Outpatients prescribed with fluvoxamine around the time of COVID-19 diagnosis are not at a reduced risk of subsequent hospitalization and death compared to their non-prescribed peers: population-based matched cohort study
et al., European Journal of Clinical Pharmacology, doi:10.1007/s00228-023-03479-3, Nov 2022 (preprint)
30th treatment shown to reduce risk in
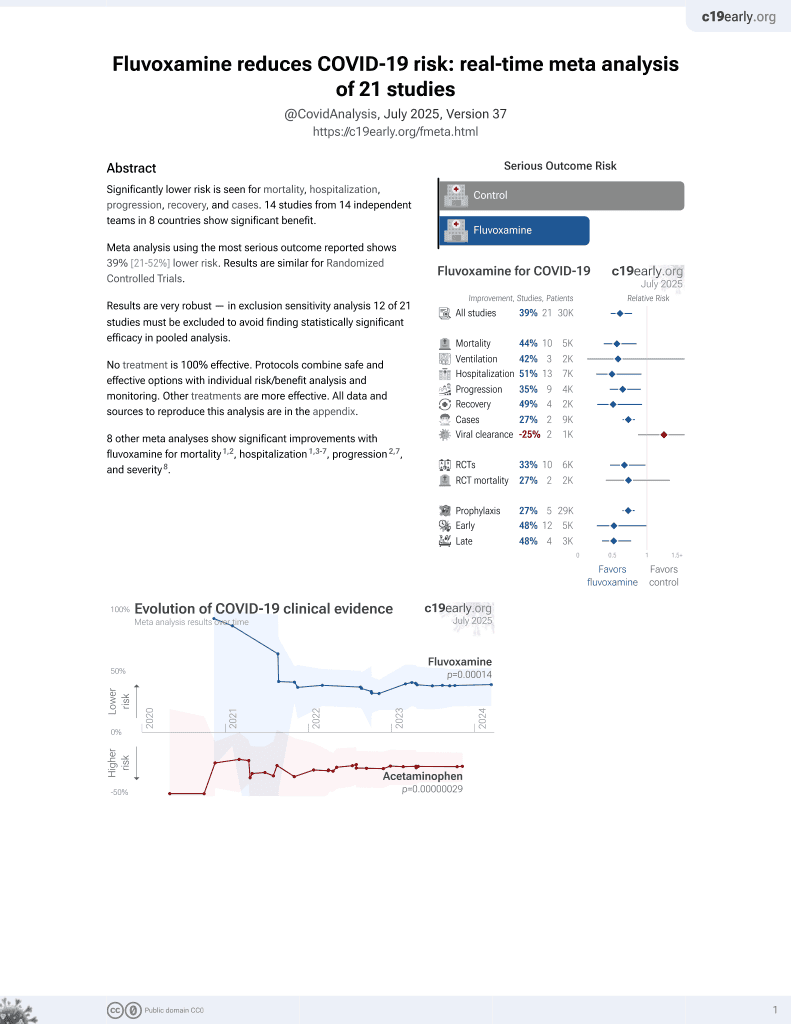
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
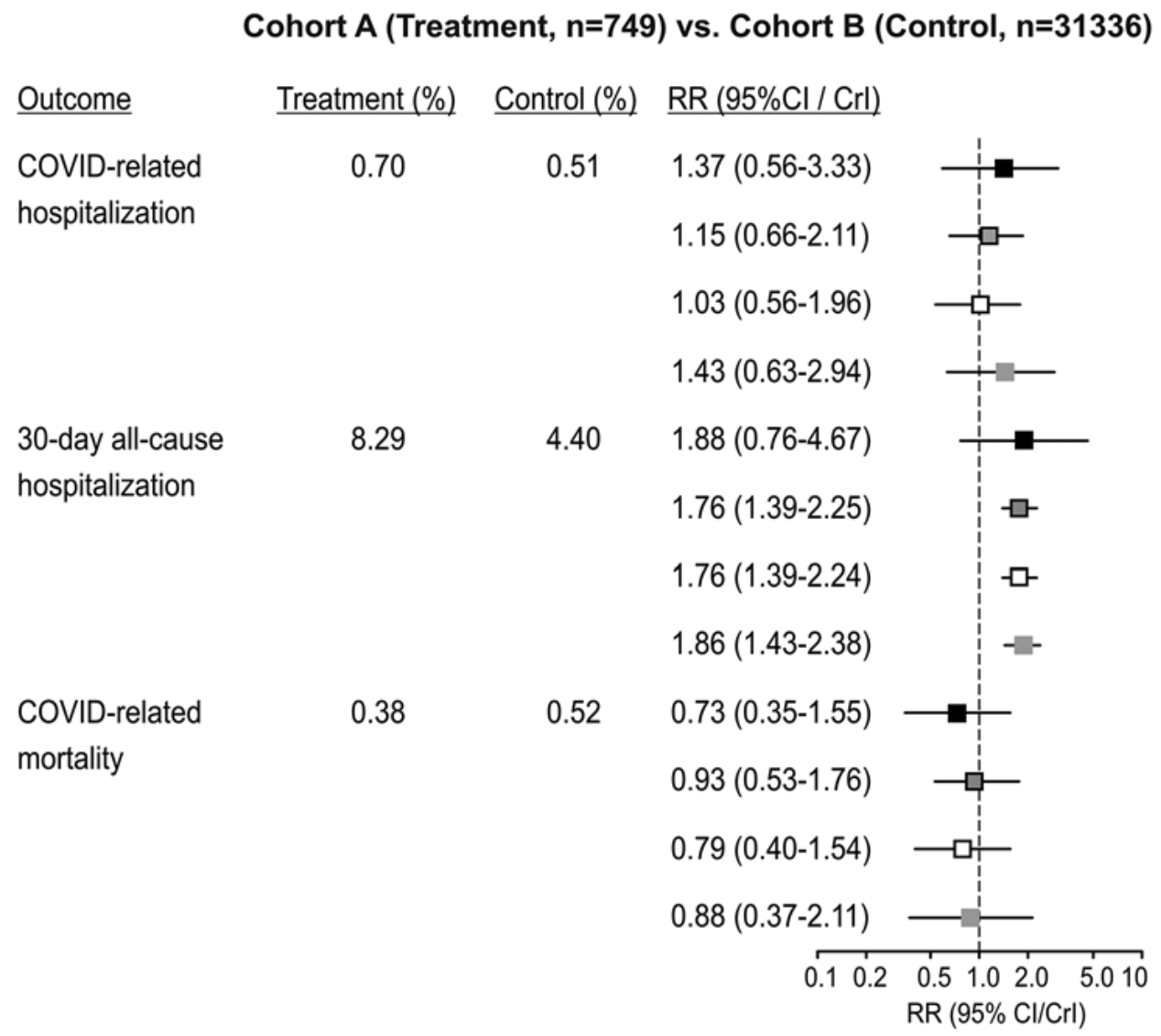
Retrospective COVID+ patients in Croatia, showing no significant difference in outcomes with fluvoxamine prophylaxis.
|
risk of death, 27.0% lower, RR 0.73, p = 0.41, treatment 749, control 31,336, cohort A vs. B, propensity score matching.
|
|
risk of hospitalization, 37.0% higher, RR 1.37, p = 0.50, treatment 749, control 31,336, cohort A vs. B, COVID-related, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Trkulja et al., 7 Nov 2022, retrospective, Croatia, peer-reviewed, 2 authors.
Contact: vladimir.trkulja@mef.hr.
Outpatients prescribed with fluvoxamine around the time of COVID-19 diagnosis are not at a reduced risk of subsequent hospitalization and death compared to their non-prescribed peers: population-based matched cohort study
European Journal of Clinical Pharmacology, doi:10.1007/s00228-023-03479-3
Purpose To assess the effect of exposure to fluvoxamine around the COVID-19 diagnosis on subsequent hospitalizations and mortality in COVID-19 outpatients in a real-life setting. Methods Using nationwide administrative data, we identified adult COVID-19 outpatients diagnosed up to August 15, 2021 and conducted two cohort studies. Study 1 included subjects prescribed fluvoxamine around the index COVID-19 diagnosis (Cohort A), their peers suffering similar psychiatric difficulties but not prescribed fluvoxamine (Cohort B) and those free of psychiatric difficulties/treatments (Cohort C). Study 2 included subjects prescribed fluvoxamine (Cohort Fluvoxamine) and their peers prescribed paroxetine (Cohort Paroxetine). Cohorts were mutually exactly matched and incidence of COVID-19-related hospitalization, 30-day all-cause hospitalization and of COVID-19-related mortality was estimated.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1007/ s00228-023-03479-3. Author contribution Vladimir Trkulja and Ivan Kodvanj designed the study, prepared and analyzed the data, drafted the manuscript and completed the final version. Both authors meet the ICMJE criteria for authorship.
Declarations Ethics approval This study used anonymized administrative data standardly collected on routine procedures, hence ethical approval was waived by the Ethics Committee of the Zagreb University School of Medicine and Croatian Institute for Public Healthy.
Competing interests The authors declare no competing interests. Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
References
Ballenger, Clinical guidelines for establishing remission in patients with depression and anxiety, J Clin Psyciatry
Beliveau, Boyne, Slater, Brenner, Arora, BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network meta-analyses, Med Res Methodol, doi:10.1186/s12874-019-0829-2
Bramante, Juling, Tignanelli, Buse, Liebovitz et al., Randomized trial of metformin, ivermectin and fluvoxamine for Covid-19, N Engl J Med
Ceban, Nogo, Carvalho, Lee, Nasri et al., Association between mood disorders and risk of COVID-19 infection, hospitalization and death. A systematic review and meta-analysis, JAMA Psychiat
Core, R: a language and environment for statistical computing
Fond, Nemani, Etchecopar-Etchart, Loundou, Goff et al., Association between mental health disorders and mortality among patients with COVID-19 in 7 countries: a systematic review and meta-analysis, JAMA Psychiat
Goodrich, Gabry, Ali, Brilleman, rstanarm: Bayesian applied regression modeling via Stan
Griffith, Morris, Tudball, Herbert, Mancano et al., Collider bias undermines our understanding of COVID-19 disease risk and severity, Nat Commun, doi:10.1038/s41467-020-19478-2
Hadley, Rhea, Jones, Li, Sotner et al., Enhancing the prediction of hospitalization from a COVID-19 agent-based model: a Bayesian method for model parameter estimation, PloS ONE, doi:10.1371/journal.pone.0264704
Heine, The episensr package: basic sensitivity analysis of epidemiological results, doi:10.5281/zenodo.4554553
Herrera-Esposito, De Los Campos, Age-specific rate of severe and critical SARS-CoV-2 infections estimated with multicountry seroprevalence studies, BMC Infect Dis, doi:10.1186/s12879-022-07262-0
Ho, Imai, King, Stuart, MatchIt : nonparametric preprocessing for parametric causal inference, J Stat Softw, doi:10.18637/jss.v042.i08
Ia, Coron aviru s Pande mic Count ry Profi le -Our World in Data
Kuran, Greer, Trivedi, Strategies to enhance the therapeutic efficacy of antidepressants: targeting residual symptoms, Expert Rev Neurother
Lenze, Fluvoxamine for early treatment of COVID-19: a fullyremote, randomized placebo controlled trial
Lenze, Mattar, Zorumski, Stevens, Schweiger et al., Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial, JAMA
Mahdi, Herman, Rethelyi, Balint, Potential role of antidepressants fluoxetine and fluvoxamine in the treatment of COVID-19, Int J Mol Sci, doi:10.3390/ijms23073812
Mas, Garcia-Vincente, Estrada-Gelnoch, Perez-Mana, Papaseit et al., Antidepressant drugs and COVID-19: a review of basic and clinical evidence, J Clin Med, doi:10.3390/jcm11144038
Mccarthy, Naggie, Boulware, Lindsell, Stewart et al., placebo-controlled, randomized platform clinical trial, doi:10.1101/2022.10.17.22281178
Oskotsky, Marić, Tang, Oskotsky, Wong et al., Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants, JAMA Network Open, doi:10.1001/jamanetworkopen.2021.33090
Reis, Moreira-Silva, Silva, Thabane, Milagres, Effect of early treatment with fluvoxamine on risk of emergeny care and hospitalizations among patients with COVID-19: the TOGETHER randomized platform trial, Lancet Glob Health, doi:10.1016/S2214-109X(21)00448-4
Ros, Aguera, De La Gandara, Rojo, De Pedro, Potentiation strategies for treatment-resistant depression, Acta Psyhciatr Scand Suppl
Schneeweiss, Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics, Pharmacoepidemiol Drug Saf
Schwarzer, Carpenter, Rucker, Meta-analysis with R
Seo, Kim, Bae, Park, Chung et al., Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial, Infect Chemother
Strom, Study design available for pharmacoepidemiological studies
Sukhatme, Reiersen, Vayttaden, Sukhatme, Fluvoxiamine: a review of its mechanism of action and its role in COVID-19, Frontiers Pharmacol, doi:10.3389/fphar.2021.652688
Trkulja, Fluvoxamine for COVID-19 outpatients: for the time being, we might prefer to curb our optimism, Br J Clin Pharmacol
Vai, Mazza, Colli, Foiselle, Allen et al., Mental disorders and risk of COVID-19-related mortality, hospitalization, and intensive care unit admission: a systematic review and meta-analysis, Lancet Psychiatry
Van Valkenhoef, Lu, De Brock, Hillege, Ades et al., Automating network meta-analysis, Res Synth. Methods
Zheng, Sun, Cai, Zhang, Ng et al., Antidepressants for COVID-19: a systematic review, J Affective Disord
DOI record:
{
"DOI": "10.1007/s00228-023-03479-3",
"ISSN": [
"0031-6970",
"1432-1041"
],
"URL": "http://dx.doi.org/10.1007/s00228-023-03479-3",
"alternative-id": [
"3479"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "5 November 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "14 March 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "24 March 2023"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study used anonymized administrative data standardly collected on routine procedures, hence ethical approval was waived by the Ethics Committee of the Zagreb University School of Medicine and Croatian Institute for Public Healthy."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The authors declare no competing interests."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0968-1194",
"affiliation": [],
"authenticated-orcid": false,
"family": "Trkulja",
"given": "Vladimir",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1359-3701",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kodvanj",
"given": "Ivan",
"sequence": "additional"
}
],
"container-title": "European Journal of Clinical Pharmacology",
"container-title-short": "Eur J Clin Pharmacol",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
24
]
],
"date-time": "2023-03-24T07:02:56Z",
"timestamp": 1679641376000
},
"deposited": {
"date-parts": [
[
2023,
3,
24
]
],
"date-time": "2023-03-24T09:03:23Z",
"timestamp": 1679648603000
},
"funder": [
{
"DOI": "10.13039/501100007682",
"award": [
"no grant id",
"no grant id"
],
"doi-asserted-by": "publisher",
"name": "Medicinski Fakultet, Sveučilište u Zagrebu"
}
],
"indexed": {
"date-parts": [
[
2023,
3,
25
]
],
"date-time": "2023-03-25T04:57:36Z",
"timestamp": 1679720256928
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
3,
24
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
24
]
],
"date-time": "2023-03-24T00:00:00Z",
"timestamp": 1679616000000
}
},
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
24
]
],
"date-time": "2023-03-24T00:00:00Z",
"timestamp": 1679616000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s00228-023-03479-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s00228-023-03479-3/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s00228-023-03479-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2023,
3,
24
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
24
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1001/jama.2020.22760",
"author": "EJ Lenze",
"doi-asserted-by": "publisher",
"first-page": "2292",
"journal-title": "JAMA",
"key": "3479_CR1",
"unstructured": "Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE et al (2020) Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA 324:2292–2300",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/S2214-109X(21)00448-4",
"author": "G Reis",
"doi-asserted-by": "publisher",
"first-page": "e42",
"journal-title": "Lancet Glob Health",
"key": "3479_CR2",
"unstructured": "Reis G, dos Santos Moreira-Silva EA, Medeiros Silva DC, Thabane L, Cruz Milagres A et al (2022) Effect of early treatment with fluvoxamine on risk of emergeny care and hospitalizations among patients with COVID-19: the TOGETHER randomized platform trial. Lancet Glob Health 10:e42-51. https://doi.org/10.1016/S2214-109X(21)00448-4",
"volume": "10",
"year": "2022"
},
{
"key": "3479_CR3",
"unstructured": "Lenze E. Fluvoxamine for early treatment of COVID-19: a fully-remote, randomized placebo controlled trial. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04668950. Accessed 26 Sept 2022"
},
{
"DOI": "10.3947/ic.2021.0142",
"author": "H Seo",
"doi-asserted-by": "publisher",
"first-page": "102",
"journal-title": "Infect Chemother",
"key": "3479_CR4",
"unstructured": "Seo H, Kim H, Bae S, Park S, Chung H, Sung HS et al (2022) Fluvoxamine treatment of patients with symptomatic COVID-19 in a community treatment center: a preliminary result of randomized controlled trial. Infect Chemother 54:102–113",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2201662",
"author": "CT Bramante",
"doi-asserted-by": "publisher",
"first-page": "599",
"journal-title": "N Engl J Med",
"key": "3479_CR5",
"unstructured": "Bramante CT, Juling JD, Tignanelli CJ, Buse JB, Liebovitz DM, Nicklas JM et al (2022) Randomized trial of metformin, ivermectin and fluvoxamine for Covid-19. N Engl J Med 387:599–610",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1101/2022.10.17.22281178",
"doi-asserted-by": "publisher",
"key": "3479_CR6",
"unstructured": "McCarthy MW, Naggie S, Boulware DR, Lindsell CJ, Stewart TG, Felker GM et al (2022) Fluvoxamine for outpatient treatment of COVID-19: a decentralized, placebo-controlled, randomized platform clinical trial. medRxiv. https://doi.org/10.1101/2022.10.17.22281178"
},
{
"DOI": "10.3389/fphar.2021.652688",
"doi-asserted-by": "publisher",
"key": "3479_CR7",
"unstructured": "Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV (2021) Fluvoxiamine: a review of its mechanism of action and its role in COVID-19. Frontiers Pharmacol 12:652688. https://doi.org/10.3389/fphar.2021.652688"
},
{
"DOI": "10.1016/j.jad.2022.03.059",
"author": "W Zheng",
"doi-asserted-by": "publisher",
"first-page": "108",
"journal-title": "J Affective Disord",
"key": "3479_CR8",
"unstructured": "Zheng W, Sun HL, Cai H, Zhang Q, Ng CH, Xiang YT (2022) Antidepressants for COVID-19: a systematic review. J Affective Disord 307:108–114",
"volume": "307",
"year": "2022"
},
{
"DOI": "10.3390/jcm11144038",
"author": "M Mas",
"doi-asserted-by": "publisher",
"first-page": "4038",
"journal-title": "J Clin Med",
"key": "3479_CR9",
"unstructured": "Mas M, Garcia-Vincente JA, Estrada-Gelnoch A, Perez-Mana C, Papaseit E, Torrens M et al (2022) Antidepressant drugs and COVID-19: a review of basic and clinical evidence. J Clin Med 11:4038. https://doi.org/10.3390/jcm11144038",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1002/9781119701101",
"doi-asserted-by": "crossref",
"key": "3479_CR10",
"unstructured": "Strom BL (2021) Study design available for pharmacoepidemiological studies. In: Strom BL, Kimmel E, Hennessy S, eds. Textbook of pharmacoepidemiology, 3rd ed. Hoboken, NJ: Wiley-Blackwell 20–46"
},
{
"DOI": "10.1016/S2215-0366(21)00232-7",
"author": "B Vai",
"doi-asserted-by": "publisher",
"first-page": "797",
"journal-title": "Lancet Psychiatry",
"key": "3479_CR11",
"unstructured": "Vai B, Mazza MG, Delli Colli C, Foiselle M, Allen B, Benedetti F et al (2021) Mental disorders and risk of COVID-19-related mortality, hospitalization, and intensive care unit admission: a systematic review and meta-analysis. Lancet Psychiatry 8:797–812",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1001/jamapsychiatry.2021.2274",
"author": "G Fond",
"doi-asserted-by": "publisher",
"first-page": "1208",
"journal-title": "JAMA Psychiat",
"key": "3479_CR12",
"unstructured": "Fond G, Nemani K, Etchecopar-Etchart D, Loundou A, Goff DC, Lee SW et al (2021) Association between mental health disorders and mortality among patients with COVID-19 in 7 countries: a systematic review and meta-analysis. JAMA Psychiat 78:1208–1217",
"volume": "78",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-19478-2",
"author": "GJ Griffith",
"doi-asserted-by": "publisher",
"first-page": "5749",
"issue": "1",
"journal-title": "Nat Commun",
"key": "3479_CR13",
"unstructured": "Griffith GJ, Morris TT, Tudball MJ, Herbert A, Mancano G, Pike L et al (2020) Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun 11(1):5749. https://doi.org/10.1038/s41467-020-19478-2",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1001/jamapsychiatry.2021.1818",
"author": "F Ceban",
"doi-asserted-by": "publisher",
"first-page": "1079",
"journal-title": "JAMA Psychiat",
"key": "3479_CR14",
"unstructured": "Ceban F, Nogo D, Carvalho IP, Lee Y, Nasri F, Xiong J et al (2021) Association between mood disorders and risk of COVID-19 infection, hospitalization and death. A systematic review and meta-analysis. JAMA Psychiat 78:1079–1091",
"volume": "78",
"year": "2021"
},
{
"DOI": "10.3390/ijms23073812",
"author": "M Mahdi",
"doi-asserted-by": "publisher",
"first-page": "3812",
"journal-title": "Int J Mol Sci",
"key": "3479_CR15",
"unstructured": "Mahdi M, Herman L, Rethelyi JM, Balint L (2022) Potential role of antidepressants fluoxetine and fluvoxamine in the treatment of COVID-19. Int J Mol Sci 23:3812. https://doi.org/10.3390/ijms23073812",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.33090",
"doi-asserted-by": "publisher",
"key": "3479_CR16",
"unstructured": "Oskotsky T, Marić I, Tang A, Oskotsky B, Wong RJ, Aghaeepour N et al (2021) Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Network Open 4(11):e2133090. https://doi.org/10.1001/jamanetworkopen.2021.33090"
},
{
"DOI": "10.18637/jss.v042.i08",
"doi-asserted-by": "publisher",
"key": "3479_CR17",
"unstructured": "Ho DE, Imai K, King G, Stuart EA (2011) MatchIt : nonparametric preprocessing for parametric causal inference. J Stat Softw 42(8):1–28. https://doi.org/10.18637/jss.v042.i08"
},
{
"key": "3479_CR18",
"unstructured": "R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria"
},
{
"DOI": "10.1111/bcp.15451",
"author": "V Trkulja",
"doi-asserted-by": "publisher",
"first-page": "4654",
"journal-title": "Br J Clin Pharmacol",
"key": "3479_CR19",
"unstructured": "Trkulja V (2022) Fluvoxamine for COVID-19 outpatients: for the time being, we might prefer to curb our optimism. Br J Clin Pharmacol 88:4654–4656",
"volume": "88",
"year": "2022"
},
{
"key": "3479_CR20",
"unstructured": "Goodrich B, Gabry J, Ali I, Brilleman S (2022) rstanarm: Bayesian applied regression modeling via Stan. R package version 2.21.3 https://mc-stan.org/rstanarm/"
},
{
"author": "G Schwarzer",
"first-page": "187",
"journal-title": "Springer International Publishing Switzerland",
"key": "3479_CR21",
"unstructured": "Schwarzer G, Carpenter JR, Rucker G (2015) Meta-analysis with R. Springer International Publishing Switzerland 2015:187–216",
"volume": "2015",
"year": "2015"
},
{
"DOI": "10.1186/s12874-019-0829-2",
"author": "A Beliveau",
"doi-asserted-by": "publisher",
"first-page": "196",
"journal-title": "Med Res Methodology",
"key": "3479_CR22",
"unstructured": "Beliveau A, Boyne DJ, Slater J, Brenner D, Arora P (2019) BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network meta-analyses. Med Res Methodol 19:196. https://doi.org/10.1186/s12874-019-0829-2",
"volume": "19",
"year": "2019"
},
{
"author": "G van Valkenhoef",
"first-page": "285",
"journal-title": "Methods",
"key": "3479_CR23",
"unstructured": "van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton J (2012) Automating network meta-analysis. Res Synth. Methods 3:285–299",
"volume": "3",
"year": "2012"
},
{
"DOI": "10.1002/pds.1200",
"author": "S Schneeweiss",
"doi-asserted-by": "publisher",
"first-page": "291",
"journal-title": "Pharmacoepidemiol Drug Saf",
"key": "3479_CR24",
"unstructured": "Schneeweiss S (2006) Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 15:291–303",
"volume": "15",
"year": "2006"
},
{
"DOI": "10.5281/zenodo.4554553",
"doi-asserted-by": "publisher",
"key": "3479_CR25",
"unstructured": "Heine D (2021) The episensr package: basic sensitivity analysis of epidemiological results. https://doi.org/10.5281/zenodo.4554553, R package version 1.1.0. https://dhaine.github.io/episensr/"
},
{
"DOI": "10.1586/ern.09.53",
"author": "BT Kuran",
"doi-asserted-by": "publisher",
"first-page": "975",
"journal-title": "Expert Rev Neurother",
"key": "3479_CR26",
"unstructured": "Kuran BT, Greer TL, Trivedi MH (2010) Strategies to enhance the therapeutic efficacy of antidepressants: targeting residual symptoms. Expert Rev Neurother 9:975–984",
"volume": "9",
"year": "2010"
},
{
"DOI": "10.1111/j.1600-0447.2005.00676.x",
"author": "S Ros",
"doi-asserted-by": "publisher",
"first-page": "14",
"journal-title": "Acta Psyhciatr Scand Suppl",
"key": "3479_CR27",
"unstructured": "Ros S, Aguera L, de la Gandara J, Rojo JE, de Pedro JM (2005) Potentiation strategies for treatment-resistant depression. Acta Psyhciatr Scand Suppl 35:14–24",
"volume": "35",
"year": "2005"
},
{
"author": "JC Ballenger",
"first-page": "29",
"issue": "Suppl. 22",
"journal-title": "J Clin Psyciatry",
"key": "3479_CR28",
"unstructured": "Ballenger JC (1999) Clinical guidelines for establishing remission in patients with depression and anxiety. J Clin Psyciatry 60(Suppl. 22):29–34",
"volume": "60",
"year": "1999"
},
{
"DOI": "10.1371/journal.pone.0264704",
"doi-asserted-by": "publisher",
"key": "3479_CR29",
"unstructured": "Hadley E, Rhea S, Jones K, Li L, Sotner M, Bobashev G (2022). Enhancing the prediction of hospitalization from a COVID-19 agent-based model: a Bayesian method for model parameter estimation. PloS ONE 17(3):e0264704. https://doi.org/10.1371/journal.pone.0264704"
},
{
"DOI": "10.1186/s12879-022-07262-0",
"author": "D Herrera-Esposito",
"doi-asserted-by": "publisher",
"first-page": "311",
"journal-title": "BMC Infect Dis",
"key": "3479_CR30",
"unstructured": "Herrera-Esposito D, de los Campos G, (2022) Age-specific rate of severe and critical SARS-CoV-2 infections estimated with multi-country seroprevalence studies. BMC Infect Dis 22:311. https://doi.org/10.1186/s12879-022-07262-0",
"volume": "22",
"year": "2022"
},
{
"key": "3479_CR31",
"unstructured": "Croatia: Coronavirus Pandemic Country Profile - Our World in Data. Accessed 20 Sept 2022"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s00228-023-03479-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology",
"General Medicine"
],
"subtitle": [],
"title": "Outpatients prescribed with fluvoxamine around the time of COVID-19 diagnosis are not at a reduced risk of subsequent hospitalization and death compared to their non-prescribed peers: population-based matched cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}