Evaluation of Low-Dose Aspirin use among Critically Ill Patients with COVID-19: A Multicenter Propensity Score Matched Study
et al., Journal of Intensive Care Medicine, doi:10.1177/08850666221093229, Sep 2021
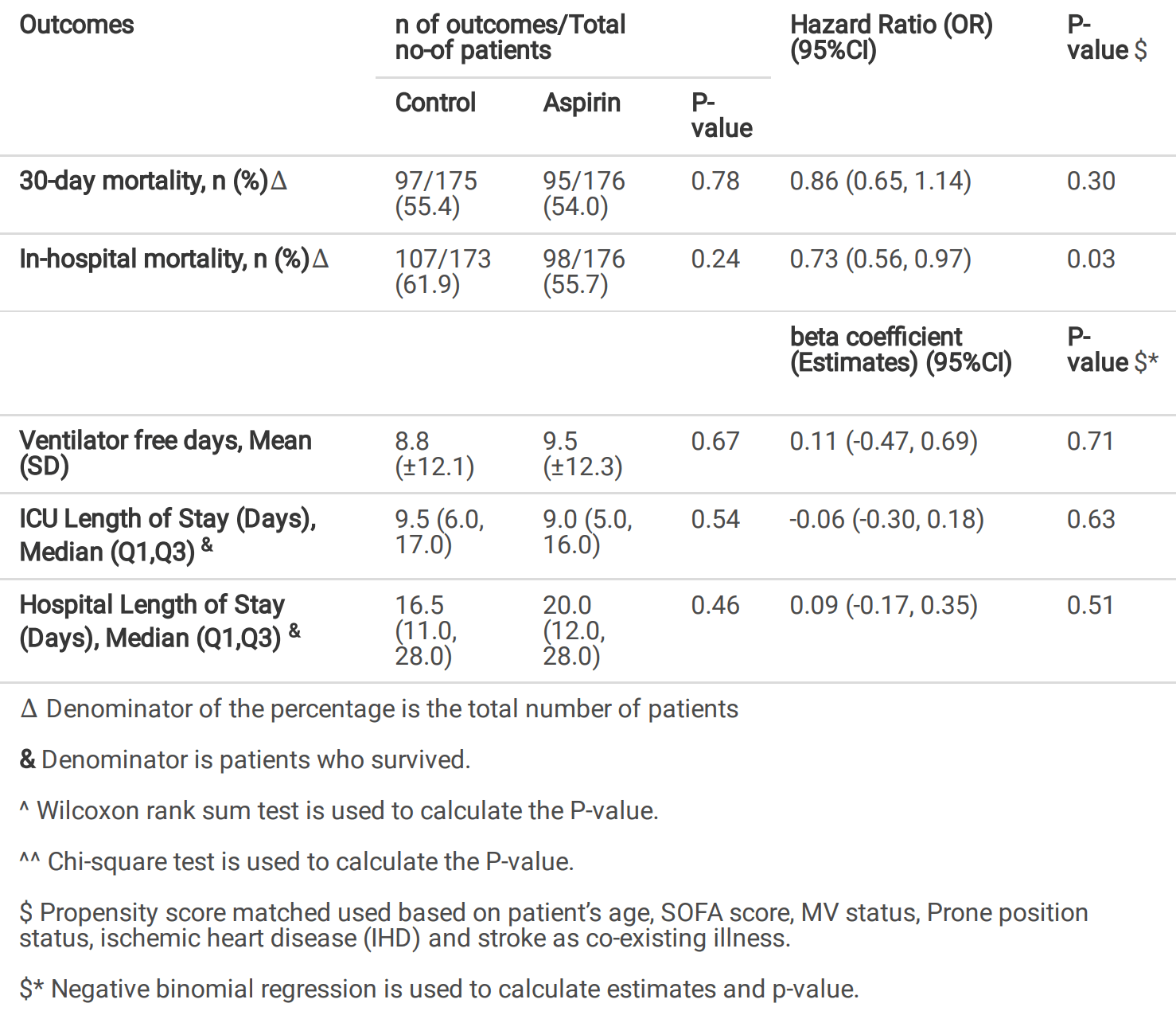
Retrospective 1,033 critical condition patients, showing lower in-hospital mortality with aspirin in PSM analysis. Patients receiving aspirin also had a higher risk of significant bleeding, although not reaching statistical significance. Authors note that the use of aspirin during an ICU stay should be tailored to each patient.
|
risk of death, 27.0% lower, HR 0.73, p = 0.03, treatment 98 of 176 (55.7%), control 107 of 173 (61.8%), adjusted per study, in-hospital mortality, multivariable Cox proportional hazards.
|
|
risk of death, 14.0% lower, HR 0.86, p = 0.30, treatment 95 of 176 (54.0%), control 97 of 175 (55.4%), adjusted per study, day 30, multivariable Cox proportional hazards.
|
|
ICU time, 5.3% lower, relative time 0.95, p = 0.54, treatment median 9.0 IQR 11.0 n=176, control median 9.5 IQR 11.0 n=175.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Al Harthi et al., 3 Sep 2021, retrospective, propensity score matching, Saudi Arabia, peer-reviewed, 21 authors.
Evaluation of low-dose aspirin use among COVID-19 critically ill patients: A Multicenter Propensity Score Matched Study
doi:10.21203/rs.3.rs-872891/v1
Background Multiple medications with anti-in ammatory effects have been used to manage the hyper-in ammatory response associated with COVID-19. Aspirin is used widely as a cardioprotective agent due to its antiplatelet and anti-in ammatory properties. Its role in hospitalized COVID-19 patients has been assessed and evaluated in the literature. However, no data regards its role in COVID-19 critically ill patients. Therefore, this study aims to evaluate the use of low-dose aspirin (81-100 mg) and its impact on outcomes in COVID-19 critically ill patients.
Method This is a multicenter, retrospective cohort study for all adult critically ill patients with con rmed COVID-19 admitted to Intensive Care Units (ICUs) between March 1, 2020, and March 31, 2021. Eligible patients were classi ed into two groups based on aspirin use during ICU stay. The primary outcome is the in-hospital mortality; other outcomes were considered secondary. Propensity score-matched used based on patient's age, SOFA score, MV status within 24 hours of ICU admission, prone position status, ischemic heart disease (IHD), and stroke as co-existing illness. We considered a P value of < 0.05 statistically signi cant.
Results A total of 1033 patients were eligible; 352 patients were included after propensity score matching (1:1 ratio). The in-hospital mortality (HR (95%CI): 0.73 (0.56, 0.97), p-value=0.03) were lower in patients who received aspirin during hospital stay. On the other hand, patients who received aspirin have a higher risk of major bleeding compared to the control group (OR (95%CI): 2.92 (0.91, 9.36), p-value=0.07); but was not statistically signi cant.
Conclusion Aspirin use in COVID-19 critically ill patients may have a mortality bene t; nevertheless, it may be linked with an increased risk of signi cant bleeding. The bene t-risk evaluation for aspirin usage during an ICU stay should be tailored to each patient.
Author contributions All authors contributed to data collections, analysis, drafted, revised, and approved the nal version of the manuscript.
Funding None.
Ethics approval and consent to participate The study was approved in March 2021 by King Abdullah International Medical Research Center Institutional Review Board, Riyadh, Saudi Arabia (Ref.# NRC21R/058/02). Participants' con dentiality was strictly observed throughout the study by using anonymous unique serial number for each subject and restricting data only to the investigators. Informed consent was not required due to the research's method as per the policy of the governmental and local research center.
Consent for publication Not applicable.
Competing interests No author has a con ict of interest in this study.
References
Aghajani, Moradi, Amini, Tehrani, Pourheidar et al., Decreased inhospital mortality associated with aspirin administration in hospitalized patients due to severe COVID-19, J Med Virol, doi:10.1002/jmv.27053
Ahmed, Merrell, Ismail, Joudeh, Riley et al., Rationales and uncertainties for aspirin use in COVID-19: a narrative review, Fam Med community Heal
Berardis, Lucisano, 'ettorre, Pellegrini, Lepore et al., Association of aspirin use with major bleeding in patients with and without diabetes, JAMA
Chernysh, Nagaswami, Kosolapova, Peshkova, Cuker et al., Con rmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis, Thromb Res, doi:10.1038/s41598-020-59526-x
Chow, Khanna, Kethireddy, Yamane, Levine et al., Aspirin Use Is Associated With Decreased Mechanical Ventilation, Intensive Care Unit Admission, and In-Hospital Mortality in Hospitalized Patients With Coronavirus Disease 2019, Anesth Analg
Cook, Fuller, Guyatt, Marshall, Leasa et al., Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group, N Engl J Med
Goligher, Bradbury, Mcverry, Lawler, Berger et al., Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19, N Engl J Med
Group, Horby, Pessoa-Amorim, Staplin, Emberson et al., COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Hayakawa, Management of disseminated intravascular coagulation: current insights on antithrombin and thrombomodulin treatments, Open Access Emerg Med
Hottz, Azevedo-Quintanilha, Palhinha, Teixeira, Barreto et al., Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19, Blood, doi:10.1182/blood.2020007252
Johnson, Department, Surgery, St, Catholic et al., The Novel Aspirin as Breakthrough Drug for COVID-19: A Narrative Review, Iberoam J Med
Lauzier, Arnold, Rabbat, Heels-Ansdell, Zarychanski et al., Risk factors and impact of major bleeding in critically ill patients receiving heparin thromboprophylaxis, Intensive Care Med
Lin, Chen, Acute kidney injury classi cation: AKIN and RIFLE criteria in critical patients, World J Crit care Med
Mcneil, Wolfe, Woods, Tonkin, Donnan et al., Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly, N Engl J Med
Meizlish, Goshua, Liu, Fine, Amin et al., Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: a propensity score-matched analysis, medRxiv
Osborne, Veigulis, Arreola, Mahajan, Röösli et al., Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration, PLoS One
Rodriguez-Roisin, Pulmonary gas exchange in acute respiratory failure, Eur J Anaesthesiol
Sadeghipour, Talasaz, Rashidi, Sharif-Kashani, Beigmohammadi et al., Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial, JAMA
Sahai, Bhandari, Godwin, Mcintyre, Chung et al., Effect of aspirin on shortterm outcomes in hospitalized patients with COVID-19, Vasc Med, doi:10.1177/1358863X211012754
Saleh K Bin, Ha Z A, Alsulaiman, Aljuhani, Alharbi, Alharbi, Clinical characteristics and outcomes of patients with heart failure admitted to the intensive care unit with coronavirus disease 2019 (COVID-19): A multicenter cohort study, Am Hear J plus Cardiol Res Pract
Schulman, Kearon, De nition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients, J Thromb Haemost
Wu, Mcgoogan, Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention, JAMA
Xu, Zhou, Xu, Mechanism of thrombocytopenia in COVID-19 patients, Ann Hematol, doi:10.1007/s00277-020-04019-0
Yuan, Chen, Li, Chen, Wang et al., Mortality and pre-hospitalization use of low-dose aspirin in COVID-19 patients with coronary artery disease, J Cell Mol Med, doi:10.1111/jcmm.16198
Zhang, Cao, Jiang, Xiao, Li et al., Pro le of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients, J Thromb Thrombolysis, doi:10.1007/s11239-020-02182-9
Zheng, Roddick, Association of Aspirin Use for Primary Prevention With Cardiovascular Events and Bleeding Events: A Systematic Review and Meta-analysis, JAMA
DOI record:
{
"DOI": "10.1177/08850666221093229",
"ISSN": [
"0885-0666",
"1525-1489"
],
"URL": "http://dx.doi.org/10.1177/08850666221093229",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p> Aspirin is widely used as a cardioprotective agent due to its antiplatelet and anti-inflammatory properties. The literature has assessed and evaluated its role in hospitalized COVID-19 patients. However, no data are available regarding its role in COVID-19 critically ill patients. This study aimed to evaluate the use of low-dose aspirin (81-100 mg) and its impact on outcomes in critically ill patients with COVID-19. </jats:p></jats:sec><jats:sec><jats:title>Method</jats:title><jats:p> A multicenter, retrospective cohort study of all critically ill adult patients with confirmed COVID-19 admitted to intensive care units (ICUs) between March 1, 2020, and March 31, 2021. Eligible patients were classified into two groups based on aspirin use during ICU stay. The primary outcome was in-hospital mortality, and other outcomes were considered secondary. Propensity score matching was used (1:1 ratio) based on the selected criteria. </jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p> A total of 1033 patients were eligible, and 352 patients were included after propensity score matching. The in-hospital mortality (HR 0.73 [0.56, 0.97], p = 0.03) was lower in patients who received aspirin during stay. Conversely, patients who received aspirin had a higher odds of major bleeding than those in the control group (OR 2.92 [0.91, 9.36], p = 0.07); however, this was not statistically significant. Additionally, subgroup analysis showed a possible mortality benefit for patients who used aspirin therapy prior to hospitalization and continued during ICU stay (HR 0.72 [0.52, 1.01], p = 0.05), but not with the new initiation of aspirin (HR 1.22 [0.68, 2.20], p = 0.50). </jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p> Continuation of aspirin therapy during ICU stay in critically ill patients with COVID-19 who were receiving it prior to ICU admission may have a mortality benefit; nevertheless, it may be associated with an increased risk of significant bleeding. Appropriate evaluation for safety versus benefits of utilizing aspirin therapy during ICU stay in COVID19 critically ill patients is highly recommended. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/08850666221093229"
],
"author": [
{
"affiliation": [
{
"name": "Pharmaceutical Care Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Al Harthi",
"given": "Abdullah F.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pharmacy Practice, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia"
}
],
"family": "Aljuhani",
"given": "Ohoud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy Practice, College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia"
}
],
"family": "Korayem",
"given": "Ghazwa B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Care Services, King Salman Specialist Hospital, Hail Health Cluster, Ministry of Health, Hail, Saudi Arabia"
}
],
"family": "Altebainawi",
"given": "Ali F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Pharmacy, University of Hail, Hail Saudi Arabia"
}
],
"family": "Alenezi",
"given": "Raghdah S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Care Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Al Harbi",
"given": "Shmeylan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Care Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Gramish",
"given": "Jawaher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Care Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Kensara",
"given": "Raed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy Practice, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia"
}
],
"family": "Hafidh",
"given": "Awattif",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Care Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Al Enazi",
"given": "Huda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy Practice, College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia"
}
],
"family": "Alawad",
"given": "Ahad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy Practice, College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia"
}
],
"family": "Alotaibi",
"given": "Rand",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
}
],
"family": "Alshehri",
"given": "Abdulaziz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
}
],
"family": "Alhuthaili",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Vishwakarma",
"given": "Ramesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Care Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "bin Saleh",
"given": "Khalid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Family Medicine Department, King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Alsulaiman",
"given": "Thamer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Care Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Alqahtani",
"given": "Rahaf Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
},
{
"name": "College of Pharmacy, King Saud University, Riyadh, Saudi Arabia"
}
],
"family": "Hussain",
"given": "Sajid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Family Medicine Department, King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Almazrou",
"given": "Saja",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5547-2043",
"affiliation": [
{
"name": "Pharmaceutical Care Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
},
{
"name": "Saudi Critical Care Pharmacy Research (SCAPE) Platform, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Al Sulaiman",
"given": "Khalid",
"sequence": "additional"
}
],
"container-title": [
"Journal of Intensive Care Medicine"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2022,
4,
22
]
],
"date-time": "2022-04-22T06:32:08Z",
"timestamp": 1650609128000
},
"deposited": {
"date-parts": [
[
2022,
4,
22
]
],
"date-time": "2022-04-22T06:32:14Z",
"timestamp": 1650609134000
},
"indexed": {
"date-parts": [
[
2022,
4,
23
]
],
"date-time": "2022-04-23T10:11:17Z",
"timestamp": 1650708677629
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0885-0666"
},
{
"type": "electronic",
"value": "1525-1489"
}
],
"issued": {
"date-parts": [
[
2022,
4,
21
]
]
},
"language": "en",
"license": [
{
"URL": "http://journals.sagepub.com/page/policies/text-and-data-mining-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
21
]
],
"date-time": "2022-04-21T00:00:00Z",
"timestamp": 1650499200000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/08850666221093229",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/08850666221093229",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/08850666221093229",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "088506662210932",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2022,
4,
21
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
21
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "bibr1-08850666221093229"
},
{
"DOI": "10.1136/fmch-2020-000741",
"author": "Ahmed HA S",
"doi-asserted-by": "crossref",
"first-page": "e000741",
"issue": "2",
"journal-title": "Fam Med Commun Health",
"key": "bibr2-08850666221093229",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.ahjo.2021.100033",
"doi-asserted-by": "publisher",
"key": "bibr3-08850666221093229"
},
{
"DOI": "10.1007/s11239-020-02182-9",
"doi-asserted-by": "publisher",
"key": "bibr4-08850666221093229"
},
{
"DOI": "10.1038/s41598-020-59526-x",
"doi-asserted-by": "publisher",
"key": "bibr5-08850666221093229"
},
{
"DOI": "10.1016/j.thromres.2020.04.041",
"doi-asserted-by": "publisher",
"key": "bibr6-08850666221093229"
},
{
"DOI": "10.1001/jama.2021.4152",
"author": "INSPIRATION Investigators",
"doi-asserted-by": "crossref",
"first-page": "1620",
"issue": "16",
"journal-title": "JAMA",
"key": "bibr7-08850666221093229",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103417",
"doi-asserted-by": "publisher",
"key": "bibr8-08850666221093229"
},
{
"DOI": "10.1007/s00277-020-04019-0",
"doi-asserted-by": "publisher",
"key": "bibr9-08850666221093229"
},
{
"DOI": "10.1182/blood.2020007252",
"doi-asserted-by": "publisher",
"key": "bibr10-08850666221093229"
},
{
"DOI": "10.1002/ajh.26102",
"doi-asserted-by": "publisher",
"key": "bibr11-08850666221093229"
},
{
"DOI": "10.1213/ANE.0000000000005292",
"doi-asserted-by": "publisher",
"key": "bibr12-08850666221093229"
},
{
"DOI": "10.1371/journal.pone.0246825",
"doi-asserted-by": "publisher",
"key": "bibr13-08850666221093229"
},
{
"key": "bibr14-08850666221093229",
"unstructured": "COVID19. Ministry of Health (MOH). https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Pages/covid19.aspx. Published 2021. Accessed February 22, 2022."
},
{
"DOI": "10.1111/j.1538-7836.2005.01204.x",
"doi-asserted-by": "publisher",
"key": "bibr15-08850666221093229"
},
{
"DOI": "10.5492/wjccm.v1.i2.40",
"doi-asserted-by": "publisher",
"key": "bibr16-08850666221093229"
},
{
"author": "Rodriguez-Roisin R",
"first-page": "5",
"issue": "1",
"journal-title": "Eur J Anaesthesiol",
"key": "bibr17-08850666221093229",
"volume": "11",
"year": "1994"
},
{
"DOI": "10.1002/jmv.27053",
"doi-asserted-by": "publisher",
"key": "bibr18-08850666221093229"
},
{
"author": "Sahai A",
"journal-title": "Preprint. Res Sq",
"key": "bibr19-08850666221093229",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"doi-asserted-by": "publisher",
"key": "bibr20-08850666221093229"
},
{
"DOI": "10.1111/jcmm.16198",
"doi-asserted-by": "publisher",
"key": "bibr21-08850666221093229"
},
{
"DOI": "10.53986/ibjm.2020.0058",
"doi-asserted-by": "publisher",
"key": "bibr22-08850666221093229"
},
{
"DOI": "10.1001/jama.2018.20578",
"doi-asserted-by": "publisher",
"key": "bibr23-08850666221093229"
},
{
"DOI": "10.1056/NEJMoa1805819",
"doi-asserted-by": "publisher",
"key": "bibr24-08850666221093229"
},
{
"DOI": "10.1001/jama.2012.5034",
"doi-asserted-by": "publisher",
"key": "bibr25-08850666221093229"
},
{
"DOI": "10.1056/NEJM199402103300601",
"doi-asserted-by": "publisher",
"key": "bibr26-08850666221093229"
},
{
"DOI": "10.1007/s00134-013-3044-3",
"doi-asserted-by": "publisher",
"key": "bibr27-08850666221093229"
},
{
"DOI": "10.2147/OAEM.S135909",
"doi-asserted-by": "publisher",
"key": "bibr28-08850666221093229"
},
{
"key": "bibr29-08850666221093229",
"unstructured": "NCT04365309. Protective Effect of Aspirin on COVID-19 Patients [Internet]. https://clinicaltrials.gov/show/NCT04365309. 2020 [cited 2021 Sep 3]. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02103526/full."
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/08850666221093229"
}
},
"score": 1,
"short-container-title": [
"J Intensive Care Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Critical Care and Intensive Care Medicine"
],
"subtitle": [],
"title": [
"Evaluation of Low-Dose Aspirin use among Critically Ill Patients with COVID-19: A Multicenter Propensity Score Matched Study"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy"
}