesen C, et al. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173(7):761–7.",
"volume": "173",
"year": "2011"
},
{
"key": "1344_CR45",
"unstructured": "Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed 10 May 2023."
},
{
"DOI": "10.1093/cid/ciac186",
"author": "BD Pollock",
"doi-asserted-by": "publisher",
"first-page": "1239",
"issue": "7",
"journal-title": "Clin Infect Dis",
"key": "1344_CR46",
"unstructured": "Pollock BD, Storlie CB, Tande AJ, Sampathkumar P. Real-world incidence of breakthrough coronavirus disease 2019 hospitalization after vaccination vs natural infection in a large, local, empaneled primary care population using time-to-event analysis. Clin Infect Dis. 2022;75(7):1239–41.",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1016/j.vaccine.2018.07.028",
"author": "MG Thompson",
"doi-asserted-by": "publisher",
"first-page": "5916",
"issue": "39",
"journal-title": "Vaccine",
"key": "1344_CR47",
"unstructured": "Thompson MG, Pierse N, Huand S, et al. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine. 2018;36(39):5916–25.",
"volume": "36",
"year": "2018"
},
{
"DOI": "10.1093/ofid/ofac282",
"doi-asserted-by": "crossref",
"key": "1344_CR48",
"unstructured": "Birk NK, Jain S, Massoud L, et al. Real-world experience of sotrovimab in high-risk, immunocompromised COVID-19 patients. Open Forum Infect Dis 2022;9:ofac282."
},
{
"DOI": "10.3390/jcm12030864",
"author": "J Calderón-Parra",
"doi-asserted-by": "publisher",
"first-page": "864",
"journal-title": "J Clin Med",
"key": "1344_CR49",
"unstructured": "Calderón-Parra J, Guisado-Vasco P, Montejano-Sánchez R, et al. Use of monoclonal antibodies in immunocompromised patients hospitalized with severe COVID-19: a retrospective multicenter cohort. J Clin Med. 2023;12:864.",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1093/infdis/jiac206",
"author": "NR Aggarwal",
"doi-asserted-by": "publisher",
"first-page": "2129",
"journal-title": "J Infect Dis",
"key": "1344_CR50",
"unstructured": "Aggarwal NR, Beaty LE, Bennett TD, et al. Real-world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients. J Infect Dis. 2022;226:2129–36.",
"volume": "226",
"year": "2022"
},
{
"key": "1344_CR51",
"unstructured": "US Department of Health & Human Services. State/territory-coordinated distribution of sotrovimab. 17 December 2021. Available from: https://aspr.hhs.gov/COVID-19/Therapeutics/updates/Pages/important-update-17December2021.aspx. Accessed 10 May 2023."
},
{
"DOI": "10.1016/j.ijid.2022.10.002",
"doi-asserted-by": "crossref",
"key": "1344_CR52",
"unstructured": "Aggarwal NR, Beaty LE, Bennett TD, et al. Change in effectiveness of sotrovimab for preventing hospitalization and mortality for at-risk COVID-19 outpatients during an Omicron BA.1 and BA.1.1-predominant phase. Int J Infect Dis. 2023;128:310–7."
},
{
"DOI": "10.1101/2023.05.12.23289914",
"doi-asserted-by": "publisher",
"key": "1344_CR53",
"unstructured": "The OpenSAFELY Collaborative, Tazare J, Nab L, et al. Effectiveness of sotrovimab and molnupiravir in community settings in England across the Omicron BA.1 and BA.2 sublineages: emulated target trials using the OpenSAFELY platform. medRxiv. 2023. https://doi.org/10.1101/2023.05.12.23289914. Preprint. Accessed 3 October 2023."
},
{
"key": "1344_CR54",
"unstructured": "World Health Organization. Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID-19 pandemic. 2023. Available from: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. Accessed 6 December 2023."
}
],
"reference-count": 54,
"references-count": 54,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40261-024-01344-4"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Real-World Effectiveness of Sotrovimab for the Early Treatment of COVID-19: Evidence from the US National COVID Cohort Collaborative (N3C)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}
