Mitigating the risks of post-acute sequelae of SARS-CoV-2 infection (PASC) with intranasal chlorpheniramine: perspectives from the ACCROS studies
et al., BMC Infectious Diseases, doi:10.1186/s12879-024-10211-8, ACCROS, Nov 2024
11th treatment shown to reduce risk in
December 2020, now with p = 0.000052 from 17 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
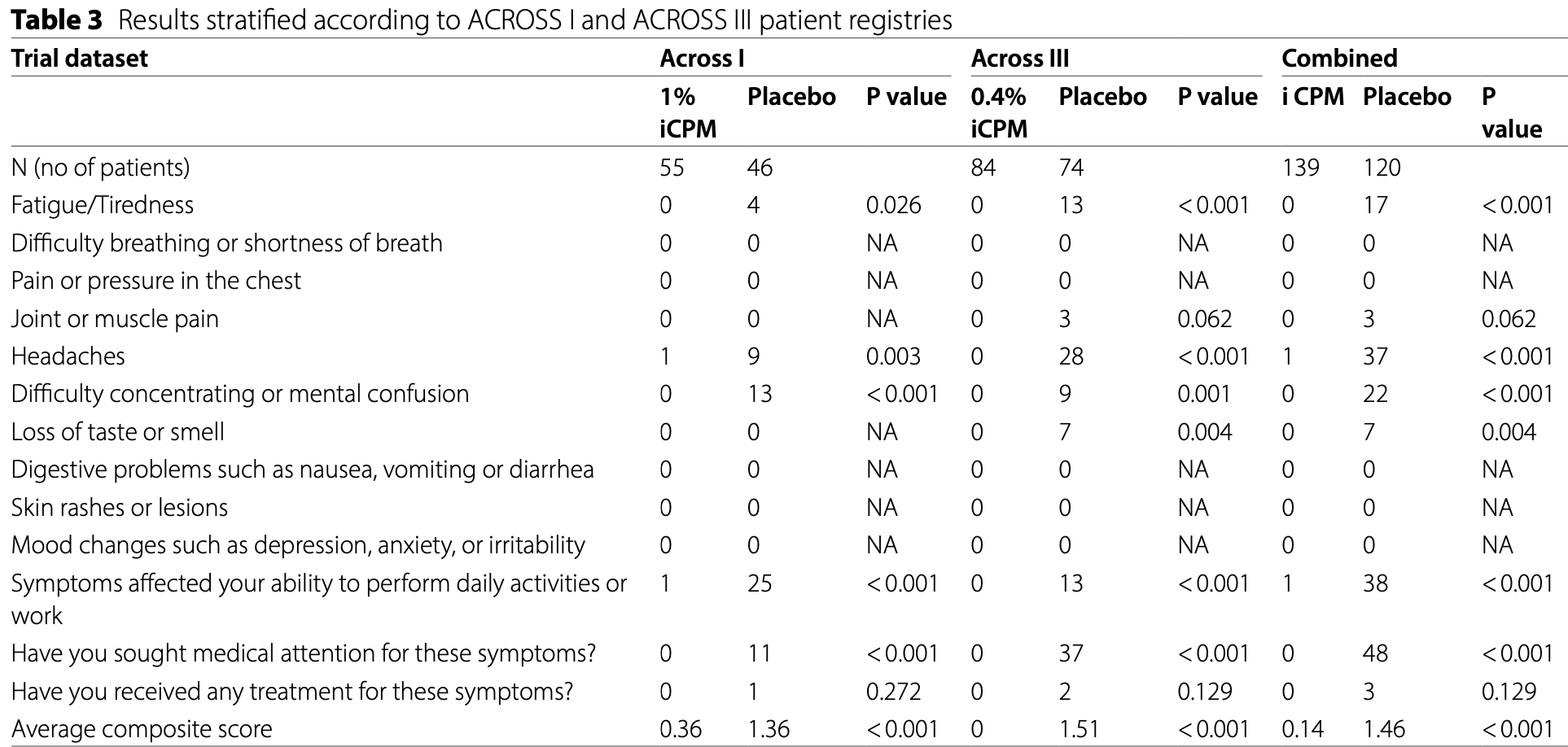
Prospective study of 259 COVID-19 outpatients from the ACROSS-I and ACROSS-III RCTs showing significantly lower long COVID with intranasal chlorpheniramine (iCPM) compared to placebo. 72% of placebo patients experienced at least one PASC symptom compared to 0.7% with iCPM (p<0.001). Treated patients had significantly lower rates of fatigue, headaches, difficulty concentrating, and impaired daily functioning.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
Study covers antihistamine H1RAs and chlorpheniramine.
|
relative long COVID score, 90.4% better, RR 0.10, p < 0.001, treatment 139, control 120, relative average composite long COVID score.
|
|
risk of long COVID, 97.7% lower, RR 0.02, p < 0.001, treatment 1 of 139 (0.7%), control 38 of 120 (31.7%), NNT 3.2, affecting daily function.
|
|
risk of long COVID, 99.0% lower, RR 0.010, p < 0.001, treatment 0 of 139 (0.0%), control 48 of 120 (40.0%), NNT 2.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), medical attention.
|
|
risk of long COVID, 97.3% lower, RR 0.03, p < 0.001, treatment 0 of 139 (0.0%), control 17 of 120 (14.2%), NNT 7.1, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), fatigue.
|
|
risk of long COVID, 97.7% lower, RR 0.02, p < 0.001, treatment 1 of 139 (0.7%), control 37 of 120 (30.8%), NNT 3.3, headaches.
|
|
risk of long COVID, 86.6% lower, RR 0.13, p = 0.10, treatment 0 of 139 (0.0%), control 3 of 120 (2.5%), NNT 40, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), joint/muscle pain.
|
|
risk of long COVID, 97.9% lower, RR 0.02, p < 0.001, treatment 0 of 139 (0.0%), control 22 of 120 (18.3%), NNT 5.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), difficulty concentrating.
|
|
risk of long COVID, 93.8% lower, RR 0.06, p = 0.004, treatment 0 of 139 (0.0%), control 7 of 120 (5.8%), NNT 17, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), loss of taste/smell.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Valerio-Pascua et al., Mitigating the risks of post-acute sequelae of SARS-CoV-2 infection (PASC) with intranasal chlorpheniramine: perspectives from the ACCROS studies, BMC Infectious Diseases, doi:10.1186/s12879-024-10211-8.
2.
Sanchez-Gonzalez et al., Intranasal Chlorpheniramine Maleate for the treatment of COVID-19: Translational and Clinical Evidence, Medical Research Archives, doi:10.18103/mra.v10i3.2752.
Valerio-Pascua et al., 26 Nov 2024, prospective, Honduras, peer-reviewed, mean age 46.0, 14 authors, study period December 2021 - March 2022, ACCROS trial.
Contact: rahaghf@ccf.org.
Mitigating the risks of post-acute sequelae of SARS-CoV-2 infection (PASC) with intranasal chlorpheniramine: perspectives from the ACCROS studies
BMC Infectious Diseases, doi:10.1186/s12879-024-10211-8
Background The World Health Organization (WHO) declared the end of the COVID-19 (SARS-CoV-2) global public health emergency on May 5, 2023, but its long-term consequences have still been haunting the global population. Post-acute sequelae of COVID-19 (PASC) and long-term COVID-19 are serious concerns and present with various symptoms. Intranasal chlorpheniramine (iCPM) has been shown to decrease the viral burden of SARS-COV-2. iCPM uses decreased COVID-19 disease progression and severity in Accelerating COVID-19 Clinical Recovery in an Outpatient Setting (ACROSS)-I & III randomized control trials (RCT). Methods This prospective survey study included 259 participants in ACROSS I and III RCTs. We compared the effect of iCPM versus placebo on the reduction of PASC symptoms. A PASC questionnaire containing 17 questions regarding the most common PASC symptoms was used in this study. T-test and Pearson chi-square statistics were performed according to continuous and categorical data using STATA 17.0 Basic Edition software.
Findings The iCPM cohort had a lower proportion of patients with fatigue or tiredness vs. placebo (0 Vs 17, 21, p < 0.001). iCPM cohort had a lower proportion of patients with difficulty concentrating or mental confusion (0 vs. 22, 27, p < 0.001). iCPM cohort had also a lower number of patients with difficulty in the ability to perform daily activities or work vs. placebo (1 Vs 38, 48, p < 0.001). A smaller number of patients in the iCPM cohort sought medical attention for PACS symptoms compared to placebo (0 vs. 48, 68, p < 0.001). Interpretation The use of intranasal chlorpheniramine shows promise in preventing COVID-19 progression to the often-debilitating post-COVID-19 syndrome PASC. The association between iCPM use and a lower prevalence of PASC symptoms is strong. Further studies are needed to establish the role of ICPM in preventing PASC.
Declarations Ethics approval and consent to participate This study was approved by the Institutional Review Board (IRB) of the Ethics Committee of Investigation of Infectious and Zoonotic Disease at the Universidad Nacional Autónoma de Honduras. Written informed consent was obtained from all participants prior to their involvement in the study. In cases where verbal consent was required (e.g., remote participation), this consent was documented and witnessed by study personnel. No waivers of consent were requested or provided by the ethics committee, and no minors were included in the study.
Consent for publication
Competing interests The authors declare no competing interests.
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Alvarez-Moreno, Pineda, Bareño, Espitia, Rengifo, Long COVID-19 in Latin America: low prevalence, high resilience or low surveillance and difficulties accessing health care?, Travel Med Infect Dis, doi:10.1016/j.tmaid.2022.102492
Amenta, Spallone, Rodriguez-Barradas, Sahly, Atmar et al., Postacute COVID-19: an overview and approach to classification, Open Forum Infect Dis, doi:10.1093/ofid/ofaa509
Ballering, Van Zon, Hartman, Rosmalen, Corona Research et al., Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study, Lancet, doi:10.1016/S0140-6736(22)01214-4
Black, Molecular modeling and preliminary Clinical Data suggesting antiviral activity for chlorpheniramine (chlorphenamine) against COVID-19, Cureus, doi:10.7759/cureus.20980
Bowe, Xie, Al-Aly, Postacute sequelae of COVID-19 at 2 years, Nat Med, doi:10.1038/s41591-023-02521-2
Carfì, Bernabei, Landi, Against, COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19, JAMA
Chen, Haupert, Zimmermann, Shi, Fritsche et al., Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review, J Infect Dis, doi:10.1093/infdis/jiac136
Davis, Mccorkell, Vogel, Topol, Long, major findings, mechanisms and recommendations, Nat Rev Microbiol
Ejmoa, None, doi:10.1056/NEJMoa2001017
Elshaier, Mostafa, Valerio-Pascua, Tesch, Costin et al., Chlorpheniramine maleate displays multiple modes of antiviral action against SARS-CoV-2: a mechanistic study, h t t p s : / / d o i . o r g, doi:10.1101/2023.08.28.554806
Ennis, Tiligada, Histamine receptors and COVID-19, Inflamm Res, doi:10.1007/s00011-020-01422-1
Fang, Druce, Baraniuk, Anticholinergic properties of brompheniramine, chlorpheniramine, and atropine in human nasal mucosa in vitro, Am J Rhinol, doi:10.2500/105065898781390271
Greenhalgh, Knight, Court, Buxton, Husain, Management of postacute COVID-19 in primary care, BMJ
Havervall, Rosell, Phillipson, Mangsbo, Nilsson et al., Symptoms and functional impairment assessed 8 months after mild COVID-19 among healthcare workers, JAMA, doi:10.1001/jama.2021.5612
Ioannou, Berry, Rajeevan, Li, Mutalik et al., Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among U.S. veterans: a target trial emulation, Ann Intern Med, doi:10.7326/M23-1394
Kim, Bae, Chang, Kim, Long COVID prevalence and impact on quality of life 2 years after acute COVID-19, Sci Rep, doi:10.1038/s41598-023-36995-4
Kumar, Cheng, A hypothesis: bitter taste receptors as a therapeutic target for the clinical symptoms of SARS-CoV-2, Pharmazie, doi:10.1691/pharmazie.2021.2
Raman, Bluemke, Lüscher, Neubauer, Long COVID: postacute sequelae of COVID-19 with a cardiovascular focus, Eur Heart J, doi:10.1093/eurheartj/ehac031
Rubin, As their numbers grow, COVID-19 long haulers stump experts, JAMA
Subramanian, Nirantharakumar, Hughes, Myles, Williams et al., Symptoms and risk factors for long COVID in non-hospitalized adults, Nat Med, doi:10.1038/s41591-022-01909-w
Tan, Anderson, Rathore, Neill, Mantri et al., Signatures of mast cell activation are associated with severe COVID-19, bioRxiv, doi:10.1101/2021.05.31.21255594
Thaweethai, Jolley, Karlson, Levitan, Levy et al., Development of a definition of postacute sequelae of SARS-CoV-2 infection, JAMA
Thompson, Williams, Walker, Mitchell, Niedzwiedz et al., Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records, Nat Commun, doi:10.1038/s41467-022-30836-0
Torres, Go, Chohan, Camacho, Sanchez-Gonzalez et al., Chlorpheniramine maleate nasal spray in COVID-19Patients: Case Series, J Clin Exp Pharmacol, doi:10.35248/2161-1459.21.10.275
Valerio-Pascua, Mejia, Tesch, Godoy, Fuentes et al., Chlorpheniramine intranasal spray to accelerate COVID-19 clinical recovery in an outpatient setting: The ACCROS trials
Wang, Zhao, Shi, Jia, Paxlovid reduces the risk of long COVID in patients six months after hospital discharge, J Med Virol, doi:10.1002/jmv.29014
Westover, Ferrer, Vazquez, Bethencourt-Mirael, Go, In vitro virucidal effect of intranasally delivered chlorpheniramine maleate compound against severe acute respiratory syndrome coronavirus 2, Cureus, doi:10.7759/cureus.10501
Wolff, Drewitz, Ulrich, Allergic diseases as risk factors for long-COVID symptoms: systematic review of prospective cohort studies, Clin Exp Allergy, doi:10.1111/cea.14391
Yelin, Moschopoulos, Margalit, Gkrania-Klotsas, Landi et al., ESCMID rapid guidelines for assessment and management of long COVID, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.02.018
Zhu, Zhang, Li, Yang, Song, A novel coronavirus from patients with pneumonia in China, N Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.1186/s12879-024-10211-8",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-024-10211-8",
"alternative-id": [
"10211"
],
"article-number": "1348",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "4 January 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 November 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "26 November 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study was approved by the Institutional Review Board (IRB) of the Ethics Committee of Investigation of Infectious and Zoonotic Disease at the Universidad Nacional Autónoma de Honduras. Written informed consent was obtained from all participants prior to their involvement in the study. In cases where verbal consent was required (e.g., remote participation), this consent was documented and witnessed by study personnel. No waivers of consent were requested or provided by the ethics committee, and no minors were included in the study."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "N/A-Not Applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Valerio-Pascua",
"given": "Fernando",
"sequence": "first"
},
{
"affiliation": [],
"family": "Baires",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sekhon",
"given": "Anupamjeet Kaur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tesch",
"given": "Mari L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pineda",
"given": "Estela Jackeline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rizvi",
"given": "Syed A. A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Jarmanjeet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cortes-Bandy",
"given": "David Abraham",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Madril",
"given": "Amy C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Radwanski",
"given": "Jana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lewis",
"given": "Anita S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sierra-Hoffman",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stevens",
"given": "Mark L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahaghi",
"given": "Franck F.",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
11,
26
]
],
"date-time": "2024-11-26T16:13:18Z",
"timestamp": 1732637598000
},
"deposited": {
"date-parts": [
[
2024,
11,
26
]
],
"date-time": "2024-11-26T16:19:07Z",
"timestamp": 1732637947000
},
"funder": [
{
"award": [
"N/A"
],
"name": "Dr. Ferrer Biopharma"
}
],
"indexed": {
"date-parts": [
[
2024,
11,
27
]
],
"date-time": "2024-11-27T05:33:15Z",
"timestamp": 1732685595961,
"version": "3.28.2"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
11,
26
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
26
]
],
"date-time": "2024-11-26T00:00:00Z",
"timestamp": 1732579200000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
26
]
],
"date-time": "2024-11-26T00:00:00Z",
"timestamp": 1732579200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-10211-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-024-10211-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-10211-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
11,
26
]
]
},
"published-online": {
"date-parts": [
[
2024,
11,
26
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1001/jama.2020.17709",
"doi-asserted-by": "crossref",
"key": "10211_CR1",
"unstructured": "Rubin R. As their numbers grow, COVID-19 long haulers stump experts. JAMA. 2020;324(14):1381. https://jamanetwork.com/journals/jama/fullarticle/2771111"
},
{
"DOI": "10.1001/jama.2020.12603",
"doi-asserted-by": "crossref",
"key": "10211_CR2",
"unstructured": "Carfì A, Bernabei R, Landi F, Gemelli Against, COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603. https://jamanetwork.com/journals/jama/fullarticle/2768351"
},
{
"key": "10211_CR3",
"unstructured": "Clinical Services and Systems, Diseases C, Technical Advisory Group on SARS-CoV-2 Virus Evolution. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. World Health Organization; 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1. Accessed 27 Sep 2023."
},
{
"DOI": "10.1093/eurheartj/ehac031",
"author": "B Raman",
"doi-asserted-by": "publisher",
"first-page": "1157",
"issue": "11",
"journal-title": "Eur Heart J",
"key": "10211_CR4",
"unstructured": "Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43(11):1157–72. https://doi.org/10.1093/eurheartj/ehac031.",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.1038/s41579-022-00846-2",
"doi-asserted-by": "crossref",
"key": "10211_CR5",
"unstructured": "Davis HE, McCorkell L, Vogel JM, Topol EJ, Long COVID. major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133 – 46. https://www.nature.com/articles/s41579-022-00846-2. Accessed 27 Sep 2023."
},
{
"DOI": "10.1016/S0140-6736(22)01214-4",
"author": "AV Ballering",
"doi-asserted-by": "publisher",
"first-page": "452",
"issue": "10350",
"journal-title": "Lancet",
"key": "10211_CR6",
"unstructured": "Ballering AV, van Zon SKR, Olde Hartman TC, Rosmalen JGM, Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400(10350):452–61. https://doi.org/10.1016/S0140-6736(22)01214-4.",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2001017",
"author": "N Zhu",
"doi-asserted-by": "publisher",
"first-page": "727",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "10211_CR7",
"unstructured": "Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. https://doi.org/10.1056/NEJMoa2001017.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.17265/1548-6648/2020.01.004",
"doi-asserted-by": "crossref",
"key": "10211_CR8",
"unstructured": "European Centre for Disease Prevention and Control. Cluster of pneumonia cases caused by a novel coronavirus, Wuhan, China; – 17 January 2020. Volume ECDC. Stockholm; 2020."
},
{
"DOI": "10.1093/infdis/jiac136",
"author": "C Chen",
"doi-asserted-by": "publisher",
"first-page": "1593",
"issue": "9",
"journal-title": "J Infect Dis",
"key": "10211_CR9",
"unstructured": "Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226(9):1593–607. https://doi.org/10.1093/infdis/jiac136.",
"volume": "226",
"year": "2022"
},
{
"DOI": "10.7759/cureus.10501",
"doi-asserted-by": "publisher",
"key": "10211_CR10",
"unstructured": "Westover JB, Ferrer G, Vazquez H, Bethencourt-Mirael A, Go CC. In vitro virucidal effect of intranasally delivered chlorpheniramine maleate compound against severe acute respiratory syndrome coronavirus 2. Cureus. 2020;12(9). https://doi.org/10.7759/cureus.10501."
},
{
"DOI": "10.21203/rs.3.rs-2167465/v1",
"doi-asserted-by": "crossref",
"key": "10211_CR11",
"unstructured": "Valerio-Pascua F, Mejia EJP, Tesch ML, Godoy J, Fuentes CL, Erazo GB et al. Chlorpheniramine intranasal spray to accelerate COVID-19 clinical recovery in an outpatient setting: The ACCROS trials. Research Square. 2022. https://www.researchsquare.com/article/rs-2167465/v1. Accessed 29 Sep 2023."
},
{
"DOI": "10.1016/j.cmi.2022.02.018",
"author": "D Yelin",
"doi-asserted-by": "publisher",
"first-page": "955",
"issue": "7",
"journal-title": "Clin Microbiol Infect",
"key": "10211_CR12",
"unstructured": "Yelin D, Moschopoulos CD, Margalit I, Gkrania-Klotsas E, Landi F, Stahl JP, et al. ESCMID rapid guidelines for assessment and management of long COVID. Clin Microbiol Infect. 2022;28(7):955–72. https://doi.org/10.1016/j.cmi.2022.02.018.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1136/bmj.m3026",
"doi-asserted-by": "crossref",
"key": "10211_CR13",
"unstructured": "Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute COVID-19 in primary care. BMJ. 2020;370 https://www.bmj.com/content/370/bmj.m3026. Accessed 29 Sep 2023."
},
{
"DOI": "10.1093/ofid/ofaa509",
"doi-asserted-by": "publisher",
"key": "10211_CR14",
"unstructured": "Amenta EM, Spallone A, Rodriguez-Barradas MC, El Sahly HM, Atmar RL, Kulkarni PA. Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis. 2020;7(12). https://doi.org/10.1093/ofid/ofaa509."
},
{
"key": "10211_CR15",
"unstructured": "Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934. https://jamanetwork.com/journals/jama/fullarticle/2805540. Accessed 31 Oct 2023."
},
{
"DOI": "10.1016/j.tmaid.2022.102492",
"author": "CA Alvarez-Moreno",
"doi-asserted-by": "publisher",
"first-page": "102492",
"journal-title": "Travel Med Infect Dis",
"key": "10211_CR16",
"unstructured": "Alvarez-Moreno CA, Pineda J, Bareño A, Espitia R, Rengifo P. Long COVID-19 in Latin America: low prevalence, high resilience or low surveillance and difficulties accessing health care? Travel Med Infect Dis. 2023;51:102492. https://doi.org/10.1016/j.tmaid.2022.102492.",
"volume": "51",
"year": "2023"
},
{
"DOI": "10.1038/s41467-022-30836-0",
"author": "EJ Thompson",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10211_CR17",
"unstructured": "Thompson EJ, Williams DM, Walker AJ, Mitchell RE, Niedzwiedz CL, Yang TC, et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat Commun. 2022;13(1):1–11. https://doi.org/10.1038/s41467-022-30836-0.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01909-w",
"author": "A Subramanian",
"doi-asserted-by": "publisher",
"first-page": "1706",
"issue": "8",
"journal-title": "Nat Med",
"key": "10211_CR18",
"unstructured": "Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706–14. https://doi.org/10.1038/s41591-022-01909-w.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1111/cea.14391",
"author": "D Wolff",
"doi-asserted-by": "publisher",
"first-page": "961",
"issue": "9",
"journal-title": "Clin Exp Allergy",
"key": "10211_CR19",
"unstructured": "Wolff D, Drewitz KP, Ulrich A, et al. Allergic diseases as risk factors for long-COVID symptoms: systematic review of prospective cohort studies. Clin Exp Allergy. 2023;53(9):961–75. https://doi.org/10.1111/cea.14391.",
"volume": "53",
"year": "2023"
},
{
"DOI": "10.1001/jama.2021.5612",
"author": "S Havervall",
"doi-asserted-by": "publisher",
"first-page": "2015",
"issue": "19",
"journal-title": "JAMA",
"key": "10211_CR20",
"unstructured": "Havervall S, Rosell A, Phillipson M, Mangsbo SM, Nilsson P, Hober S, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among healthcare workers. JAMA. 2021;325(19):2015. https://doi.org/10.1001/jama.2021.5612.",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1038/s41598-023-36995-4",
"author": "Y Kim",
"doi-asserted-by": "publisher",
"first-page": "36995",
"issue": "1",
"journal-title": "Sci Rep",
"key": "10211_CR21",
"unstructured": "Kim Y, Bae S, Chang H-H, Kim S-W. Long COVID prevalence and impact on quality of life 2 years after acute COVID-19. Sci Rep. 2023;13(1):36995–4. https://doi.org/10.1038/s41598-023-36995-4.",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1038/s41591-023-02521-2",
"author": "B Bowe",
"doi-asserted-by": "publisher",
"first-page": "2347",
"issue": "9",
"journal-title": "Nat Med",
"key": "10211_CR22",
"unstructured": "Bowe B, Xie Y, Al-Aly Z. Postacute sequelae of COVID-19 at 2 years. Nat Med. 2023;29(9):2347–57. https://doi.org/10.1038/s41591-023-02521-2.",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1101/2021.05.31.21255594",
"doi-asserted-by": "publisher",
"key": "10211_CR23",
"unstructured": "Tan J, Anderson DE, Rathore APS, O’Neill A, Mantri CK, Saron WAA et al. Signatures of mast cell activation are associated with severe COVID-19. bioRxiv. 2021. https://doi.org/10.1101/2021.05.31.21255594"
},
{
"DOI": "10.1101/2023.08.28.554806",
"author": "YAMM Elshaier",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv",
"key": "10211_CR24",
"unstructured": "Elshaier YAMM, Mostafa A, Valerio-Pascua F, Tesch ML, Costin JM, Rahaghi FF. Chlorpheniramine maleate displays multiple modes of antiviral action against SARS-CoV-2: a mechanistic study. bioRxiv. 2023. https://doi.org/10.1101/2023.08.28.554806.",
"year": "2023"
},
{
"DOI": "10.7759/cureus.20980",
"author": "SD Black",
"doi-asserted-by": "publisher",
"first-page": "e20980",
"issue": "1",
"journal-title": "Cureus",
"key": "10211_CR25",
"unstructured": "Black SD. Molecular modeling and preliminary Clinical Data suggesting antiviral activity for chlorpheniramine (chlorphenamine) against COVID-19. Cureus. 2022;14(1):e20980. https://doi.org/10.7759/cureus.20980. PMID: 35154957; PMCID: PMC8820487.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1691/pharmazie.2021.2",
"author": "SA Kumar",
"doi-asserted-by": "publisher",
"first-page": "43",
"issue": "2",
"journal-title": "Pharmazie",
"key": "10211_CR26",
"unstructured": "Kumar SA, Cheng W. A hypothesis: bitter taste receptors as a therapeutic target for the clinical symptoms of SARS-CoV-2. Pharmazie. 2021;76(2):43–54. https://doi.org/10.1691/pharmazie.2021.2.",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.2500/105065898781390271",
"author": "S-Y Fang",
"doi-asserted-by": "publisher",
"first-page": "131",
"issue": "2",
"journal-title": "Am J Rhinol",
"key": "10211_CR27",
"unstructured": "Fang S-Y, Druce HM, Baraniuk JN. Anticholinergic properties of brompheniramine, chlorpheniramine, and atropine in human nasal mucosa in vitro. Am J Rhinol. 1998;12(2):131–4. https://doi.org/10.2500/105065898781390271.",
"volume": "12",
"year": "1998"
},
{
"DOI": "10.1007/s00011-020-01422-1",
"author": "M Ennis",
"doi-asserted-by": "publisher",
"first-page": "67",
"issue": "1",
"journal-title": "Inflamm Res",
"key": "10211_CR28",
"unstructured": "Ennis M, Tiligada K. Histamine receptors and COVID-19. Inflamm Res. 2021;70(1):67–75. https://doi.org/10.1007/s00011-020-01422-1.",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.35248/2161-1459.21.10.275",
"author": "J Torres",
"doi-asserted-by": "publisher",
"first-page": "275",
"journal-title": "J Clin Exp Pharmacol",
"key": "10211_CR29",
"unstructured": "Torres J, Go CC, Chohan F, Genesis Camacho L, Sanchez-Gonzalez MA, Ferrer G. Chlorpheniramine maleate nasal spray in COVID-19Patients: Case Series. J Clin Exp Pharmacol. 2021;10:275. https://doi.org/10.35248/2161-1459.21.10.275.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1002/jmv.29014",
"doi-asserted-by": "publisher",
"key": "10211_CR30",
"unstructured": "Wang Y, Zhao D, Xiao W, Shi J, Chen W, Jia Q, et al. Paxlovid reduces the risk of long COVID in patients six months after hospital discharge. J Med Virol. 2023;95(8). https://doi.org/10.1002/jmv.29014."
},
{
"DOI": "10.7326/M23-1394",
"author": "GN Ioannou",
"doi-asserted-by": "publisher",
"first-page": "1486",
"issue": "11",
"journal-title": "Ann Intern Med",
"key": "10211_CR31",
"unstructured": "Ioannou GN, Berry K, Rajeevan N, Li Y, Mutalik P, Yan L, et al. Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among U.S. veterans: a target trial emulation. Ann Intern Med. 2023;176(11):1486–97. https://doi.org/10.7326/M23-1394.",
"volume": "176",
"year": "2023"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-024-10211-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Mitigating the risks of post-acute sequelae of SARS-CoV-2 infection (PASC) with intranasal chlorpheniramine: perspectives from the ACCROS studies",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}
