Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial
et al., Trials, doi:10.1186/s13063-023-07114-5, NCT04883203, Jul 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
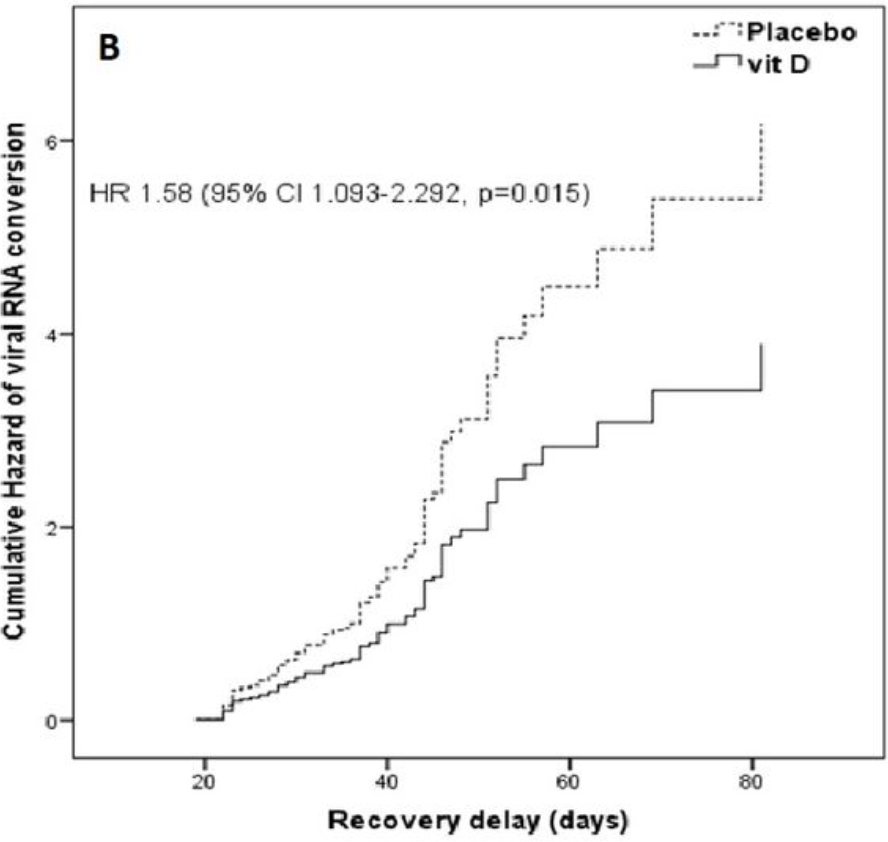
Long COVID RCT with mostly asymptomatic patients that remained PCR positive for 14 days, showing slower viral conversion with treatment. Authors report "a 30-day follow-up of our patients showed that a long-lasting COVID-19 was noted in 34.5% of patients who received vitamin D and in 38.9% of patients who belonged to the placebo group", however no number of the reported group sizes matches these percentages. Authors do not define "long-lasting COVID-19" which does not match the viral clearance at 30 days shown in Figure 2, and is also unexpected in terms of symptoms according to the baseline symptomatic status. Details and timing of PCR tests are not provided. According to Figure 3, testing appears to have been sporadic - for example ~23 treatment patients remain PCR+ at day 40, however no more than one unique Ct value per day is shown.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
Bolus treatment is less effective.
Pharmacokinetics and the potential side effects of high bolus doses suggest
that ongoing treatment spread over time is more appropriate.
Research has confirmed that lower dose regular treatment with vitamin D is more
effective than intermittent high-dose bolus treatment for various conditions,
including rickets and acute respiratory infections1,2. The biological mechanisms supporting these
findings involve the induction of enzymes such as 24-hydroxylase and
fibroblast growth factor 23 (FGF23) by high-dose bolus treatments. These
enzymes play roles in inactivating vitamin D, which can paradoxically reduce
levels of activated vitamin D and suppress its activation for extended periods
post-dosage. Evidence indicates that 24-hydroxylase activity may remain
elevated for several weeks following a bolus dose, leading to reduced levels
of the activated form of vitamin D. Additionally, FGF23 levels can increase
for at least three months after a large bolus dose, which also contributes to
the suppression of vitamin D activation1.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
|
risk of no recovery, 8.5% lower, RR 0.92, p = 0.85, treatment 20 of 57 (35.1%), control 23 of 60 (38.3%), NNT 31, approximate, reported percentages do not match group sizes, day 30.
|
|
risk of no viral clearance, 58.0% higher, HR 1.58, p = 0.01, treatment 57, control 60, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Griffin et al., Perspective: Vitamin D supplementation prevents rickets and acute respiratory infections when given as daily maintenance but not as intermittent bolus: implications for COVID-19, Clinical Medicine, doi:10.7861/clinmed.2021-0035.
Abroug et al., 21 Jul 2022, Randomized Controlled Trial, placebo-controlled, Tunisia, peer-reviewed, mean age 42.7, 16 authors, study period May 2020 - August 2020, dosage 200,000IU single dose, trial NCT04883203 (history).
Contact: hela-abr@hotmail.com.
Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial
Trials, doi:10.1186/s13063-023-07114-5
Introduction The present study aimed to determine the impact of vitamin D supplementation (VDs) on recovery delay among COVID-19 patients.
Methods We performed a randomized controlled clinical trial at the national COVID-19 containment center in Monastir (Tunisia), from May to August 2020. Simple randomization was done in a 1:1 allocation ratio. We included patients aged more than 18 years who had confirmed reverse transcription-polymerase chain reaction (RT-PCR) and who remained positive on the 14th day. The intervention group received VDs (200,000 IU/1 ml of cholecalciferol); the control group received a placebo treatment (physiological saline (1 ml)). We measured the recovery delay and the cycle threshold (Ct) values in RT-PCR for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The logrank test and hazard ratios (HR) were calculated. Results A total of 117 patients were enrolled. The mean age was 42.7 years (SD 14). Males represented 55.6%. The median duration of viral RNA conversion was 37 days (95% confidence interval (CI): 29-45.50) in the intervention group and 28 days (95% CI: 23-39) in the placebo group (p=0.010). HR was 1.58 (95% CI: 1.09-2.29, p=0.015). Ct values revealed a stable trend over time in both groups.
Conclusion VDs was not associated with a shortened recovery delay when given to patients for whom the RT-PCR remained positive on the 14th day.
Authors' contributions
Declarations Ethics approval and consent to participate This study was approved by the Human Subjects Protection Tunisia center (TN2020-NAT-INS-40) and by ClinicalTrial.gov with approval numberClinicalTrials.gov ID: NCT04883203. The research ethical considerations were respected including free, informed, written, clear and loyal consent, confidentiality, protection, and assistance
Consent for publication Not applicable.
Competing interests The authors declare that they have no competing interests. • fast, convenient online submission • thorough peer review by experienced researchers in your field
• rapid publication on acceptance • support for research data, including large and complex data types • gold Open Access which fosters wider collaboration and increased citations maximum visibility for your research: over 100M website views per year
• At BMC, research is always in progress.
Learn more biomedcentral.com/submissions Ready to submit your research Ready to submit your research ? Choose BMC and benefit from: ? Choose BMC and benefit from:
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Annweiler, Hanotte, De L'eprevier, Sabatier, Lafaie et al., Vitamin D and survival in COVID-19 patients: A quasi-experimental study, J Steroid Biochem Mol Biol
Bayat, Mundodan, Hasnain, Sallam, Khogali et al., Can the cycle threshold (Ct) value of RT-PCR test for SARS CoV2 predict infectivity among close contacts?, J Infect Public Health
Bennasrallah, Zemni, Dhouib, Sriha, Mezhoud et al., Factors associated with a prolonged negative conversion of viral RNA in patients with COVID-19, Int J Infect Dis
Brandão, Chiamolera, Biscolla, Lima, De et al., No association between vitamin D status and COVID-19 infection in São Paulo, Brazil Arch Endocrinol Metab
Brito, Ribeiro, Daltro, Da, De, The possible benefits of vitamin D in COVID-19, Nutr
Bui, Zhu, Hawkins, Cortez-Resendiz, Bellon, Vitamin D regulation of the immune system and its implications for COVID-19: A mini review, SAGE Open Med
Campi, Gennari, Merlotti, Mingiano, Frosali et al., Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy, BMC Infect Dis
Castillo, Costa, Barrios, Díaz, Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J Steroid Biochem Mol Biol
Davoudi, Najafi, Aarabi, Tayebi, Nikaeen et al., Lack of association between vitamin D insufficiency and clinical outcomes of patients with COVID-19 infection, BMC Infect Dis
Dramé, Cofais, Hentzien, Proye, Coulibaly et al., Relation between vitamin D and COVID-19 in aged people: A systematic review, Nutrients
Giannini, Passeri, Tripepi, Sella, Fusaro et al., Effectiveness of in-hospital cholecalciferol use on clinical outcomes in comorbid COVID-19 patients: A hypothesis-generating study, Nutrients
Grove, Osokogu, Al-Khudairy, Mehrabian, Zanganeh et al., Association between vitamin D supplementation or serum vitamin D level and susceptibility to SARS-CoV-2 infection or COVID-19 including clinical course, morbidity and mortality outcomes? A systematic review, BMJ Open
Hastie, Mackay, Ho, Celis-Morales, Katikireddi et al., Vitamin D concentrations and COVID-19 infection in UK Biobank, Diabetes Metab Syndr
Hu, Xing, Jia, Ni, Liang et al., Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19, Sci Total Environ
Kaufman, Niles, Kroll, Bi, Holick, SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels, PloS One
Kim, Outbreak of novel coronavirus (COVID-19): What is the role of radiologists?, Eur Radiol
Kim, Shin, How to do random allocation (randomization), Clin Orthop Surg
Lakkireddy, Gadiga, Malathi, Karra, Raju et al., Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease, Sci Rep
Mcadam, Cycle threshold values from Severe Acute Respiratory Syndrome Coronavirus-2 Reverse Transcription-Polymerase Chain Reaction Assays, Clin Lab Med
Munshi, Hussein, Toraih, Elshazli, Jardak et al., Vitamin D insufficiency as a potential culprit in critical COVID-19 patients, J Med Virol
Orchard, Baldry, Nasim-Mohi, Monck, Saeed et al., Vitamin-D levels and intensive care unit outcomes of a cohort of critically ill COVID-19 patients, Clin Chem Lab Med
Pan, Liu, Wang, Guo, Hao et al., Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China JAMA
Pizzini, Aichner, Sahanic, Böhm, Egger et al., Impact of vitamin D deficiency on COVID-19-A prospective analysis from the CovILD registry, Nutrients
Rastogi, Bhansali, Khare, Suri, Yaddanapudi et al., Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study), Postgrad Med J, doi:10.1136/postgradmedj-2020-139065
Rhee, Kanjilal, Baker, Klompas, Duration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infectivity: When is it safe to discontinue isolation?, Clin Infect Dis Off Publ Infect Dis Soc Am
Rocha, Atallah, Aldrighi, Pires, Santos Puga et al., Insufficient evidence for vitamin D use in COVID-19: A rapid systematic review, Int J Clin Pract
Sabico, Enani, Sheshah, Aljohani, Aldisi et al., Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of Symptoms in Patients with Mild to Moderate Covid-19: A randomized clinical trial, Nutrients
Sadeghi Dousari, Taatimoghadam, Satarzadeh, COVID-19 (Coronavirus Disease 2019): A new coronavirus disease, Infect Drug Resist
Schulz, Altman, Moher, Group, CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials, BMJ
Solís, Salas, Luesmabartolomé, Ballestín, The Effects of Vitamin D supplementation in COVID-19 patients: A systematic review, Int J Mol Sci
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet Lond Engl
DOI record:
{
"DOI": "10.1186/s13063-023-07114-5",
"ISSN": [
"1745-6215"
],
"URL": "http://dx.doi.org/10.1186/s13063-023-07114-5",
"abstract": "<jats:title>Abstract\n</jats:title><jats:sec>\n <jats:title>Introduction</jats:title>\n <jats:p>The present study aimed to determine the impact of vitamin D supplementation (VDs) on recovery delay among COVID-19 patients.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We performed a randomized controlled clinical trial at the national COVID-19 containment center in Monastir (Tunisia), from May to August 2020. Simple randomization was done in a 1:1 allocation ratio. We included patients aged more than 18 years who had confirmed reverse transcription-polymerase chain reaction (RT-PCR) and who remained positive on the 14th day. The intervention group received VDs (200,000 IU/1 ml of cholecalciferol); the control group received a placebo treatment (physiological saline (1 ml)). We measured the recovery delay and the cycle threshold (Ct) values in RT-PCR for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The log-rank test and hazard ratios (HR) were calculated.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 117 patients were enrolled. The mean age was 42.7 years (SD 14). Males represented 55.6%. The median duration of viral RNA conversion was 37 days (95% confidence interval (CI): 29–45.50) in the intervention group and 28 days (95% CI: 23–39) in the placebo group (<jats:italic>p</jats:italic>=0.010). HR was 1.58 (95% CI: 1.09–2.29, <jats:italic>p</jats:italic>=0.015). Ct values revealed a stable trend over time in both groups.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>VDs was not associated with a shortened recovery delay when given to patients for whom the RT-PCR remained positive on the 14th day.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Trial registration\n</jats:title>\n <jats:p>This study was approved by the Human Subjects Protection Tunisia center (TN2020-NAT-INS-40) on April 28, 2020, and by ClinicalTrial.gov on May 12, 2021 with approval number ClinicalTrials.gov ID: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04883203\">NCT04883203</jats:ext-link>.</jats:p>\n </jats:sec>",
"alternative-id": [
"7114"
],
"article-number": "123",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "19 January 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "23 January 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "20 February 2023"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study was approved by the Human Subjects Protection Tunisia center (TN2020-NAT-INS-40) and by ClinicalTrial.gov with approval numberClinicalTrials.gov ID: NCT04883203. The research ethical considerations were respected including free, informed, written, clear and loyal consent, confidentiality, protection, and assistance"
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9666-4914",
"affiliation": [],
"authenticated-orcid": false,
"family": "Abroug",
"given": "Hela",
"sequence": "first"
},
{
"affiliation": [],
"family": "Maatouk",
"given": "Amani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bennasrallah",
"given": "Cyrine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dhouib",
"given": "Wafa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ben Fredj",
"given": "Manel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zemni",
"given": "Imen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kacem",
"given": "Meriem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mhalla",
"given": "Salma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nouira",
"given": "Sarra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ben Belgacem",
"given": "Manel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nasri",
"given": "Aymen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klii",
"given": "Rim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loussaief",
"given": "Chawki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ben Alya",
"given": "Nissaf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouanene",
"given": "Ines",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belguith Sriha",
"given": "Asma",
"sequence": "additional"
}
],
"container-title": "Trials",
"container-title-short": "Trials",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
2,
20
]
],
"date-time": "2023-02-20T12:03:23Z",
"timestamp": 1676894603000
},
"deposited": {
"date-parts": [
[
2023,
2,
20
]
],
"date-time": "2023-02-20T12:09:07Z",
"timestamp": 1676894947000
},
"indexed": {
"date-parts": [
[
2023,
2,
21
]
],
"date-time": "2023-02-21T05:36:51Z",
"timestamp": 1676957811422
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
2,
20
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
20
]
],
"date-time": "2023-02-20T00:00:00Z",
"timestamp": 1676851200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
20
]
],
"date-time": "2023-02-20T00:00:00Z",
"timestamp": 1676851200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13063-023-07114-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13063-023-07114-5/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13063-023-07114-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2023,
2,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
2,
20
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"author": "A Pan",
"first-page": "1",
"issue": "19",
"journal-title": "China. JAMA.",
"key": "7114_CR1",
"unstructured": "Pan A, Liu L, Wang C, Guo H, Hao X, Wang Q, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan. China JAMA. 2020;323(19):1–9.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.2147/IDR.S259279",
"author": "A Sadeghi Dousari",
"doi-asserted-by": "publisher",
"first-page": "2819",
"issue": "13",
"journal-title": "Infect Drug Resist.",
"key": "7114_CR2",
"unstructured": "Sadeghi Dousari A, TaatiMoghadam M, Satarzadeh N. COVID-19 (Coronavirus Disease 2019): A new coronavirus disease. Infect Drug Resist. 2020;12(13):2819–28.",
"volume": "12",
"year": "2020"
},
{
"author": "H Kim",
"first-page": "1",
"journal-title": "Eur Radiol.",
"key": "7114_CR3",
"unstructured": "Kim H. Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? Eur Radiol. 2020;18:1–2.",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.nut.2021.111356",
"author": "DTM Brito",
"doi-asserted-by": "publisher",
"first-page": "111356",
"journal-title": "Nutr Burbank Los Angel Cty Calif.",
"key": "7114_CR4",
"unstructured": "Brito DTM, Ribeiro LHC, Daltro CH da C, Silva R de B. The possible benefits of vitamin D in COVID-19. Nutr Burbank Los Angel Cty Calif. 2021;91:111356.",
"volume": "91",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-043737",
"author": "A Grove",
"doi-asserted-by": "publisher",
"first-page": "e043737",
"issue": "5",
"journal-title": "BMJ Open.",
"key": "7114_CR5",
"unstructured": "Grove A, Osokogu O, Al-Khudairy L, Mehrabian A, Zanganeh M, Brown A, et al. Association between vitamin D supplementation or serum vitamin D level and susceptibility to SARS-CoV-2 infection or COVID-19 including clinical course, morbidity and mortality outcomes? A systematic review. BMJ Open. 2021;11(5):e043737.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1177/20503121211014073",
"author": "L Bui",
"doi-asserted-by": "publisher",
"first-page": "205031212110140",
"journal-title": "SAGE Open Med.",
"key": "7114_CR6",
"unstructured": "Bui L, Zhu Z, Hawkins S, Cortez-Resendiz A, Bellon A. Vitamin D regulation of the immune system and its implications for COVID-19: A mini review. SAGE Open Med. 2021;9:20503121211014070.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3390/ijms232012424",
"author": "Á Feiner Solís",
"doi-asserted-by": "publisher",
"first-page": "12424",
"issue": "20",
"journal-title": "Int J Mol Sci.",
"key": "7114_CR7",
"unstructured": "Feiner Solís Á, Avedillo Salas A, LuesmaBartolomé MJ, Santander Ballestín S. The Effects of Vitamin D supplementation in COVID-19 patients: A systematic review. Int J Mol Sci. 2022;23(20):12424.",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"author": "M Entrenas Castillo",
"doi-asserted-by": "publisher",
"first-page": "105751",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "7114_CR8",
"unstructured": "Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751.",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.3390/nu13010219",
"author": "S Giannini",
"doi-asserted-by": "publisher",
"first-page": "219",
"issue": "1",
"journal-title": "Nutrients.",
"key": "7114_CR9",
"unstructured": "Giannini S, Passeri G, Tripepi G, Sella S, Fusaro M, Arcidiacono G, et al. Effectiveness of in-hospital cholecalciferol use on clinical outcomes in comorbid COVID-19 patients: A hypothesis-generating study. Nutrients. 2021;13(1):219.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/nu13041339",
"author": "M Dramé",
"doi-asserted-by": "publisher",
"first-page": "1339",
"issue": "4",
"journal-title": "Nutrients.",
"key": "7114_CR10",
"unstructured": "Dramé M, Cofais C, Hentzien M, Proye E, Coulibaly PS, Demoustier-Tampère D, et al. Relation between vitamin D and COVID-19 in aged people: A systematic review. Nutrients. 2021;13(4):1339.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06168-7",
"author": "A Davoudi",
"doi-asserted-by": "publisher",
"first-page": "450",
"issue": "1",
"journal-title": "BMC Infect Dis.",
"key": "7114_CR11",
"unstructured": "Davoudi A, Najafi N, Aarabi M, Tayebi A, Nikaeen R, Izadyar H, et al. Lack of association between vitamin D insufficiency and clinical outcomes of patients with COVID-19 infection. BMC Infect Dis. 2021;21(1):450.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1249",
"author": "C Rhee",
"doi-asserted-by": "publisher",
"first-page": "1467",
"issue": "8",
"journal-title": "Clin Infect Dis Off Publ Infect Dis Soc Am.",
"key": "7114_CR12",
"unstructured": "Rhee C, Kanjilal S, Baker M, Klompas M. Duration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infectivity: When is it safe to discontinue isolation? Clin Infect Dis Off Publ Infect Dis Soc Am. 2021;72(8):1467–74.",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet Lond Engl.",
"key": "7114_CR13",
"unstructured": "Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl. 2020;395(10229):1054–62.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1136/bmj.c332",
"author": "KF Schulz",
"doi-asserted-by": "publisher",
"first-page": "c332",
"issue": "mar23 1",
"journal-title": "BMJ.",
"key": "7114_CR14",
"unstructured": "Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340(mar23 1):c332–c332.",
"volume": "340",
"year": "2010"
},
{
"DOI": "10.1016/j.cll.2022.02.003",
"author": "AJ McAdam",
"doi-asserted-by": "publisher",
"first-page": "237",
"issue": "2",
"journal-title": "Clin Lab Med.",
"key": "7114_CR15",
"unstructured": "McAdam AJ. Cycle threshold values from Severe Acute Respiratory Syndrome Coronavirus-2 Reverse Transcription-Polymerase Chain Reaction Assays. Clin Lab Med. 2022;42(2):237–48.",
"volume": "42",
"year": "2022"
},
{
"key": "7114_CR16",
"unstructured": "Société Française de Microbiologie (SFM). Avis du 25 septembre 2020 de la Société Française de Microbiologie (SFM) relatif à l’interprétation de la valeur de Ct (estimation de la charge virale) obtenue en cas de RT-PCR SARS-CoV-2 positive sur les prélèvements cliniques réalisés à des fins diagnostiques ou de dépistage. Version 1. 2020. Available from: https://www.sfm-microbiologie.org/wp-content/uploads/2021/01/Avis-SFM-valeur-Ct-excre%CC%81tion-virale-_-Version-def-14012021_V4.pdf. cited 2020 Oct 1"
},
{
"DOI": "10.1016/j.ijid.2021.02.089",
"author": "C Bennasrallah",
"doi-asserted-by": "publisher",
"first-page": "463",
"journal-title": "Int J Infect Dis.",
"key": "7114_CR17",
"unstructured": "Bennasrallah C, Zemni I, Dhouib W, Sriha H, Mezhoud N, Bouslama S, et al. Factors associated with a prolonged negative conversion of viral RNA in patients with COVID-19. Int J Infect Dis. 2021;105:463–9.",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.4055/cios.2014.6.1.103",
"author": "J Kim",
"doi-asserted-by": "publisher",
"first-page": "103",
"issue": "1",
"journal-title": "Clin Orthop Surg.",
"key": "7114_CR18",
"unstructured": "Kim J, Shin W. How to do random allocation (randomization). Clin Orthop Surg. 2014;6(1):103–9.",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.1002/jmv.26360",
"author": "R Munshi",
"doi-asserted-by": "publisher",
"first-page": "733",
"issue": "2",
"journal-title": "J Med Virol.",
"key": "7114_CR19",
"unstructured": "Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol. 2021;93(2):733–40.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1111/ijcp.14649",
"author": "AP Da Rocha",
"doi-asserted-by": "publisher",
"first-page": "e14649",
"issue": "11",
"journal-title": "Int J Clin Pract.",
"key": "7114_CR20",
"unstructured": "Da Rocha AP, Atallah AN, Aldrighi JM, Pires ALR, Dos Santos Puga ME, Pinto ACPN. Insufficient evidence for vitamin D use in COVID-19: A rapid systematic review. Int J Clin Pract. 2021;75(11):e14649.",
"volume": "75",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2020.04.050",
"author": "CE Hastie",
"doi-asserted-by": "publisher",
"first-page": "561",
"issue": "4",
"journal-title": "Diabetes Metab Syndr.",
"key": "7114_CR21",
"unstructured": "Hastie CE, Mackay DF, Ho F, Celis-Morales CA, Katikireddi SV, Niedzwiedz CL, et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14(4):561–5.",
"volume": "14",
"year": "2020"
},
{
"author": "CMÁ Brandão",
"first-page": "381",
"issue": "3",
"journal-title": "Brazil. Arch Endocrinol Metab.",
"key": "7114_CR22",
"unstructured": "Brandão CMÁ, Chiamolera MI, Biscolla RPM, Lima JV, De Francischi Ferrer CM, Prieto WH, et al. No association between vitamin D status and COVID-19 infection in São Paulo. Brazil Arch Endocrinol Metab. 2021;65(3):381–5.",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.3390/nu12092775",
"author": "A Pizzini",
"doi-asserted-by": "publisher",
"first-page": "E2775",
"issue": "9",
"journal-title": "Nutrients.",
"key": "7114_CR23",
"unstructured": "Pizzini A, Aichner M, Sahanic S, Böhm A, Egger A, Hoermann G, et al. Impact of vitamin D deficiency on COVID-19-A prospective analysis from the CovILD registry. Nutrients. 2020;12(9):E2775.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1136/postgradmedj-2020-139065",
"doi-asserted-by": "publisher",
"key": "7114_CR24",
"unstructured": "Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J. 2022;98(1156):87–90. https://doi.org/10.1136/postgradmedj-2020-139065."
},
{
"DOI": "10.1016/j.jsbmb.2020.105771",
"author": "C Annweiler",
"doi-asserted-by": "publisher",
"first-page": "105771",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "7114_CR25",
"unstructured": "Annweiler C, Hanotte B, Grandin de l’Eprevier C, Sabatier JM, Lafaie L, Célarier T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J Steroid Biochem Mol Biol. 2020;204:105771.",
"volume": "204",
"year": "2020"
},
{
"DOI": "10.3390/nu13072170",
"author": "S Sabico",
"doi-asserted-by": "publisher",
"first-page": "2170",
"issue": "7",
"journal-title": "Nutrients.",
"key": "7114_CR26",
"unstructured": "Sabico S, Enani MA, Sheshah E, Aljohani NJ, Aldisi DA, Alotaibi NH, et al. Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of Symptoms in Patients with Mild to Moderate Covid-19: A randomized clinical trial. Nutrients. 2021;13(7):2170.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0239252",
"author": "HW Kaufman",
"doi-asserted-by": "publisher",
"first-page": "e0239252",
"issue": "9",
"journal-title": "PloS One.",
"key": "7114_CR27",
"unstructured": "Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PloS One. 2020;15(9):e0239252.",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06281-7",
"author": "I Campi",
"doi-asserted-by": "publisher",
"first-page": "566",
"issue": "1",
"journal-title": "BMC Infect Dis.",
"key": "7114_CR28",
"unstructured": "Campi I, Gennari L, Merlotti D, Mingiano C, Frosali A, Giovanelli L, et al. Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect Dis. 2021;21(1):566.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.scitotenv.2020.138812",
"author": "X Hu",
"doi-asserted-by": "publisher",
"first-page": "138812",
"issue": "728",
"journal-title": "Sci Total Environ.",
"key": "7114_CR29",
"unstructured": "Hu X, Xing Y, Jia J, Ni W, Liang J, Zhao D, et al. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci Total Environ. 2020;1(728):138812.",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1016/j.jiph.2021.08.013",
"author": "S Al Bayat",
"doi-asserted-by": "publisher",
"first-page": "1201",
"issue": "9",
"journal-title": "J Infect Public Health.",
"key": "7114_CR30",
"unstructured": "Al Bayat S, Mundodan J, Hasnain S, Sallam M, Khogali H, Ali D, et al. Can the cycle threshold (Ct) value of RT-PCR test for SARS CoV2 predict infectivity among close contacts? J Infect Public Health. 2021;14(9):1201–5.",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-90189-4",
"author": "M Lakkireddy",
"doi-asserted-by": "publisher",
"first-page": "10641",
"issue": "1",
"journal-title": "Sci Rep.",
"key": "7114_CR31",
"unstructured": "Lakkireddy M, Gadiga SG, Malathi RD, Karra ML, Raju ISSVPM, Ragini null, et al. Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease. Sci Rep. 2021;11(1):10641.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1515/cclm-2020-1567",
"author": "L Orchard",
"doi-asserted-by": "publisher",
"first-page": "1155",
"issue": "6",
"journal-title": "Clin Chem Lab Med.",
"key": "7114_CR32",
"unstructured": "Orchard L, Baldry M, Nasim-Mohi M, Monck C, Saeed K, Grocott MPW, et al. Vitamin-D levels and intensive care unit outcomes of a cohort of critically ill COVID-19 patients. Clin Chem Lab Med. 2021;59(6):1155–63.",
"volume": "59",
"year": "2021"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-023-07114-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Medicine (miscellaneous)"
],
"subtitle": [],
"title": "Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}
