Tixagevimab/Cilgavimab for Prevention of COVID-19 during the Omicron Surge: Retrospective Analysis of National VA Electronic Data
et al., medRxiv, doi:10.1101/2022.05.28.22275716, May 2022
42nd treatment shown to reduce risk in
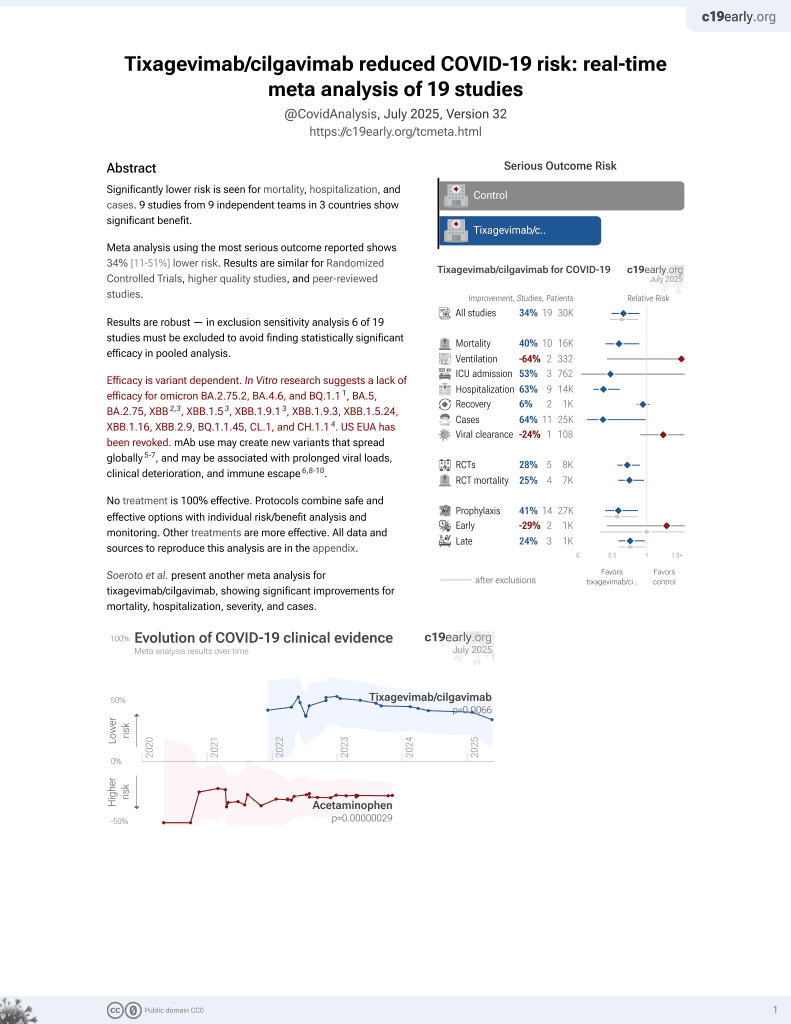
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
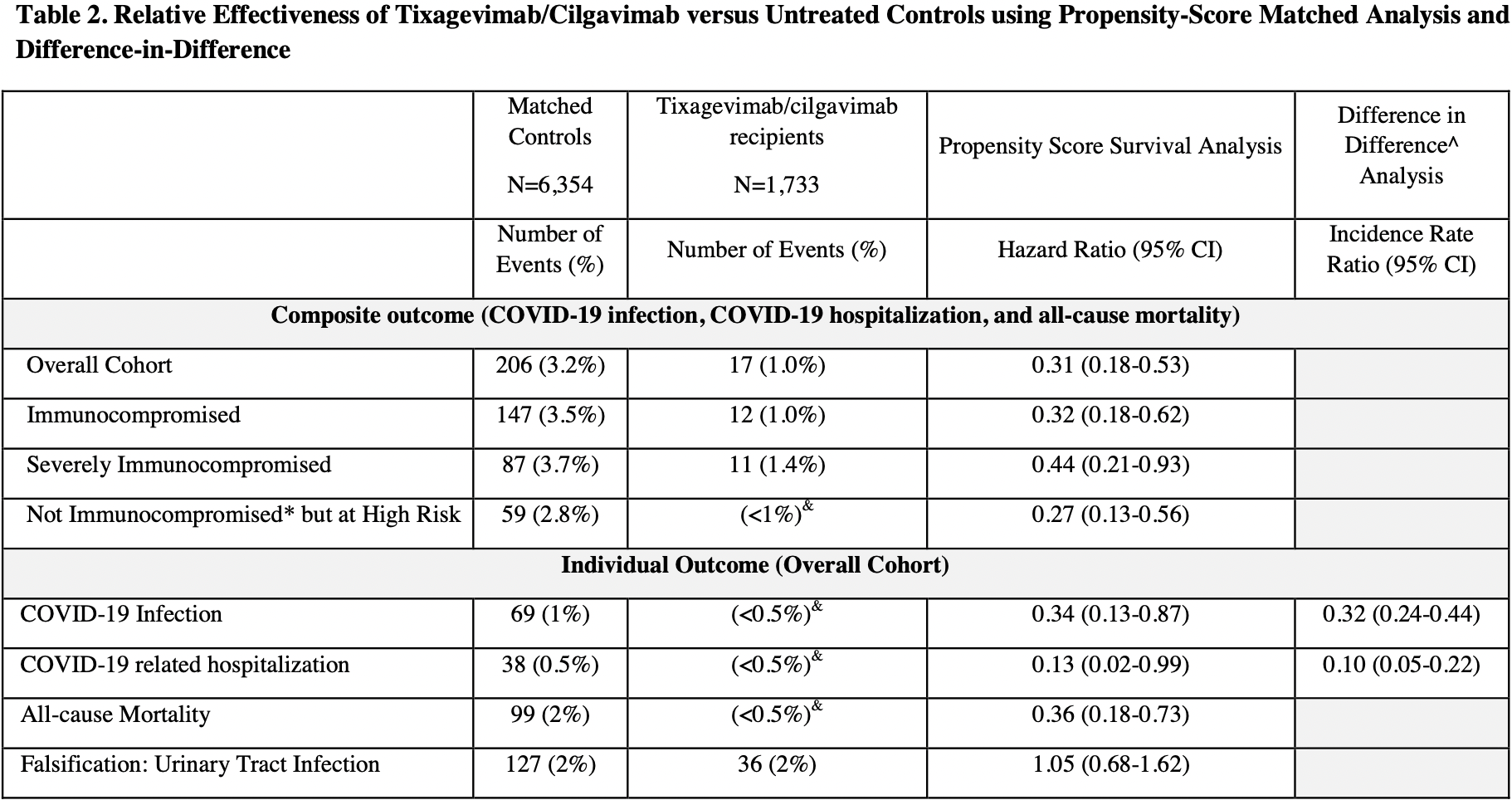
PSM retrospective 1,848 immunocompromised patients given tixagevimab/cilgavimab prophylaxis, showing lower mortality, hospitalization, and cases.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments5.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 64.0% lower, HR 0.36, p = 0.004, treatment 1,733, control 6,354.
|
|
risk of death/hospitalization/cases, 69.0% lower, HR 0.31, p < 0.001, treatment 17 of 1,733 (1.0%), control 206 of 6,354 (3.2%), NNT 44.
|
|
risk of hospitalization, 87.0% lower, HR 0.13, p = 0.04, treatment 1,733, control 6,354.
|
|
risk of case, 66.0% lower, HR 0.34, p = 0.03, treatment 1,733, control 6,354.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
3.
Uraki et al., Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate, iScience, doi:10.1016/j.isci.2023.108147.
Young-Xu et al., 29 May 2022, retrospective, propensity score matching, USA, preprint, 10 authors.
Contact: adit.ginde@cuanschutz.edu.
Tixagevimab/Cilgavimab for Prevention of COVID-19 during the Omicron Surge: Retrospective Analysis of National VA Electronic Data
doi:10.1101/2022.05.28.22275716
Background: Little is known regarding the effectiveness of tixagevimab/cilgavimab in preventing SARS-CoV-2 infection in this population, particularly after the emergence of the Omicron variant. Objective: To determine the effectiveness of tixagevimab/cilgavimab for prevention of SARS-CoV-2 infection and severe disease among immunocompromised patients. Design: Retrospective cohort study with propensity matching and difference-in-difference analyses. Setting: U.S. Department of Veterans Affairs (VA) healthcare system. Participants: Veterans age ≥18 years as of January 1, 2022, receiving VA healthcare. We compared a cohort of 1,848 patients treated with at least one dose of intramuscular tixagevimab/cilgavimab to matched controls selected from 251,756 patients who were on immunocompromised or otherwise at high risk for COVID-19. Patients were followed through April 30, 2022, or until death, whichever occurred earlier. Main Outcomes: Composite of SARS-CoV-2 infection, COVID-19-related hospitalization, and allcause mortality. We used cox proportional hazards modelling to estimate the hazard ratios (HR) and 95% CI for the association between receipt of tixagevimab/cilgavimab and outcomes. Results: Most (69%) tixagevimab/cilgavimab recipients were ≥65 years old, 92% were identified as immunocompromised in electronic data, and 73% had ≥3 mRNA vaccine doses or two doses of Ad26.COV2. Compared to propensity-matched controls, tixagevimab/cilgavimab-treated patients had a lower incidence of the composite COVID-19 outcome (17/1733 [1.0%] vs 206/6354 [3.2%]; HR 0.31; 95%CI, 0.18-0.53), and individually SARS-CoV-2 infection (HR 0.34; 95%CI, 0.13-0.87), COVID-19 hospitalization (HR 0.13; 95%CI, 0.02-0.99), and all-cause mortality (HR 0.36; 95%CI, 0.18-0.73).
Limitations: Confounding by indication and immortal time bias. Conclusions: Using national real-world data from predominantly vaccinated, immunocompromised Veterans, administration of tixagevimab/cilgavimab was associated with lower rates of SARS-CoV-2 infection, COVID-19 hospitalization, and all-cause mortality during the Omicron surge.
Declaration of Authors Competing Interests VCM has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences and ViiV. YYX, GZ, CK, JS reported receiving grants from the US Food and Drug Administration through an interagency agreement with the Veterans Health Administration and from the US Department of Veterans Affairs Office of Rural Health.
References
Adler-Milstein, Jha, No evidence found that hospitals are using new electronic health records to increase Medicare reimbursements, Health Aff (Millwood)
Angrist, Pischke, Mastering'Metrics: The Path from Cause to Effect
Austin, Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples, Stat Med, doi:10.1002/sim.3697
Bar-On, Goldberg, Mandel, Bodenheimer, Freedman et al., Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel, N Engl J Med, doi:10.1056/NEJMoa211425
Collard, Boter, Schoevers, Prevalence of frailty in community-dwelling older persons: A systematic review, J Am Geriatr Soc
Corey, Beyrer, Cohen, Michael, Bedford et al., SARS-CoV-2 Variants in Patients with Immunosuppression, N Engl J Med, doi:10.1056/NEJMsb2104756
Devoe, Gold, Mcintire, Electronic health records vs Medicaid claims: completeness of diabetes preventive care data in community health centers, Ann Fam Med
Deyo, Cherkin, Ciol, Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases, J Clin Epidemiol
Dubberke, Olsen, Stwalley, Identification of Medicare Recipients at Highest Risk for Clostridium difficile Infection in the US by Population Attributable Risk Analysis, PLoS One, doi:10.1371/journal.pone.0146822
Embi, Levy, Naleway, Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults -Nine States, January-September 2021, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7044e3
Fried, Tangen, Walston, Frailty in older adults: Evidence for a phenotype, J Gerontol A
Hoffmann, Krüger, Schulz, Cossmann, Rocha et al., The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic, Cell, doi:10.1016/j.cell.2021.12.032
Kamar, Abravanel, Marion, Couat, Izopet et al., Three doses of an mRNA Covid-19 vaccine in solid organ transplant recipients, N Engl J Med
Kennedy, Lin, Goodhand, Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD, Gut
Levin, Ustianowski, Wit, Launay, Avila et al., Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19, N Engl J Med, doi:10.1056/NEJMoa2116620.7
Mazzali, Duca, Use of administrative data in healthcare research, Intern Emerg Med
Mounzer E Agha, Blake, Chilleo, Wells, Haidar, Suboptimal Response to Coronavirus Disease 2019 Messenger RNA Vaccines in Patients With Hematologic Malignancies: A Need for Vigilance in the Postmasking Era, Open Forum Infectious Diseases, doi:.org/10.1093/ofid/ofab353
Paneth, Joyner, Casadevall, Finding evidence for treatment decisions in a pandemic, Trends Mol Med, doi:10.1016/j.molmed.2022.04.008.9
Patrick, Schneeweiss, Brookhart, The implications of propensity score variable selection strategies in pharmacoepidemiology: an empirical illustration, Pharmacoepidemiology and drug safety
Petersen, Byrne, Daw, Relationship between clinical conditions and use of Veterans Affairs healthcare among Medicare-enrolled veterans, Health Serv Res
Pizer, Falsification testing of instrumental variables methods for comparative effectiveness research, Health Serv Res
Planas, Saunders, Maes, Guivel-Benhassine, Planchais et al., Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature, doi:10.1038/s41586-021-04389-z
Psaty, Koepsell, Lin, Assessment and control for confounding by indication in observational studies, J Am Geriatr Soc
Rogers, Kazis, Comparing the health status of VA and non-VA ambulatory patients: The Veterans' Health and Medical Outcomes Studies, J Ambul Care Manage
Suissa, Immortal Time Bias in Pharmacoepidemiology, American Journal of Epidemiology, doi:10.1093/aje/kwm324
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2, N Engl J Med, doi:10.1056/NEJMc2201933
Vanblargan, Errico, Halfmann, Zost, Crowe et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nat Med, doi:10.1038/s41591-021-01678-y
Young-Xu, Van Aalst, Mahmud, Relative Vaccine Effectiveness of High-Dose Versus Standard-Dose Influenza Vaccines Among Veterans Health Administration Patients, J Infect Dis, doi:10.1093/infdis/jiy088.23
DOI record:
{
"DOI": "10.1101/2022.05.28.22275716",
"URL": "http://dx.doi.org/10.1101/2022.05.28.22275716",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Little is known regarding the effectiveness of tixagevimab/cilgavimab in preventing SARS-CoV-2 infection in this population, particularly after the emergence of the Omicron variant.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To determine the effectiveness of tixagevimab/cilgavimab for prevention of SARS-CoV-2 infection and severe disease among immunocompromised patients.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>Retrospective cohort study with propensity matching and difference-in-difference analyses.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>U.S. Department of Veterans Affairs (VA) healthcare system.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>Veterans age ≥18 years as of January 1, 2022, receiving VA healthcare. We compared a cohort of 1,848 patients treated with at least one dose of intramuscular tixagevimab/cilgavimab to matched controls selected from 251,756 patients who were on immunocompromised or otherwise at high risk for COVID-19. Patients were followed through April 30, 2022, or until death, whichever occurred earlier.</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes</jats:title><jats:p>Composite of SARS-CoV-2 infection, COVID-19-related hospitalization, and all-cause mortality. We used cox proportional hazards modelling to estimate the hazard ratios (HR) and 95% CI for the association between receipt of tixagevimab/cilgavimab and outcomes.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Most (69%) tixagevimab/cilgavimab recipients were ≥65 years old, 92% were identified as immunocompromised in electronic data, and 73% had ≥3 mRNA vaccine doses or two doses of Ad26.COV2. Compared to propensity-matched controls, tixagevimab/cilgavimab-treated patients had a lower incidence of the composite COVID-19 outcome (17/1733 [1.0%] vs 206/6354 [3.2%]; HR 0.31; 95%CI, 0.18-0.53), and individually SARS-CoV-2 infection (HR 0.34; 95%CI, 0.13-0.87), COVID-19 hospitalization (HR 0.13; 95%CI, 0.02-0.99), and all-cause mortality (HR 0.36; 95%CI, 0.18-0.73).</jats:p></jats:sec><jats:sec><jats:title>Limitations</jats:title><jats:p>Confounding by indication and immortal time bias.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Using national real-world data from predominantly vaccinated, immunocompromised Veterans, administration of tixagevimab/cilgavimab was associated with lower rates of SARS-CoV-2 infection, COVID-19 hospitalization, and all-cause mortality during the Omicron surge.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2022,
5,
29
]
]
},
"author": [
{
"affiliation": [],
"family": "Young-Xu",
"given": "Yinong",
"sequence": "first"
},
{
"affiliation": [],
"family": "Epstein",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marconi",
"given": "Vincent C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davey",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zwain",
"given": "Gabrielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Korves",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cunningham",
"given": "Fran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonomo",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ginde",
"given": "Adit A",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
5,
29
]
],
"date-time": "2022-05-29T19:25:11Z",
"timestamp": 1653852311000
},
"deposited": {
"date-parts": [
[
2022,
5,
31
]
],
"date-time": "2022-05-31T15:50:35Z",
"timestamp": 1654012235000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
5,
31
]
],
"date-time": "2022-05-31T16:16:13Z",
"timestamp": 1654013773655
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
5,
29
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.05.28.22275716",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
5,
29
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
5,
29
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1056/nejmsb2104756",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.1"
},
{
"DOI": "10.15585/mmwr.mm7044e3",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.2"
},
{
"DOI": "10.1056/nejmc2108861",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.3"
},
{
"DOI": "10.1136/gutjnl-2021-324789",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.4"
},
{
"DOI": "10.1093/ofid/ofab353",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.5"
},
{
"DOI": "10.1056/NEJMoa2116620",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.6"
},
{
"key": "2022053108501042000_2022.05.28.22275716v1.7",
"unstructured": "https://www.fda.gov/media/154701/download, last accessed May 9, 2022"
},
{
"DOI": "10.1016/j.molmed.2022.04.008",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.8"
},
{
"key": "2022053108501042000_2022.05.28.22275716v1.9",
"unstructured": "https://www.hsrd.research.va.gov/for_researchers/cdw.cfm"
},
{
"key": "2022053108501042000_2022.05.28.22275716v1.10",
"unstructured": "https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/3544-notes.pdf"
},
{
"key": "2022053108501042000_2022.05.28.22275716v1.11",
"unstructured": "https://www.pbm.va.gov/"
},
{
"key": "2022053108501042000_2022.05.28.22275716v1.12",
"unstructured": "https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-revisions-evusheld-dosing"
},
{
"DOI": "10.1111/j.1475-6773.2010.01107.x",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.13"
},
{
"DOI": "10.1016/0895-4356(92)90133-8",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.14"
},
{
"key": "2022053108501042000_2022.05.28.22275716v1.15",
"unstructured": "https://covid.cdc.gov/covid-data-tracker/#variant-proportions"
},
{
"DOI": "10.1111/1475-6773.12355",
"article-title": "Falsification testing of instrumental variables methods for comparative effectiveness research",
"doi-asserted-by": "crossref",
"first-page": "790",
"issue": "2",
"journal-title": "Health Serv Res",
"key": "2022053108501042000_2022.05.28.22275716v1.16",
"volume": "51",
"year": "2016"
},
{
"DOI": "10.1002/pds.2098",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.17"
},
{
"DOI": "10.1002/sim.3697",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.18"
},
{
"DOI": "10.1093/aje/kwm324",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.19"
},
{
"DOI": "10.1371/journal.pone.0146822",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.20"
},
{
"key": "2022053108501042000_2022.05.28.22275716v1.21",
"unstructured": "Angrist JD , Pischke JS . Mastering’Metrics: The Path from Cause to Effect. Princeton, NJ: Princeton University Press, 2014."
},
{
"DOI": "10.1093/infdis/jiy088",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.22"
},
{
"key": "2022053108501042000_2022.05.28.22275716v1.23",
"unstructured": "https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/"
},
{
"DOI": "10.1056/NEJMOA2114255/SUPPL_FILE/NEJMOA2114255_DISCLOSURES.PDF",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.24"
},
{
"DOI": "10.1056/NEJMc2201933",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.25"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.26"
},
{
"DOI": "10.1016/j.cell.2021.12.032",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.27"
},
{
"DOI": "10.1038/s41591-021-01678-y",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.28"
},
{
"DOI": "10.1377/hlthaff.2014.0023",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.29"
},
{
"DOI": "10.1370/afm.1279",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.30"
},
{
"DOI": "10.1111/j.1532-5415.1999.tb01603.x",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.31"
},
{
"DOI": "10.1111/j.1532-5415.2012.04054.x",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.32"
},
{
"DOI": "10.1159/000048110",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.33"
},
{
"DOI": "10.1097/00004479-200407000-00009",
"doi-asserted-by": "publisher",
"key": "2022053108501042000_2022.05.28.22275716v1.34"
},
{
"DOI": "10.1007/s11739-015-1213-9",
"article-title": "Use of administrative data in healthcare research",
"doi-asserted-by": "crossref",
"first-page": "517",
"issue": "4",
"journal-title": "Intern Emerg Med",
"key": "2022053108501042000_2022.05.28.22275716v1.35",
"volume": "10",
"year": "2015"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.05.28.22275716"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Tixagevimab/Cilgavimab for Prevention of COVID-19 during the Omicron Surge: Retrospective Analysis of National VA Electronic Data",
"type": "posted-content"
}