Human bronchopulmonary disposition and plasma pharmacokinetics of oral bemnifosbuvir (AT-527), an experimental guanosine nucleotide prodrug for COVID-19
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkae122, NCT04877769, Jan 2024 (preprint)
Phase 1 study showing effective lung delivery and safety of the oral COVID-19 antiviral candidate bemnifosbuvir (AT-527) at 550mg twice daily. Authors found AT-527 550mg BID achieved sustained antiviral drug levels in lung fluids that exceeded the EC90 target concentration of 0.5μM required to inhibit SARS-CoV-2 replication in human airway cells. Although AT-527 underwent rapid systemic clearance, it demonstrated good lung distribution, and its active metabolite was detected in alveolar macrophages. The 550 mg BID regimen was well tolerated over 2.5 days with mostly mild adverse effects.
Zhou et al., 8 Jan 2024, United Kingdom, peer-reviewed, 9 authors, study period May 2021 - June 2021, trial NCT04877769 (history).
Contact: zhou.xj@ateapharma.com.
Human bronchopulmonary disposition and plasma pharmacokinetics of oral bemnifosbuvir (AT-527), an experimental guanosine nucleotide prodrug for COVID-19
doi:10.1093/jac/dkae122/7665247
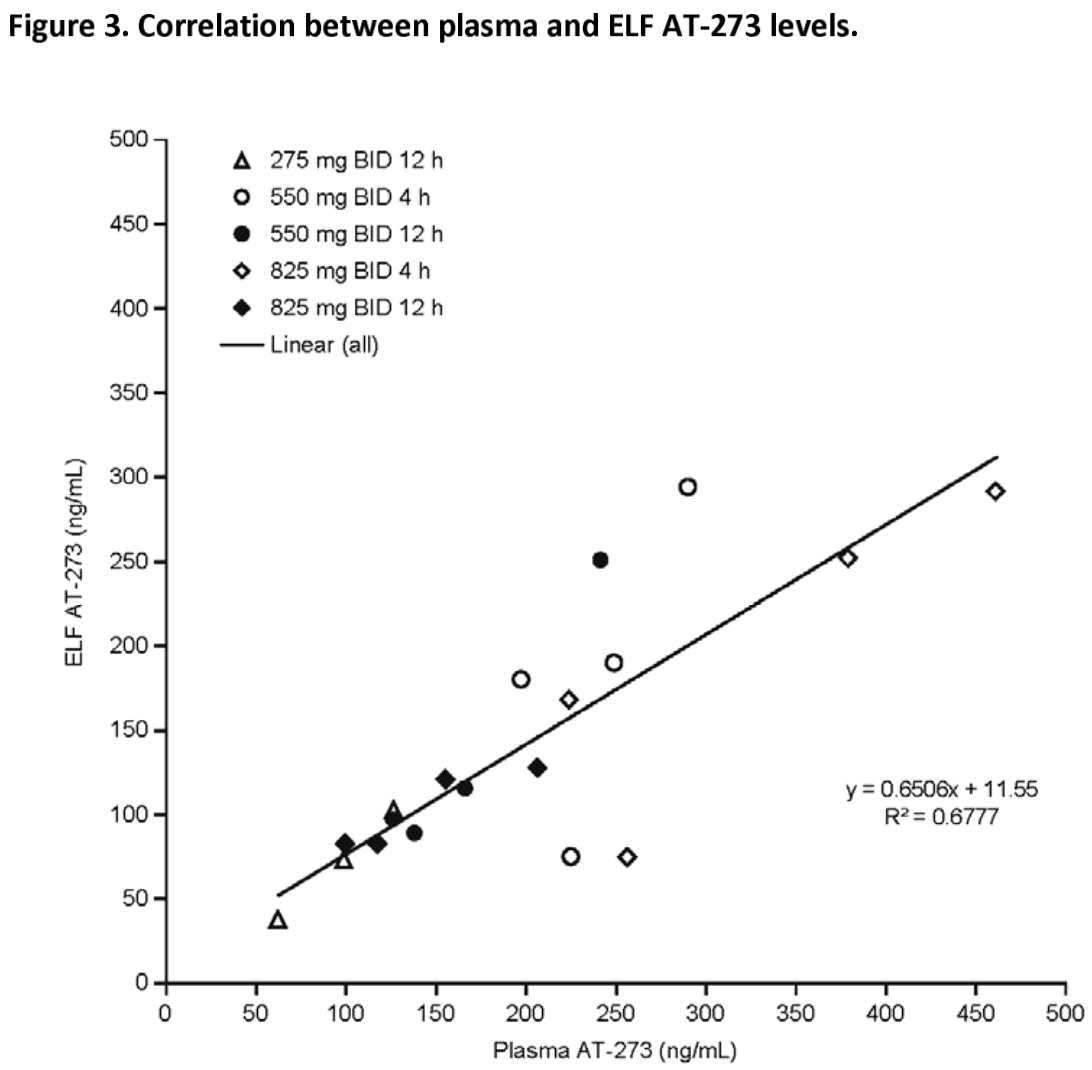
Background: Bemnifosbuvir (AT-527) is a novel oral guanosine nucleotide antiviral drug for the treatment of persons with COVID-19. Direct assessment of drug disposition in the lungs, via bronchoalveolar lavage, is necessary to ensure antiviral drug levels at the primary site of SARS-CoV-2 infection are achieved. Objectives: This Phase 1 study in healthy subjects aimed to assess the bronchopulmonary pharmacokinetics, safety and tolerability of repeated doses of bemnifosbuvir. Methods: A total of 24 subjects were assigned to receive bemnifosbuvir twice daily at doses of 275, 550 or 825 mg for up to 3.5 days. Results: AT-511, the free base of bemnifosbuvir, was largely eliminated from the plasma within 6 h post dose in all dosing groups. Antiviral drug levels of bemnifosbuvir were consistently achieved in the lungs with bemnifosbuvir 550 mg twice daily. The mean level of the guanosine nucleoside metabolite AT-273, the surrogate of the active triphosphate metabolite of the drug, measured in the epithelial lining fluid of the lungs was 0.62 µM at 4-5 h post dose. This exceeded the target in vitro 90% effective concentration (EC 90 ) of 0.5 µM for antiviral drug exposure against SARS-CoV-2 replication in human airway epithelial cells. Bemnifosbuvir was well tolerated across all doses tested, and most treatment-emergent adverse events reported were mild in severity and resolved.
Conclusions: The favourable pharmacokinetics and safety profile of bemnifosbuvir demonstrates its potential as an oral antiviral treatment for COVID-19, with 550 mg bemnifosbuvir twice daily currently under further clinical evaluation in persons with COVID-19.
Supplementary data Supplementary Methods are available as Supplementary data at JAC Online.
References
Berliba, Bogus, Vanhoutte, Safety, pharmacokinetics and antiviral activity of AT-527, a novel purine nucleotide prodrug, in HCV-infected subjects with and without cirrhosis, Antimicrob Agents Chemother, doi:10.1128/AAC.01201-19
Boffito, Dolan, Singh, A phase 2 randomized trial evaluating the antiviral activity and safety of the direct-acting antiviral bemnifosbuvir in ambulatory patients with mild or moderate COVID-19 (MOONSONG Study), Microbiol Spectr, doi:10.1128/spectrum.00077-23
Bösmüller, Matter, Fend, The pulmonary pathology of COVID-19, Virchows Arch, doi:10.1007/s00428-021-03053-1
Candel, Tyrkalska, Álvarez-Santacruz, The nasopharyngeal microbiome in COVID-19, Emerg Microbes Infect, doi:10.1080/22221751.2023.2165970
Cox, Wolf, Plemper, Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets, Nat Microbiol, doi:10.1038/s41564-020-00835-2
Driouich, Cochin, Lingas, Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model, Nat Commun, doi:10.1038/s41467-021-21992-w
Elezkurtaj, Greuel, Ihlow, Causes of death and comorbidities in hospitalized patients with COVID-19, Sci Rep, doi:10.1038/s41598-021-82862-5
Good, Moussa, Zhou, Preclinical evaluation of AT-527, a novel guanosine nucleotide prodrug with potent, pan-genotypic activity against hepatitis C virus, PLoS One, doi:10.1371/journal.pone.0227104
Good, Westover, Jung, AT-527, a double prodrug of a guanosine nucleotide analog, is a potent inhibitor of SARS-CoV-2 in vitro and a promising oral antiviral for treatment of COVID-19, Antimicrob Agents Chemother, doi:10.1128/AAC.02479-20
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe COVID-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Gülhan, Eryüksel, İdriz Oğlu, Pharmacokinetic characterization of favipiravir in patients with COVID-19, Br J Clin Pharmacol, doi:10.1111/bcp.15227
Hoefnagel, Koopmans, Burger, Role of the inhibitory quotient in HIV therapy, Antivir Ther, doi:10.1177/135965350501000802
Horga, Kuritzkes, Kowalczyk, Phase II study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19, Future Virol, doi:10.2217/fvl-2023-0064
Horga, Saenz, Yilmaz, Oral bemnifosbuvir (AT-527) vs placebo in patients with mild-to-moderate COVID-19 in an outpatient setting (MORNINGSKY), Future Virol, doi:10.2217/fvl-2023-0115
Huang, Bassett, Acute phase reaction in healthy volunteers after bronchoscopy with lavage, Chest, doi:10.1378/chest.129.6.1565
Leegwater, Moes, Bosma, Population pharmacokinetics of remdesivir and GS-441524 in hospitalized COVID-19 patients, Antimicrob Agents Chemother, doi:10.1128/aac.00254-22
Mungur, Berliba, Bourgeois, A combination of AT-527, a pan-genotypic guanosine nucleotide prodrug, and daclatasvir was welltolerated and effective in HCV-infected subjects, J Hepatol, doi:10.1016/S0168-8278(20)31210-1
Painter, Holman, Bush, Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2, Antimicrob Agents Chemother, doi:10.1128/AAC.02428-20
Pharmaceuticals, Inc, Bemnifosbuvir hemisulfate (BEM; AT-527) investigator's brochure, Investigator's Brochure
Roque, Proudfoot, Mathys, A review of nasopharyngeal swab and saliva tests for SARS-CoV-2 infection: disease timelines, relative sensitivities, and test optimization, J Surg Oncol, doi:10.1002/jso.26561
Sahin, Akbal-Dagistan, Culha, Antivirals and the potential benefits of orally inhaled drug administration in COVID-19 treatment, J Pharm Sci, doi:10.1016/j.xphs.2022.06.004
Shannon, Fattorini, Sama, A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase, Nat Commun, doi:10.1038/s41467-022-28113-1
Sharp, Dohme, Fact sheet for healthcare providers: emergency use authorization for Lagevrio ™ (molnupiravir) capsules
Singh, De, Antiviral agents for the treatment of COVID-19: progress and challenges, Cell Rep Med, doi:10.1016/j.xcrm.2022.100549
Toussi, Neutel, Navarro, Pharmacokinetics of oral nirmatrelvir/ritonavir, a protease inhibitor for treatment of COVID-19, in subjects with renal impairment, Clin Pharmacol Ther, doi:10.1002/cpt.2688
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wang, Chen, Lung tissue distribution of drugs as a key factor for COVID-19 treatment, Br J Pharmacol, doi:10.1111/bph.15102
Zhou, Horga, Morelli, High lung levels of active triphosphate predicted with oral AT-527 in COVID patients, Top Antivir Med
Zhou, Montrond, Pietropaolo, Pharmacokinetics and metabolism of [14C]-bemnifosbuvir in healthy male participants
Zhou, None
DOI record:
{
"DOI": "10.1093/jac/dkae122",
"ISSN": [
"0305-7453",
"1460-2091"
],
"URL": "http://dx.doi.org/10.1093/jac/dkae122",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Bemnifosbuvir (AT-527) is a novel oral guanosine nucleotide antiviral drug for the treatment of persons with COVID-19. Direct assessment of drug disposition in the lungs, via bronchoalveolar lavage, is necessary to ensure antiviral drug levels at the primary site of SARS-CoV-2 infection are achieved.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Objectives</jats:title>\n <jats:p>This Phase 1 study in healthy subjects aimed to assess the bronchopulmonary pharmacokinetics, safety and tolerability of repeated doses of bemnifosbuvir.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>A total of 24 subjects were assigned to receive bemnifosbuvir twice daily at doses of 275, 550 or 825 mg for up to 3.5 days.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>AT-511, the free base of bemnifosbuvir, was largely eliminated from the plasma within 6 h post dose in all dosing groups. Antiviral drug levels of bemnifosbuvir were consistently achieved in the lungs with bemnifosbuvir 550 mg twice daily. The mean level of the guanosine nucleoside metabolite AT-273, the surrogate of the active triphosphate metabolite of the drug, measured in the epithelial lining fluid of the lungs was 0.62 µM at 4–5 h post dose. This exceeded the target in vitro 90% effective concentration (EC90) of 0.5 µM for antiviral drug exposure against SARS-CoV-2 replication in human airway epithelial cells. Bemnifosbuvir was well tolerated across all doses tested, and most treatment-emergent adverse events reported were mild in severity and resolved.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>The favourable pharmacokinetics and safety profile of bemnifosbuvir demonstrates its potential as an oral antiviral treatment for COVID-19, with 550 mg bemnifosbuvir twice daily currently under further clinical evaluation in persons with COVID-19.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc. , Boston, MA , USA"
}
],
"family": "Zhou",
"given": "Xiao-Jian",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc. , Boston, MA , USA"
}
],
"family": "Horga",
"given": "Arantxa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hammersmith Medicines Research Ltd , London , UK"
}
],
"family": "Puri",
"given": "Adeep",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Antiviral Pharmacology Laboratory, University of Nebraska Medical Center , Omaha, NE , USA"
}
],
"family": "Winchester",
"given": "Lee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc. , Boston, MA , USA"
}
],
"family": "Montrond",
"given": "Maureen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc. , Boston, MA , USA"
}
],
"family": "Pietropaolo",
"given": "Keith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc. , Boston, MA , USA"
}
],
"family": "Belanger",
"given": "Bruce",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3703-7849",
"affiliation": [
{
"name": "Antiviral Pharmacology Laboratory, University of Nebraska Medical Center , Omaha, NE , USA"
}
],
"authenticated-orcid": false,
"family": "Fletcher",
"given": "Courtney V",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Atea Pharmaceuticals, Inc. , Boston, MA , USA"
}
],
"family": "Hammond",
"given": "Janet",
"sequence": "additional"
}
],
"container-title": "Journal of Antimicrobial Chemotherapy",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
5,
6
]
],
"date-time": "2024-05-06T08:13:37Z",
"timestamp": 1714983217000
},
"deposited": {
"date-parts": [
[
2024,
5,
6
]
],
"date-time": "2024-05-06T08:13:53Z",
"timestamp": 1714983233000
},
"funder": [
{
"name": "Atea Pharmaceuticals, Inc"
},
{
"name": "Atea Pharmaceuticals, Inc"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
7
]
],
"date-time": "2024-05-07T00:28:14Z",
"timestamp": 1715041694460
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
5,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
6
]
],
"date-time": "2024-05-06T00:00:00Z",
"timestamp": 1714953600000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkae122/57415276/dkae122.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkae122/57415276/dkae122.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2024,
5,
6
]
]
},
"published-online": {
"date-parts": [
[
2024,
5,
6
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "World Health Organization",
"key": "2024050608132818200_dkae122-B1"
},
{
"DOI": "10.1002/jso.26561",
"article-title": "A review of nasopharyngeal swab and saliva tests for SARS-CoV-2 infection: disease timelines, relative sensitivities, and test optimization",
"author": "Roque",
"doi-asserted-by": "crossref",
"first-page": "465",
"journal-title": "J Surg Oncol",
"key": "2024050608132818200_dkae122-B2",
"volume": "124",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2023.2165970",
"article-title": "The nasopharyngeal microbiome in COVID-19",
"author": "Candel",
"doi-asserted-by": "crossref",
"first-page": "e2165970",
"journal-title": "Emerg Microbes Infect",
"key": "2024050608132818200_dkae122-B3",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1007/s00428-021-03053-1",
"article-title": "The pulmonary pathology of COVID-19",
"author": "Bösmüller",
"doi-asserted-by": "crossref",
"first-page": "137",
"journal-title": "Virchows Arch",
"key": "2024050608132818200_dkae122-B4",
"volume": "478",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-82862-5",
"article-title": "Causes of death and comorbidities in hospitalized patients with COVID-19",
"author": "Elezkurtaj",
"doi-asserted-by": "crossref",
"first-page": "4263",
"journal-title": "Sci Rep",
"key": "2024050608132818200_dkae122-B5",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1128/aac.00254-22",
"article-title": "Population pharmacokinetics of remdesivir and GS-441524 in hospitalized COVID-19 patients",
"author": "Leegwater",
"doi-asserted-by": "crossref",
"first-page": "e0025422",
"journal-title": "Antimicrob Agents Chemother",
"key": "2024050608132818200_dkae122-B6",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1128/AAC.02428-20",
"article-title": "Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2",
"author": "Painter",
"doi-asserted-by": "crossref",
"first-page": "e02428-20",
"journal-title": "Antimicrob Agents Chemother",
"key": "2024050608132818200_dkae122-B7",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1002/cpt.2688",
"article-title": "Pharmacokinetics of oral nirmatrelvir/ritonavir, a protease inhibitor for treatment of COVID-19, in subjects with renal impairment",
"author": "Toussi",
"doi-asserted-by": "crossref",
"first-page": "892",
"journal-title": "Clin Pharmacol Ther",
"key": "2024050608132818200_dkae122-B8",
"volume": "112",
"year": "2022"
},
{
"DOI": "10.1016/j.xphs.2022.06.004",
"article-title": "Antivirals and the potential benefits of orally inhaled drug administration in COVID-19 treatment",
"author": "Sahin",
"doi-asserted-by": "crossref",
"first-page": "2652",
"journal-title": "J Pharm Sci",
"key": "2024050608132818200_dkae122-B9",
"volume": "111",
"year": "2022"
},
{
"DOI": "10.1111/bph.15102",
"article-title": "Lung tissue distribution of drugs as a key factor for COVID-19 treatment",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "4995",
"journal-title": "Br J Pharmacol",
"key": "2024050608132818200_dkae122-B10",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01201-19",
"article-title": "Safety, pharmacokinetics and antiviral activity of AT-527, a novel purine nucleotide prodrug, in HCV-infected subjects with and without cirrhosis",
"author": "Berliba",
"doi-asserted-by": "crossref",
"first-page": "e01201-19",
"journal-title": "Antimicrob Agents Chemother",
"key": "2024050608132818200_dkae122-B11",
"volume": "63",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0227104",
"article-title": "Preclinical evaluation of AT-527, a novel guanosine nucleotide prodrug with potent, pan-genotypic activity against hepatitis C virus",
"author": "Good",
"doi-asserted-by": "crossref",
"first-page": "e0227104",
"journal-title": "PLoS One",
"key": "2024050608132818200_dkae122-B12",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1128/AAC.02479-20",
"article-title": "AT-527, a double prodrug of a guanosine nucleotide analog, is a potent inhibitor of SARS-CoV-2 in vitro and a promising oral antiviral for treatment of COVID-19",
"author": "Good",
"doi-asserted-by": "crossref",
"first-page": "e02479-20",
"journal-title": "Antimicrob Agents Chemother",
"key": "2024050608132818200_dkae122-B13",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1038/s41467-022-28113-1",
"article-title": "A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase",
"author": "Shannon",
"doi-asserted-by": "crossref",
"first-page": "621",
"journal-title": "Nat Commun",
"key": "2024050608132818200_dkae122-B14",
"volume": "13",
"year": "2022"
},
{
"article-title": "Pharmacokinetics and metabolism of [14C]-bemnifosbuvir in healthy male participants",
"author": "Zhou",
"key": "2024050608132818200_dkae122-B15"
},
{
"author": "Atea Pharmaceuticals, Inc",
"key": "2024050608132818200_dkae122-B16"
},
{
"article-title": "High lung levels of active triphosphate predicted with oral AT-527 in COVID patients",
"author": "Zhou",
"first-page": "127",
"journal-title": "Top Antivir Med",
"key": "2024050608132818200_dkae122-B17",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1128/spectrum.00077-23",
"article-title": "A phase 2 randomized trial evaluating the antiviral activity and safety of the direct-acting antiviral bemnifosbuvir in ambulatory patients with mild or moderate COVID-19 (MOONSONG Study)",
"author": "Boffito",
"doi-asserted-by": "crossref",
"first-page": "e0007723",
"journal-title": "Microbiol Spectr",
"key": "2024050608132818200_dkae122-B18",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1378/chest.129.6.1565",
"article-title": "Acute phase reaction in healthy volunteers after bronchoscopy with lavage",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "1565",
"journal-title": "Chest",
"key": "2024050608132818200_dkae122-B19",
"volume": "129",
"year": "2006"
},
{
"DOI": "10.1016/S0168-8278(20)31210-1",
"article-title": "A combination of AT-527, a pan-genotypic guanosine nucleotide prodrug, and daclatasvir was well-tolerated and effective in HCV-infected subjects",
"author": "Mungur",
"doi-asserted-by": "crossref",
"first-page": "S357",
"journal-title": "J Hepatol",
"key": "2024050608132818200_dkae122-B20",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.2217/fvl-2023-0064",
"article-title": "Phase II study of bemnifosbuvir in high-risk participants in a hospital setting with moderate COVID-19",
"author": "Horga",
"doi-asserted-by": "crossref",
"first-page": "489",
"journal-title": "Future Virol",
"key": "2024050608132818200_dkae122-B21",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.2217/fvl-2023-0115",
"article-title": "Oral bemnifosbuvir (AT-527) vs placebo in patients with mild-to-moderate COVID-19 in an outpatient setting (MORNINGSKY)",
"author": "Horga",
"doi-asserted-by": "crossref",
"first-page": "839",
"journal-title": "Future Virol",
"key": "2024050608132818200_dkae122-B22",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.1016/j.xcrm.2022.100549",
"article-title": "Antiviral agents for the treatment of COVID-19: progress and challenges",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "100549",
"journal-title": "Cell Rep Med",
"key": "2024050608132818200_dkae122-B23",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1177/135965350501000802",
"article-title": "Role of the inhibitory quotient in HIV therapy",
"author": "Hoefnagel",
"doi-asserted-by": "crossref",
"first-page": "879",
"journal-title": "Antivir Ther",
"key": "2024050608132818200_dkae122-B24",
"volume": "10",
"year": "2005"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "2024050608132818200_dkae122-B25",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1038/s41564-020-00835-2",
"article-title": "Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets",
"author": "Cox",
"doi-asserted-by": "crossref",
"first-page": "11",
"journal-title": "Nat Microbiol",
"key": "2024050608132818200_dkae122-B26",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-21992-w",
"article-title": "Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model",
"author": "Driouich",
"doi-asserted-by": "crossref",
"first-page": "1735",
"journal-title": "Nat Commun",
"key": "2024050608132818200_dkae122-B27",
"volume": "12",
"year": "2021"
},
{
"author": "Gilead Sciences",
"key": "2024050608132818200_dkae122-B28"
},
{
"author": "Merck Sharp & Dohme LLC",
"key": "2024050608132818200_dkae122-B29"
},
{
"DOI": "10.1111/bcp.15227",
"article-title": "Pharmacokinetic characterization of favipiravir in patients with COVID-19",
"author": "Gülhan",
"doi-asserted-by": "crossref",
"first-page": "3516",
"journal-title": "Br J Clin Pharmacol",
"key": "2024050608132818200_dkae122-B30",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe COVID-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "2024050608132818200_dkae122-B31",
"volume": "386",
"year": "2022"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jac/advance-article/doi/10.1093/jac/dkae122/7665247"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Human bronchopulmonary disposition and plasma pharmacokinetics of oral bemnifosbuvir (AT-527), an experimental guanosine nucleotide prodrug for COVID-19",
"type": "journal-article"
}