Therapeutic Efficacy of AFree Oral Spray on the Symptoms and Course of Moderate and Severe COVID-19 in the Field Hospital
et al., In Vivo, doi:10.21873/invivo.13262, Jun 2023
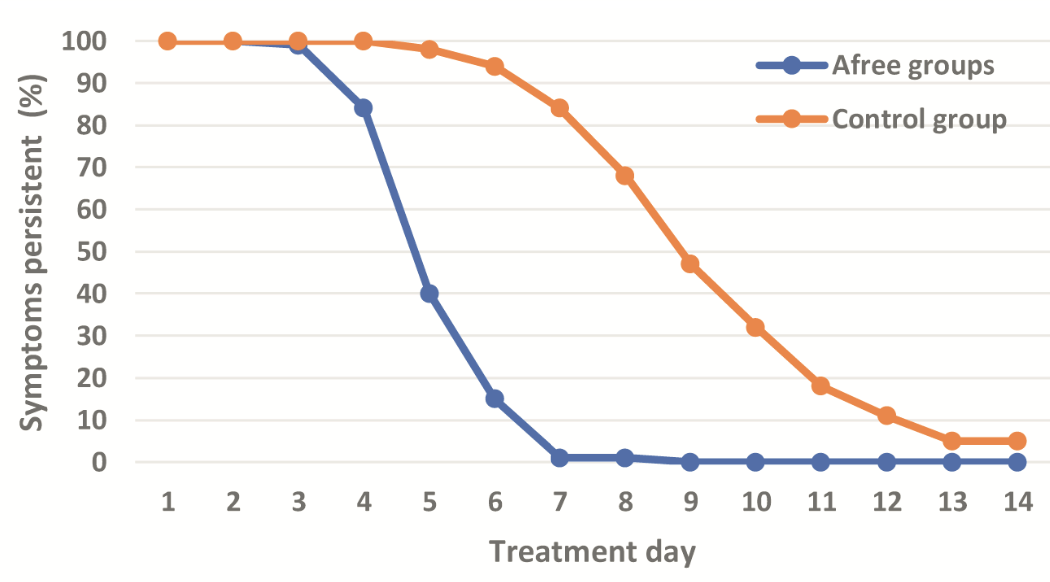
RCT 200 hospitalized patients in Vietnam, showing faster recovery with an oral spray containing zinc, propolis, xylitol, ginger, and DMSO.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects (early treatment may be more beneficial).
This study is excluded in meta-analysis:
many combined treatments which may significantly contribute to the effect seen.
Study covers propolis and zinc.
|
risk of no recovery, 84.0% lower, RR 0.16, p < 0.001, treatment 15 of 100 (15.0%), control 94 of 100 (94.0%), NNT 1.3, mid-recovery, day 6.
|
|
risk of no recovery, 90.9% lower, RR 0.09, p = 0.06, treatment 0 of 100 (0.0%), control 5 of 100 (5.0%), NNT 20, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 14.
|
|
risk of no recovery, 98.5% lower, RR 0.02, p < 0.001, treatment 0 of 100 (0.0%), control 32 of 100 (32.0%), NNT 3.1, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 10.
|
|
risk of no recovery, 98.8% lower, RR 0.01, p < 0.001, treatment 1 of 100 (1.0%), control 84 of 100 (84.0%), NNT 1.2, day 7.
|
|
risk of no recovery, 59.2% lower, RR 0.41, p < 0.001, treatment 40 of 100 (40.0%), control 98 of 100 (98.0%), NNT 1.7, day 5.
|
|
risk of no recovery, 16.0% lower, RR 0.84, p < 0.001, treatment 84 of 100 (84.0%), control 100 of 100 (100.0%), NNT 6.2, day 4.
|
|
risk of no recovery, 2.0% lower, RR 0.98, p = 0.50, treatment 98 of 100 (98.0%), control 100 of 100 (100.0%), NNT 50, day 3.
|
|
risk of no viral clearance, 77.8% lower, RR 0.22, p = 0.06, treatment 2 of 100 (2.0%), control 9 of 100 (9.0%), NNT 14, 4th test.
|
|
risk of no viral clearance, 84.2% lower, RR 0.16, p < 0.001, treatment 3 of 100 (3.0%), control 19 of 100 (19.0%), NNT 6.2, 3rd test.
|
|
risk of no viral clearance, 25.9% lower, RR 0.74, p = 0.32, treatment 20 of 100 (20.0%), control 27 of 100 (27.0%), NNT 14, 2nd test.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Tran et al., 27 Jun 2023, Single Blind Randomized Controlled Trial, Vietnam, peer-reviewed, 8 authors, this trial uses multiple treatments in the treatment arm (combined with ginger, xylitol, zinc, and DMSO) - results of individual treatments may vary.
Contact: baxuanho@usc.edu.
Therapeutic Efficacy of AFree Oral Spray on the Symptoms and Course of Moderate and Severe COVID-19 in the Field Hospital
In Vivo, doi:10.21873/invivo.13262
Background/Aim: A prospective randomized, openlabel, single-blinded clinical trial was conducted to evaluate the efficacy of AFree on the symptoms and course of moderate and severe COVID-19 in the field hospital. Patients and Methods: Two hundred hospitalized patients diagnosed with COVID-19 were enrolled. The patients were randomized into 100 patients in the interventional AFree group and 100 in the control group. The AFree group patients were treated with AFree oral spray in conjunction with the standard COVID-19 treatment protocol, while the control group of patients were treated with only standard care. Results: Patients of the AFree group demonstrated a remarkedly faster improvement in all COVID-19-related symptoms, resulting in a shorter time for complete recovery than the control group. More importantly, they showed a shorter time for complete viral clearance. Adding AFree to the standard of care protocol also significantly improved the restoration of taste and smell and reduced lung infiltration. Additionally, the patients in the AFree group also exhibited fewer adverse effects related to treatment. Conclusion: AFree oral spray is a simple-to-use, safe, and effective adjunctive treatment for moderate and severe COVID-19 cases. AFree oral spray was demonstrated to potentially be effective for COVID-19 prevention.
Conflicts of Interest The Authors declare no conflicts of interest regarding the data presented in this publication.
Authors' Contributions
References
Chopra, Sivaraman, Radhakrishnan, Balakrishnan, Narayana, Can povidone iodine gargle/mouthrinse inactivate SARS-CoV-2 and decrease the risk of nosocomial and community transmission during the COVID-19 pandemic? An evidence-based update, Japanese Dental Science Review, doi:10.1016/j.jdsr.2021.03.001
Fang, Roth, 'ng, Tamm, Han et al., Zinc salicylate reduces airway smooth muscle cells remodelling by blocking mTOR and activating p21(Waf1/Cip1), The Journal of Nutritional Biochemistry, doi:10.1016/j.jnutbio.2020.108563
Han, Hoang, Opinions on the current pandemic of COVID-19: Use functional food to boost our immune functions, Journal of Infection and Public Health, doi:10.1016/j.jiph.2020.08.014
Hoang, Han, A possible application of hinokitiol as a natural zinc ionophore and anti-infective agent for the prevention and treatment of COVID-19 and viral infections, Medical Hypotheses, doi:10.1016/j.mehy.2020.110333
Hoang, Han, Micronutrient zinc roles in adjunctive therapy for COVID-19 by enhancing patients immunoregulation and tolerance to the pathogen, Reviews in Medical Microbiology, doi:10.1097/MRM.0000000000000263
Hoang, Hoang, Han, Zinc Iodide in combination with Dimethyl Sulfoxide for treatment of SARS-CoV-2 and other viral infections, Med Hypotheses, doi:10.1016/j.mehy.2020.109866
Hoang, Nguyen, Nguyen, Fang, Han et al., Application of dimethyl sulfoxide as a therapeutic agent and drug vehicle for eye diseases, Journal of Ocular Pharmacology and Therapeutics, doi:10.1089/jop.2021.0043
Hoang, Shaw, Fang, Han, Possible application of highdose vitamin C in the prevention and therapy of coronavirus infection, Journal of Global Antimicrobial Resistance, doi:10.1016/j.jgar.2020.09.025
Peng, Xu, Li, Cheng, Zhou et al., Transmission routes of 2019-nCoV and controls in dental practice, International Journal of Oral Science, doi:10.1038/s41368-020-0075-9
To, Tsang, Yip, Chan, Wu et al., Consistent detection of 2019 novel Coronavirus in saliva, Clinical Infectious Diseases, doi:10.1093/cid/ciaa149
Xu, Cui, Duan, Zhang, Zhou et al., Saliva: potential diagnostic value and transmission of 2019-nCoV, International Journal of Oral Science, doi:10.1038/s41368-020-0080-z
Zhang, Liu, Potential interventions for novel coronavirus in China: A systematic review, Journal of Medical Virology, doi:10.1002/jmv.25707
DOI record:
{
"DOI": "10.21873/invivo.13262",
"ISSN": [
"0258-851X",
"1791-7549"
],
"URL": "http://dx.doi.org/10.21873/invivo.13262",
"alternative-id": [
"10.21873/invivo.13262"
],
"author": [
{
"affiliation": [],
"family": "TRAN",
"given": "DUONG T.",
"sequence": "first"
},
{
"affiliation": [],
"family": "PHAM",
"given": "TRUONG N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "NGUYEN",
"given": "NHUNG H.T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "TRAN",
"given": "HAU D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "HOANG",
"given": "HUY Q.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "NGUYEN",
"given": "ANH K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "HAN",
"given": "BO",
"sequence": "additional"
},
{
"affiliation": [],
"family": "HOANG",
"given": "BA X.",
"sequence": "additional"
}
],
"container-title": "In Vivo",
"container-title-short": "In Vivo",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
6,
27
]
],
"date-time": "2023-06-27T15:21:03Z",
"timestamp": 1687879263000
},
"deposited": {
"date-parts": [
[
2023,
6,
27
]
],
"date-time": "2023-06-27T15:22:06Z",
"timestamp": 1687879326000
},
"indexed": {
"date-parts": [
[
2023,
6,
28
]
],
"date-time": "2023-06-28T04:27:18Z",
"timestamp": 1687926438496
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2023,
6,
27
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.21873/invivo.13262",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "9336",
"original-title": [],
"page": "1743-1750",
"prefix": "10.21873",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
27
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "Anticancer Research USA Inc.",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://iv.iiarjournals.org/lookup/doi/10.21873/invivo.13262"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology",
"General Biochemistry, Genetics and Molecular Biology",
"Cancer Research"
],
"subtitle": [],
"title": "Therapeutic Efficacy of AFree Oral Spray on the Symptoms and Course of Moderate and Severe COVID-19 in the Field Hospital",
"type": "journal-article",
"volume": "37"
}
tran