A Phase 2 Trial of the Effect of Antiandrogen Therapy on COVID-19 Outcome: No Evidence of Benefit, Supported by Epidemiology and In Vitro Data
et al., European Urology, doi:10.1016/j.eururo.2021.12.013, NCT04475601, Dec 2021
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
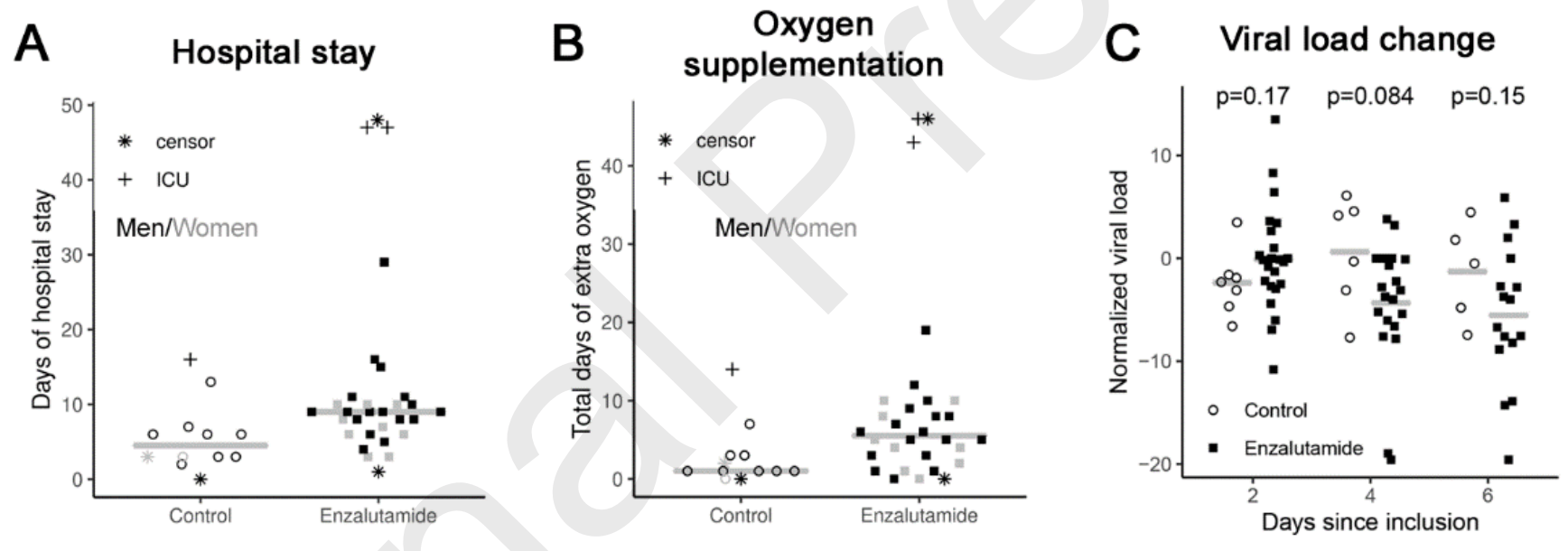
Very small late stage RCT with 10 control patients and 29 enzalutamide patients, showing mixed results. Discharge and hospitalization time favored the control group, while viral load reduction was better with treatment on days 4&6 (day 4 ΔCt -5.6 p = 0.084), and the only death occurred in the control group. 27% of enzalutamide patients had diabetes compared to 0% of the control group. This paper also includes a retrospective study which is listed separately, and an in vitro HBEC study showing no significant differences (p = 0.084). The supplementary data is not currently available.
|
risk of death, 79.6% lower, RR 0.20, p = 0.26, treatment 0 of 29 (0.0%), control 1 of 10 (10.0%), NNT 10.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 31.0% lower, RR 0.69, p = 1.00, treatment 2 of 29 (6.9%), control 1 of 10 (10.0%), NNT 32.
|
|
risk of no hospital discharge, 132.6% higher, RR 2.33, p = 0.03, treatment 29, control 10, inverted to make RR<1 favor treatment, primary outcome.
|
|
hospitalization time, 50.0% higher, relative time 1.50, p = 0.01, treatment 29, control 10.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Welén et al., 14 Dec 2021, Randomized Controlled Trial, Sweden, peer-reviewed, 27 authors, study period 15 July, 2020 - 29 May, 2021, average treatment delay 9.5 days, trial NCT04475601 (history).
Contact: andreas.josefsson@umu.se.
A Phase 2 Trial of the Effect of Antiandrogen Therapy on COVID-19 Outcome: No Evidence of Benefit, Supported by Epidemiology and In Vitro Data
European Urology, doi:10.1016/j.eururo.2021.12.013
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Andreas Josefsson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
Cadegiani, Mccoy, Wambier, Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial, Cureus
Chan, Zhang, Yuan, Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility, Clin Infect Dis
Charlson, Pompei, Ales, Mackenzie, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J Chronic Dis
Charlson, Szatrowski, Peterson, Gold, Validation of a combined comorbidity index, J Clin Epidemiol
Dalpiaz, Lamas, Caliman, Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats, PLoS One
Ghazizadeh, Majd, Richter, Androgen regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men, doi:10.1101/2020.05.12.091082
Gibbons, De Vries, Krauwinkel, Pharmacokinetic drug interaction studies with enzalutamide, Clin Pharmacokinet
Horby, Lim, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
Karimi, Nowroozi, Alilou, Amini, Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer: a systematic review and meta-analysis, Urol J, doi:10.22037/uj.v18i.6691
Koskinen, Carpen, Honkanen, Androgen deprivation and SARS-CoV-2 in men with prostate cancer, Ann Oncol
Leach, Mohr, Giotis, The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells, Nat Commun
Li, Han, Dai, Distinct mechanisms for TMPRSS2 expression explain organspecific inhibition of SARS-CoV-2 infection by enzalutamide, Nat Commun
Ludvigsson, Appelros, Askling, Adaptation of the Charlson comorbidity index for register-based research in Sweden, Clin Epidemiol
Lyon, Li, Cullen, 5-Reductase inhibitors are associated with reduced risk of SARS-CoV-2 infection: a matched-pair, registry-based analysis, J Urol, doi:10.1097/ju.0000000000002180
Mccoy, Goren, Cadegiani, Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebocontrolled trial, Front Med
Mikkonen, Pihlajamaa, Sahu, Zhang, Janne, Androgen receptor and androgen-dependent gene expression in lung, Mol Cell Endocrinol
Montopoli, Zumerle, Vettor, Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532), Ann Oncol
Patel, Zhong, Liaw, Does androgen deprivation therapy protect against severe complications from COVID-19?, Ann Oncol
Peckham, De Gruijter, Raine, Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission, Nat Commun
Qiao, Wang, Mannan, Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2, Proc Natl Acad Sci U S A
Schwartzberg, Elias, A phase I/Ib Study of enzalutamide alone and in combination with endocrine therapies in women with advanced breast cancer, Clin Cancer Res
Wambier, Vano-Galvan, Mccoy, Androgenetic alopecia present in the majority of hospitalized COVID-19 patients: the "Gabrin sign, J Am Acad Dermatol
Welén, Rosendal, Gisslén, Lenman, Freyhult et al., Josefsson This study shows no effect of enzalutamide on hospitalized COVID-19 patients or a decrease in the risk of COVID-19 severity from any androgen-inhibiting drug in the population. Collectively, there is evidence of no beneficial role of androgen inhibition in COVID-19
Williamson, Walker, Bhaskaran, Factors associated with COVID-19-related death using OpenSAFELY, Nature
Zhang, Tan, Ling, Viral and host factors related to the clinical outcome of COVID-19, Nature
DOI record:
{
"DOI": "10.1016/j.eururo.2021.12.013",
"ISSN": [
"0302-2838"
],
"URL": "http://dx.doi.org/10.1016/j.eururo.2021.12.013",
"alternative-id": [
"S0302283821022247"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A Phase 2 Trial of the Effect of Antiandrogen Therapy on COVID-19 Outcome: No Evidence of Benefit, Supported by Epidemiology and In Vitro Data"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "European Urology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eururo.2021.12.013"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier B.V. on behalf of European Association of Urology."
}
],
"author": [
{
"affiliation": [],
"family": "Welén",
"given": "Karin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rosendal",
"given": "Ebba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gisslén",
"given": "Magnus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lenman",
"given": "Annasara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Freyhult",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fonseca-Rodríguez",
"given": "Osvaldo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bremell",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stranne",
"given": "Johan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balkhed",
"given": "Åse Östholm",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Niward",
"given": "Katarina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Repo",
"given": "Johanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinsson",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Henningsson",
"given": "Anna J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Styrke",
"given": "Johan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Angelin",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lindquist",
"given": "Elisabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allard",
"given": "Annika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Becker",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rudolfsson",
"given": "Stina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buckland",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carlsson",
"given": "Camilla Thellenberg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bjartell",
"given": "Anders",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nilsson",
"given": "Anna C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahlm",
"given": "Clas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Connolly",
"given": "Anne-Marie Fors",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Överby",
"given": "Anna K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Josefsson",
"given": "Andreas",
"sequence": "additional"
}
],
"container-title": "European Urology",
"container-title-short": "European Urology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T22:42:54Z",
"timestamp": 1639608174000
},
"deposited": {
"date-parts": [
[
2022,
2,
26
]
],
"date-time": "2022-02-26T03:38:20Z",
"timestamp": 1645846700000
},
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T10:40:13Z",
"timestamp": 1711622413583
},
"is-referenced-by-count": 38,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
3
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2022,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
1
]
],
"date-time": "2022-03-01T00:00:00Z",
"timestamp": 1646092800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T00:00:00Z",
"timestamp": 1639526400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0302283821022247?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0302283821022247?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "285-293",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
3
]
]
},
"published-print": {
"date-parts": [
[
2022,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41467-020-19741-6",
"article-title": "Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission",
"author": "Peckham",
"doi-asserted-by": "crossref",
"first-page": "6317",
"journal-title": "Nat Commun",
"key": "10.1016/j.eururo.2021.12.013_b0005",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"article-title": "Factors associated with COVID-19-related death using OpenSAFELY",
"author": "Williamson",
"doi-asserted-by": "crossref",
"first-page": "430",
"journal-title": "Nature",
"key": "10.1016/j.eururo.2021.12.013_b0010",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1101/2020.05.12.091082",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eururo.2021.12.013_b0015",
"unstructured": "Ghazizadeh Z, Majd H, Richter M, et al. Androgen regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men. BioRxiv preprint. https://doi.org/10.1101/2020.05.12.091082"
},
{
"DOI": "10.1038/s41467-021-24342-y",
"article-title": "The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells",
"author": "Leach",
"doi-asserted-by": "crossref",
"first-page": "4068",
"journal-title": "Nat Commun",
"key": "10.1016/j.eururo.2021.12.013_b0020",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.mce.2009.12.022",
"article-title": "Androgen receptor and androgen-dependent gene expression in lung",
"author": "Mikkonen",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Mol Cell Endocrinol",
"key": "10.1016/j.eururo.2021.12.013_b0025",
"volume": "317",
"year": "2010"
},
{
"article-title": "Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats",
"author": "Dalpiaz",
"journal-title": "PLoS One",
"key": "10.1016/j.eururo.2021.12.013_b0030",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.1016/j.annonc.2020.04.479",
"article-title": "Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532)",
"author": "Montopoli",
"doi-asserted-by": "crossref",
"first-page": "1040",
"journal-title": "Ann Oncol",
"key": "10.1016/j.eururo.2021.12.013_b0035",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.06.023",
"article-title": "Does androgen deprivation therapy protect against severe complications from COVID-19?",
"author": "Patel",
"doi-asserted-by": "crossref",
"first-page": "1419",
"journal-title": "Ann Oncol",
"key": "10.1016/j.eururo.2021.12.013_b0040",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.jaad.2020.05.079",
"article-title": "Androgenetic alopecia present in the majority of hospitalized COVID-19 patients: the “Gabrin sign”",
"author": "Wambier",
"doi-asserted-by": "crossref",
"first-page": "680",
"journal-title": "J Am Acad Dermatol",
"key": "10.1016/j.eururo.2021.12.013_b0045",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.06.015",
"article-title": "Androgen deprivation and SARS-CoV-2 in men with prostate cancer",
"author": "Koskinen",
"doi-asserted-by": "crossref",
"first-page": "1417",
"journal-title": "Ann Oncol",
"key": "10.1016/j.eururo.2021.12.013_b0050",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/0895-4356(94)90129-5",
"article-title": "Validation of a combined comorbidity index",
"author": "Charlson",
"doi-asserted-by": "crossref",
"first-page": "1245",
"journal-title": "J Clin Epidemiol",
"key": "10.1016/j.eururo.2021.12.013_b0055",
"volume": "47",
"year": "1994"
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"article-title": "A new method of classifying prognostic comorbidity in longitudinal studies: development and validation",
"author": "Charlson",
"doi-asserted-by": "crossref",
"first-page": "373",
"journal-title": "J Chronic Dis",
"key": "10.1016/j.eururo.2021.12.013_b0060",
"volume": "40",
"year": "1987"
},
{
"DOI": "10.2147/CLEP.S282475",
"article-title": "Adaptation of the Charlson comorbidity index for register-based research in Sweden",
"author": "Ludvigsson",
"doi-asserted-by": "crossref",
"first-page": "21",
"journal-title": "Clin Epidemiol",
"key": "10.1016/j.eururo.2021.12.013_b0065",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1007/s40262-015-0283-1",
"article-title": "Pharmacokinetic drug interaction studies with enzalutamide",
"author": "Gibbons",
"doi-asserted-by": "crossref",
"first-page": "1057",
"journal-title": "Clin Pharmacokinet",
"key": "10.1016/j.eururo.2021.12.013_b0070",
"volume": "54",
"year": "2015"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eururo.2021.12.013_b0075",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1097/JU.0000000000002180",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eururo.2021.12.013_b0080",
"unstructured": "Lyon M, Li J, Cullen J, et al. 5α-Reductase inhibitors are associated with reduced risk of SARS-CoV-2 infection: a matched-pair, registry-based analysis. J Urol. In press. https://doi.org/10.1097/ju.0000000000002180."
},
{
"article-title": "Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial",
"author": "Cadegiani",
"journal-title": "Cureus",
"key": "10.1016/j.eururo.2021.12.013_b0085",
"volume": "13",
"year": "2021"
},
{
"article-title": "Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2",
"author": "Qiao",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "10.1016/j.eururo.2021.12.013_b0090",
"volume": "118",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-21171-x",
"article-title": "Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "866",
"journal-title": "Nat Commun",
"key": "10.1016/j.eururo.2021.12.013_b0095",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2021.668698",
"article-title": "Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial",
"author": "McCoy",
"doi-asserted-by": "crossref",
"journal-title": "Front Med",
"key": "10.1016/j.eururo.2021.12.013_b0100",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2355-0",
"article-title": "Viral and host factors related to the clinical outcome of COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "437",
"journal-title": "Nature",
"key": "10.1016/j.eururo.2021.12.013_b0105",
"volume": "583",
"year": "2020"
},
{
"key": "10.1016/j.eururo.2021.12.013_b0110",
"unstructured": "Karimi A, Nowroozi A, Alilou S, Amini E. Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer: a systematic review and meta-analysis. Urol J. In press. https://doi.org/10.22037/uj.v18i.6691."
},
{
"DOI": "10.1158/1078-0432.CCR-16-2339",
"article-title": "A phase I/Ib Study of enzalutamide alone and in combination with endocrine therapies in women with advanced breast cancer",
"author": "Schwartzberg",
"doi-asserted-by": "crossref",
"first-page": "4046",
"journal-title": "Clin Cancer Res",
"key": "10.1016/j.eururo.2021.12.013_b0115",
"volume": "23",
"year": "2017"
},
{
"DOI": "10.1093/cid/ciaa644",
"article-title": "Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "2428",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.eururo.2021.12.013_b0120",
"volume": "71",
"year": "2020"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0302283821022247"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Urology"
],
"subtitle": [],
"title": "A Phase 2 Trial of the Effect of Antiandrogen Therapy on COVID-19 Outcome: No Evidence of Benefit, Supported by Epidemiology and In Vitro Data",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "81"
}
